Unravelling Impaired Hypoalgesia at Rest and in Response to Exercise in Patients with Chronic Whiplash-Associated Disorders: Effects of a Single Administration of Selective Serotonin Reuptake Inhibitor versus Selective Norepinephrine Reuptake Inhibitor
Abstract
1. Introduction
2. Materials and Methods
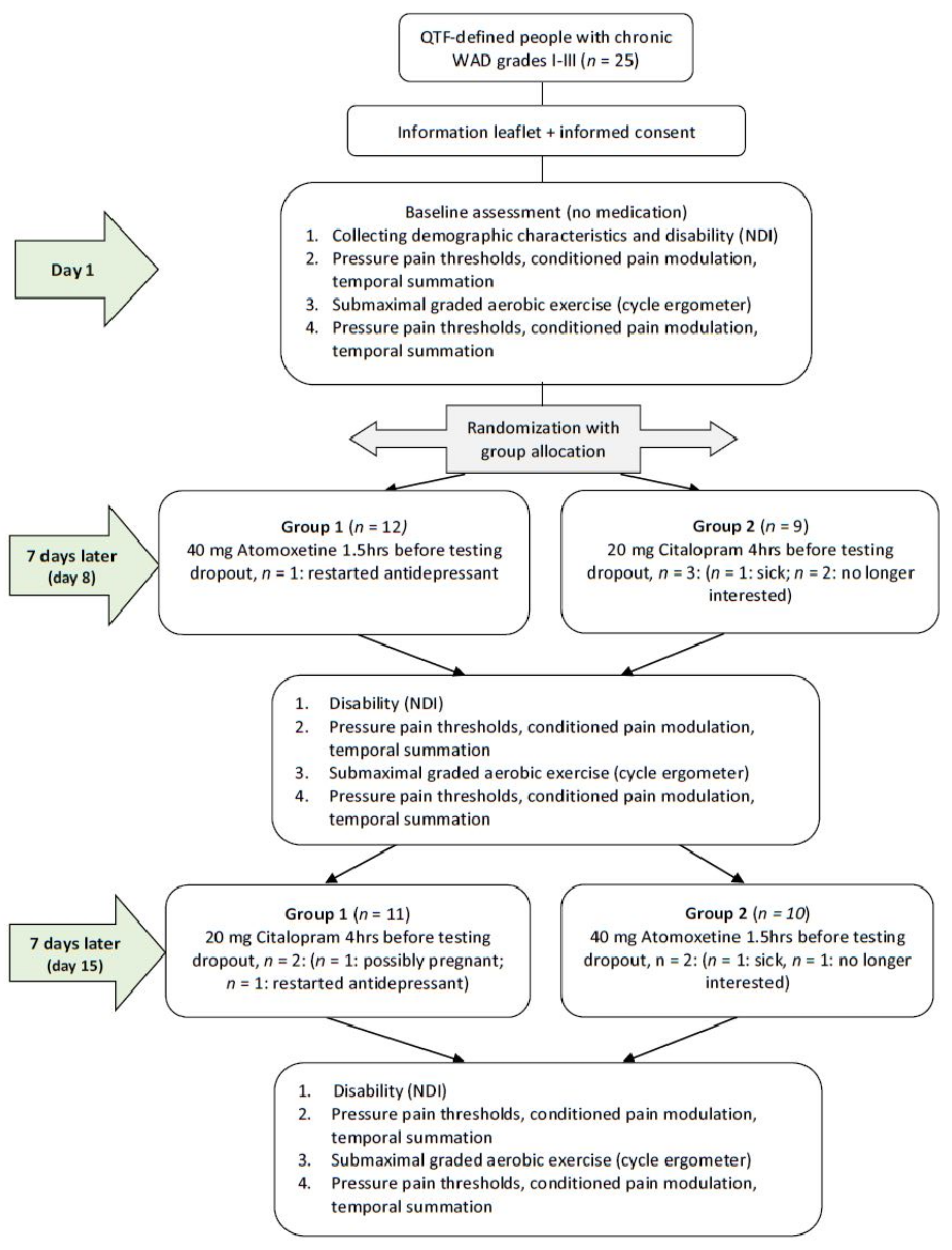
2.1. Study Design and Setting
2.2. Participants
2.3. Procedure
2.4. Medication Administration
2.5. Submaximal Aerobic Exercise
2.6. Demographic Characteristics
2.7. Self-Reported Measures
2.7.1. Neck-Pain-Related Disability
2.7.2. Self-Reported Pain Intensity
2.7.3. Experimental Pain Measurements–Quantitative Sensory Testing
2.8. Statistical Analysis
3. Results
3.1. Group Characteristics and Self-Reported Measures
3.2. The Isolated Effect of a Single Dose of a SSRI or a Selective NRI on Self-Reported and Experimental Pain Measurements at Rest in Patients with Chronic WAD
3.3. The Isolated Effect of a Single Dose of a SSRI or a Selective NRI on Exercise-Induced Hypoalgesia in Patients with Chronic WAD
3.4. Confounding Effect of Occupational Status on the Effect of a Single Dose of a SSRI or a Selective NRI on Exercise-Induced Hypoalgesia in Patients with Chronic WAD
4. Discussion
4.1. Neither a Single Dose of SSRI Nor Selective NRI Influence Hypoalgesia at Rest in Patients with Chronic WAD
4.2. Neither a Single Dose of SSRI Nor Selective NRI Influenced Exercise-Induced Hypoalgesia in Patients with Chronic WAD
4.3. Occupational Status Influenced the Effect of a Single Dose of SSRI and Selective NRI on Hypoalgesia at Rest and in Response to Exercise in Patients with Chronic WAD
4.4. Study Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Bannister, K.; Dickenson, A.H. What do monoamines do in pain modulation? Curr. Opin. Support Palliat. Care 2016, 10, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Pertovaara, A. Noradrenergic pain modulation. Prog. Neurobiol. 2006, 80, 53–83. [Google Scholar] [CrossRef]
- Marks, D.; Shah, M.; Patkar, A.; Masand, P.; Park, G.-Y.; Pae, C.-U. Serotonin-norepinephrine reuptake inhibitors for pain control: Premise and promise. Curr. Neuropharmacol. 2009, 7, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Leysen, L.; Vanlauwe, J.; Logghe, T.; Ickmans, K.; Polli, A.; Malfliet, A.; Coppieters, I.; Huysmans, E. Treatment of central sensitization in patients with chronic pain: Time for change? Expert Opin. Pharmacother. 2019, 20, 1961–1970. [Google Scholar] [CrossRef]
- Sterling, M. Physiotherapy management of whiplash-associated disorders (WAD). J. Physiother. 2014, 60, 5–12. [Google Scholar] [CrossRef]
- Spitzer, W.O.J.S. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: Redefining “whiplash” and its management. Spine 1995, 20 (Suppl. 8), 1S–73S. [Google Scholar]
- Van Oosterwijck, J.; Nijs, J.; Meeus, M.; Paul, L. Evidence for central sensitization in chronic whiplash: A systematic literature review. Eur. J. Pain. 2013, 17, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152 (Suppl. 3), S2–S15. [Google Scholar] [CrossRef]
- Rice, D.; Nijs, J.; Kosek, E.; Wideman, T.; Hasenbring, M.I.; Koltyn, K.; Graven-Nielsen, T.; Polli, A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain. 2019, 20, 1249–1266. [Google Scholar] [CrossRef]
- Nijs, J.; George, S.Z.; Clauw, D.J.; Fernández-De-Las-Peñas, C.; Kosek, E.; Ickmans, K.; Fernández-Carnero, J.; Polli, A.; Kapreli, E.; Huysmans, E.; et al. Central sensitisation in chronic pain conditions: Latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021, 3, e383–e392. [Google Scholar] [CrossRef]
- Daenen, L.; Nijs, J.; Roussel, N.; Wouters, K.; Van Loo, M.; Cras, P. Dysfunctional pain inhibition in patients with chronic whiplash-associated disorders: An experimental study. Clin. Rheumatol. 2013, 32, 23–31. [Google Scholar] [CrossRef]
- Van Oosterwijck, J.; Nijs, J.; Meeus, M.; Van Loo, M.; Paul, L. Lack of endogenous pain inhibition during exercise in people with chronic whiplash associated disorders: An experimental study. J. Pain. 2012, 13, 242–254. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Shepanski, M.A.; Ruble, S.B.; Valic, Z.; Buckwalter, J.B.; Clifford, P. Intensity and duration threshold for aerobic exercise-induced analgesia to pressure pain. Arch. Phys. Med. Rehabil. 2004, 85, 1183–1187. [Google Scholar] [CrossRef]
- Meeus, M.; Hermans, L.; Ickmans, K.; Struyf, F.; Van Cauwenbergh, D.; Bronckaerts, L.; De Clerck, L.S.; Moorken, G.; Hans, G.; Grosemans, S.; et al. Endogenous pain modulation in response to exercise in patients with rheumatoid arthritis, patients with chronic fatigue syndrome and comorbid fibromyalgia, and healthy controls: A double-blind randomized controlled trial. Pain. Pract. 2015, 15, 98–106. [Google Scholar] [CrossRef]
- Da Silva Santos, R.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. J. Physiol. Pharmacol. 2018, 69, 3–13. [Google Scholar]
- Brown, B.S.; Payne, T.; Kim, C.; Moore, G.; Krebs, P.; Martin, W. Chronic response of rat brain norepinephrine and serotonin levels to endurance training. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 46, 19–23. [Google Scholar] [CrossRef]
- Smith, A.; Ritchie, C.; Pedler, A.; McCamley, K.; Roberts, K.; Sterling, M. Exercise induced hypoalgesia is elicited by isometric, but not aerobic exercise in individuals with chronic whiplash associated disorders. Scand. J. Pain 2017, 15, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, J.M.; Heisey, D.M. The Abuse of Power. Am. Stat. 2001, 55, 19–24. [Google Scholar] [CrossRef]
- Nijs, J.; Inghelbrecht, E.; Daenen, L.; Hachimi-Idrissi, S.; Hens, L.; Willems, B.; Roussel, N.; Cras, P.; Wouters, K.; Bernheim, J. Recruitment bias in chronic pain research: Whiplash as a model. Clin. Rheumatol. 2011, 30, 1481–1489. [Google Scholar] [CrossRef]
- Coppieters, I.; Nijs, J.; Meeus, M.; De Kooning, M.; Rheel, E.; Huysmans, E.; Pas, R.; Van Bogaert, W.; Hubloue, I.; Ickmans, K. The Role of Serotonergic and Noradrenergic Descending Pathways on Performance-Based Cognitive Functioning at Rest and in Response to Exercise in People with Chronic Whiplash-Associated Disorders: A Randomized Controlled Crossover Study. Clin. Pract. 2023, 13, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Ickmans, K.; Malfliet, A.; De Kooning, M.; Goudman, L.; Hubloue, I.; Schmitz, T.; Goubert, D.; Ferrandiz, M.E.A. Lack of Gender and Age Differences in Pain Measurements Following Exercise in People with Chronic Whiplash-Associated Disorders. Pain. Physician 2017, 20, E829–E840. [Google Scholar] [CrossRef]
- Copieters, I.; Ickmans, K.; Huysmans, E. Brain neurotransmission, exercise and cognitive functioning in people with chronic whiplash-associated disorders: A randomized controlled crossover study. In Proceedings of the EFIC Congress 2019—Pain in Europe XI, Valencia, Spain, 4–7 September 2019. [Google Scholar]
- Basbaum, A.I.; Fields, H.L. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984, 7, 309–338. [Google Scholar] [CrossRef]
- Willetts, J.; Lippa, A.; Beer, B. Clinical development of citalopram. J. Clin. Psychopharmacol. 1999, 19 (Suppl. 1), 36s–46s. [Google Scholar] [CrossRef]
- Richter, T.; Paluch, Z.; Alusik, S. The non-antidepressant effects of citalopram: A clinician’s perspective. Neuro Endocrinol. Lett. 2014, 35, 7–12. [Google Scholar]
- Garnock-Jones, K.P.; Keating, G.M. Atomoxetine: A review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr. Drugs 2009, 11, 203–226. [Google Scholar] [CrossRef]
- Bymaster, F.P.; Bs, J.S.K.; Nelson, D.L.; Hemrick-Luecke, S.K.; Threlkeld, P.G.; Heiligenstein, J.H.; Morin, S.M.; Gehlert, D.R.; Perry, K.W. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002, 27, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Telford RD, R.D.; Minikin, B.R.; Hahn, A.G.; Hooper, L.A. A simple method for the assessment of general fitness: The tri-level profile. Aust. J. Sci. Med. Sport 1989, 21, 6–9. [Google Scholar]
- Wallman, K.E.; Morton, A.R.; Goodman, C.; Grove, J.R. Physiological responses during a submaximal cycle test in chronic fatigue syndrome. Med. Sci. Sports Exerc. 2004, 36, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H. The Neck Disability Index: State-of-the-art, 1991–2008. J. Manip. Physiol. Ther. 2008, 31, 491–502. [Google Scholar] [CrossRef]
- Vernon, H.; Mior, S. The Neck Disability Index: A study of reliability and validity. J. Manip. Physiol. Ther. 1991, 14, 409–415. [Google Scholar]
- Jorritsma, W.; de Vries, G.E.; Geertzen, J.H.B.; Dijkstra, P.U.; Reneman, M.F. Neck Pain and Disability Scale and the Neck Disability Index: Reproducibility of the Dutch Language Versions. Eur. Spine J. 2010, 19, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Jorritsma, W.; de Vries, G.E.; Dijkstra, P.U.; Geertzen, J.H.B.; Reneman, M.F. Neck Pain and Disability Scale and Neck Disability Index: Validity of Dutch language versions. Eur. Spine J. 2012, 21, 93–100. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. 11), S240–S252. [Google Scholar]
- Nijs, J.; Thielemans, A. Kinesiophobia and symptomatology in chronic fatigue syndrome: A psychometric study of two questionnaires. Psychol. Psychother. 2008, 81 Pt 3, 273–283. [Google Scholar] [CrossRef]
- Cathcart, S.; Winefield, A.H.; Lushington, K.; Rolan, P. Noxious inhibition of temporal summation is impaired in chronic tension-type headache. Headache 2010, 50, 403–412. [Google Scholar] [CrossRef]
- Cathcart, S.; Winefield, A.; Rolan, P.; Lushington, K. Reliability of temporal summation and diffuse noxious inhibitory control. Pain. Res. Manag. 2009, 14, 433–438. [Google Scholar] [CrossRef]
- Yarnitsky, D.; Bouhassira, D.; Drewes, A.; Fillingim, R.; Granot, M.; Hansson, P.; Landau, R.; Marchand, S.; Matre, D.; Nilsen, K.; et al. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur. J. Pain. 2015, 19, 805–806. [Google Scholar] [CrossRef]
- Yarnitsky, D.; Arendt-Nielsen, L.; Bouhassira, D.; Edwards, R.R.; Fillingim, R.B.; Granot, M.; Hansson, P.; Lautenbacher, S.; Marchand, S.; Wilder-Smith, O. Recommendations on terminology and practice of psychophysical DNIC testing. Eur. J. Pain. 2010, 14, 339. [Google Scholar] [CrossRef]
- Bannister, K.; Patel, R.; Goncalves, L.; Townson, L.; Dickenson, A.H. Diffuse noxious inhibitory controls and nerve injury: Restoring an imbalance between descending monoamine inhibitions and facilitations. Pain 2015, 156, 1803–1811. [Google Scholar] [CrossRef]
- Yarnitsky, D.; Granot, M.; Nahman-Averbuch, H.; Khamaisi, M.; Granovsky, Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012, 153, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Chappell, A.S.; Ossanna, M.J.; Liu-Seifert, H.; Iyengar, S.; Skljarevski, V.; Li, L.C.; Bennett, R.M.; Collins, H. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: A 13-week, randomized, placebo-controlled trial. Pain 2009, 146, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Lunn, M.P.; Hughes, R.A.; Wiffen, P.J. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst. Rev. 2014, CD007115. [Google Scholar] [CrossRef]
- Pergolizzi, J.V., Jr.; Raffa, R.B.; Taylor, R.; Rodriguez, G.; Nalamachu, S.; Langley, P. A review of duloxetine 60 mg once-daily dosing for the management of diabetic peripheral neuropathic pain, fibromyalgia, and chronic musculoskeletal pain due to chronic osteoarthritis pain and low back pain. Pain. Pract. 2013, 13, 239–252. [Google Scholar] [CrossRef]
- Skljarevski, V.; Desaiah, D.; Liu-Seifert, H.; Zhang, Q.; Chappell, A.S.; Detke, M.J.; Iyengar, S.; Atkinson, J.H.; Backonja, M. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine 2010, 35, E578–E585. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, A.B.; Backonja, M.; Farrar, J.T.; Finnerup, N.B.; Jensen, T.S.; Kalso, E.A.; Loeser, J.D.; Miaskowski, C.; Nurmikko, T.J.; et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain 2007, 132, 237–251. [Google Scholar] [CrossRef]
- Sokunbi, O.; Watt, P.; Moore, A. Changes in plasma concentration of serotonin in response to spinal stabilisation exercises in chronic low back pain patient. Niger. Q. J. Hosp. Med. 2007, 17, 108–111. [Google Scholar] [CrossRef]
- Meeus, M.; Ickmans, K.; De Clerck, L.S.; Moorkens, G.; Hans, G.; Grosemans, S.; Nijs, J. Serotonergic descending inhibition in chronic pain: Design, preliminary results and early cessation of a randomized controlled trial. In Vivo 2011, 25, 1019–1025. [Google Scholar]
- De Souza, G.G.; Duarte, I.D.; de Castro Perez, A. Differential involvement of central and peripheral alpha2 adrenoreceptors in the antinociception induced by aerobic and resistance exercise. Anesth. Analg. 2013, 116, 703–711. [Google Scholar] [CrossRef]
- Fitzcharles, M.-A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Arendt-Nielsen, L. Central sensitization in humans: Assessment and pharmacology. Handb. Exp. Pharmacol. 2015, 227, 79–102. [Google Scholar] [PubMed]
- Lemming, D.; Sörensen, J.; Graven-Nielsen, T.; Arendt-Nielsen, L.; Gerdle, B. The responses to pharmacological challenges and experimental pain in patients with chronic whiplash-associated pain. Clin. J. Pain. 2005, 21, 412–421. [Google Scholar] [CrossRef] [PubMed]
| Chronic WAD (n = 25) | ||||
|---|---|---|---|---|
| Age, years | 40.7 (10.7) | |||
| Sex, n | 10 men (40%) 15 women (60%) | |||
| Body mass, kg | 73.8 (14.4) | |||
| Height, cm | 171.7 (8.8) | |||
| BMI, kg/m2 | 24.9 (3.3) | |||
| Disease duration, months | 55.1 (84.2) | |||
| Occupational status, n | 9 inactive (36%) 6 part-time (24%) 8 full-time (32%); 2 student (8%) 0 retired (0%) | |||
| Education level, n | 0 primary education (0%) 9 secondary education (36%) 11 bachelor’s degree (44%) 3 master’s degree (12%) | |||
| Antidepressants, n | 1 (4%) | |||
| Analgesics, n | 8 (32%) a | |||
| Anti-epileptica, n | 0 (0%) | |||
| Legal conflict, n | 13 no (52%); 1 employer (4%); 9 insurance company (36%); 1 employer and insurance company (4%) | |||
| Successful blinding of patients | 96% | |||
| Successful blinding of assessors | 100% | |||
| No Medication (n = 25) | After Intake Citalopram (n = 20) | After Intake Atomoxetine (n = 22) | p Valueb | |
| Neck Disability Index, /100 | 40.24 (34.97–45.51) | 38.65 (33.25–44.05) | 38.73 (33.38–44.08) | >0.05 |
| Submaximal aerobic exercise characteristics | ||||
| Resting heart rate, beats per minute | 82.96 c (77.78–88.14) | 84.74 (79.05–90.42) | 91.59 c (86.12–97.06) | <0.05 c |
| Duration aerobic cycling exercise, minutes | 4.80 (4.26–5.34) | 5.12 c (4.55–5.69) | 4.49 c (3.94–5.05) | <0.05 c |
| Max Wattage | 120 (106.54–133.46) | 128.05 c (113.86–142.24) | 112.30 c (98.41–126.18) | <0.05 c |
| Medication Condition | Estimated Marginal Means (SE) | |||
|---|---|---|---|---|
| Pre-Exercise | f-Score | p-Value | ||
| PPT at the shoulder ab, kg/cm2 | ||||
| No medication | 1.767 | (0.158) | 0.814 | 0.450 |
| Citalopram | 1.898 | (0.166) | ||
| Atomoxetine | 1.837 | (0.162) | ||
| TS at the shoulder b, VNRS | ||||
| No medication | 1.454 | (0.328) | 0.273 | 0.763 |
| Citalopram | 1.654 | (0.355) | ||
| Atomoxetine | 1.431 | (0.340) | ||
| CPM at the shoulder b, VNRS | ||||
| No medication | −0.460 | (0.224) | 1.024 | 0.368 |
| Citalopram | −0.001 | (0.250) | ||
| Atomoxetine | −0.360 | (0.238) | ||
| PPT at the calf ab, kg/cm2 | ||||
| No medication | 3.123 | (0.342) | 0.091 | 0.913 |
| Citalopram | 3.176 | (0.356) | ||
| Atomoxetine | 3.088 | (0.349) | ||
| TS at the calf b, VNRS | ||||
| No medication | 1.798 | (0.412) | 1.304 | 0.283 |
| Citalopram | 1.761 | (0.439) | ||
| Atomoxetine | 1.319 | (0.424) | ||
| CPM at the calf b, VNRS | ||||
| No medication | 0.738 | (0.244) | 0.383 | 0.683 |
| Citalopram | 0.707 | (0.279) | ||
| Atomoxetine | 0.467 | (0.258) | ||
| Cuff pressure VNRS 3 b | ||||
| No medication | 88.67 | (12.60) | 2.480 | 0.096 |
| Citalopram | 71.77 | (13.10) | ||
| Atomoxetine | 85.06 | (12.82) | ||
| VAS pain b | ||||
| No medication | 52.9 | (4.1) | 1.348 | 0.262 |
| Citalopram | 49.0 | (4.3) | ||
| Atomoxetine | 49.6 | (4.2) | ||
| Medication Condition | Estimated Marginal Means (SE) | Main Effect of Condition | Main Effect of Time | Interaction Effect | Bonferroni Post Hoc Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Exercise | Post-Exercise | 24-h Post-Exercise | f-Score | p-Value | f-Score | p-Value | f-Score | p-Value | |||||
| PPT at the shoulder a, kg/cm2 | |||||||||||||
| No medication | 1.675 | (0.140) | 1.657 | (0.140) | 2.005 | 0.14 | 0.165 | 0.685 | 0.486 | 0.616 | |||
| Citalopram | 1.791 | (0.145) | 1.776 | (0.145) | |||||||||
| Atomoxetine | 1.734 | (0.143) | 1.834 | (0.143) | |||||||||
| TS at the shoulder b, VNRS | |||||||||||||
| No medication | 1.384 | (0.311) | 1.584 | (0.311) | 0.187 | 0.829 | 0.016 | 0.900 | 0.995 | 0.373 | |||
| Citalopram | 1.513 | (0.330) | 1.213 | (0.330) | |||||||||
| Atomoxetine | 1.350 | (0.320) | 1.509 | (0.320) | |||||||||
| CPM at the shoulder b, VNRS | |||||||||||||
| No medication | −0.460 | (0.202) | 0.100 | (0.202) | 0.898 | 0.410 | 5.408 | 0.022 | 0.405 | 0.668 | Main effect of time Pre-exercise < Post-exercise mean difference −0.394 (SE 0.169) p = 0.022 | ||
| Citalopram | −0.011 | (0.226) | 0.180 | (0.220) | |||||||||
| Atomoxetine | −0.353 | (0.215) | 0.078 | (0.215) | |||||||||
| PPT at the calf ab, kg/cm2 | |||||||||||||
| No medication | 3.073 | (0.342) | 2.890 | (0.342) | 0.713 | 0.492 | 0.582 | 0.447 | 1.53 | 0.221 | |||
| Citalopram | 3.107 | (0.349) | 2.924 | (0.349) | |||||||||
| Atomoxetine | 3.033 | (0.345) | 3.190 | (0.345) | |||||||||
| TS at the calf b, VNRS | |||||||||||||
| No medication | 1.841 | (0.408) | 1.341 | (0.408) | 1.071 | 0.346 | 0.611 | 0.436 | 2.332 | 0.102 | |||
| Citalopram | 1.833 | (0.421) | 1.708 | (0.421) | |||||||||
| Atomoxetine | 1.353 | (0.414) | 1.626 | (0.414) | |||||||||
| CPM at the calf b, VNRS | |||||||||||||
| No medication | 0.668 | (0.237) | 0.548 | (0.237) | |||||||||
| Citalopram | 0.641 | (0.264) | 0.416 | (0.264) | 0.197 | 0.821 | 0.286 | 0.594 | 0.237 | 0.790 | |||
| Atomoxetine | 0.445 | (0.250) | 0.514 | (0.250) | |||||||||
| Cuff pressure VNRS 3 b | |||||||||||||
| No medication | 88.7 | (12.6) | 77.26 | (12.6) | |||||||||
| Citalopram | 72.4 | (12.95) | 74.35 | (12.95) | 2.262 | 0.109 | 0.795 | 0.375 | 0.896 | 0.412 | |||
| Atomoxetine | 85.09 | (12.77) | 83.12 | (12.77) | |||||||||
| VAS pain b | |||||||||||||
| No medication | 52.1 | (4.8) | 52.2 | (4.8) | 56.6 | (4.8) | |||||||
| Citalopram | 47.4 | (5.1) | 47.2 | (5.1) | 52.6 | (5.1) | 1.347 | 0.263 | 1.644 | 0.196 | 0.616 | 0.652 | |
| Atomoxetine | 52.7 | (4.9) | 47.1 | (4.9) | 49.2 | (4.9) | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Kooning, M.; Coppieters, I.; Huysmans, E.; Nijs, J.; Meeus, M.; Voogt, L.; Hendriks, E.; Ickmans, K. Unravelling Impaired Hypoalgesia at Rest and in Response to Exercise in Patients with Chronic Whiplash-Associated Disorders: Effects of a Single Administration of Selective Serotonin Reuptake Inhibitor versus Selective Norepinephrine Reuptake Inhibitor. J. Clin. Med. 2023, 12, 4977. https://doi.org/10.3390/jcm12154977
De Kooning M, Coppieters I, Huysmans E, Nijs J, Meeus M, Voogt L, Hendriks E, Ickmans K. Unravelling Impaired Hypoalgesia at Rest and in Response to Exercise in Patients with Chronic Whiplash-Associated Disorders: Effects of a Single Administration of Selective Serotonin Reuptake Inhibitor versus Selective Norepinephrine Reuptake Inhibitor. Journal of Clinical Medicine. 2023; 12(15):4977. https://doi.org/10.3390/jcm12154977
Chicago/Turabian StyleDe Kooning, Margot, Iris Coppieters, Eva Huysmans, Jo Nijs, Mira Meeus, Lennard Voogt, Erwin Hendriks, and Kelly Ickmans. 2023. "Unravelling Impaired Hypoalgesia at Rest and in Response to Exercise in Patients with Chronic Whiplash-Associated Disorders: Effects of a Single Administration of Selective Serotonin Reuptake Inhibitor versus Selective Norepinephrine Reuptake Inhibitor" Journal of Clinical Medicine 12, no. 15: 4977. https://doi.org/10.3390/jcm12154977
APA StyleDe Kooning, M., Coppieters, I., Huysmans, E., Nijs, J., Meeus, M., Voogt, L., Hendriks, E., & Ickmans, K. (2023). Unravelling Impaired Hypoalgesia at Rest and in Response to Exercise in Patients with Chronic Whiplash-Associated Disorders: Effects of a Single Administration of Selective Serotonin Reuptake Inhibitor versus Selective Norepinephrine Reuptake Inhibitor. Journal of Clinical Medicine, 12(15), 4977. https://doi.org/10.3390/jcm12154977






