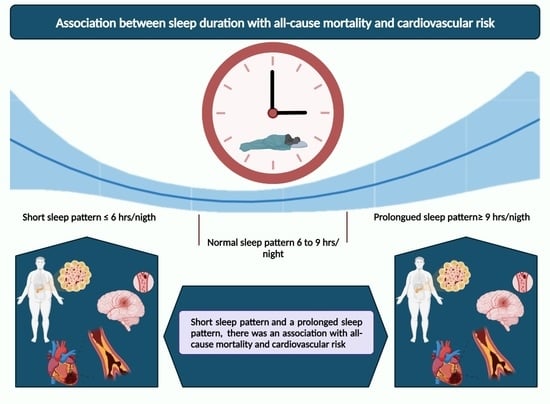
The U-Shaped Association between Sleep Duration, All-Cause Mortality and Cardiovascular Risk in a Hispanic/Latino Clinically Based Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
Sleep Assessment
2.2. Primary Exposure: Total Sleep Time
2.3. Covariates
2.4. Outcome Assessment
2.5. Statistical Analysis
3. Results
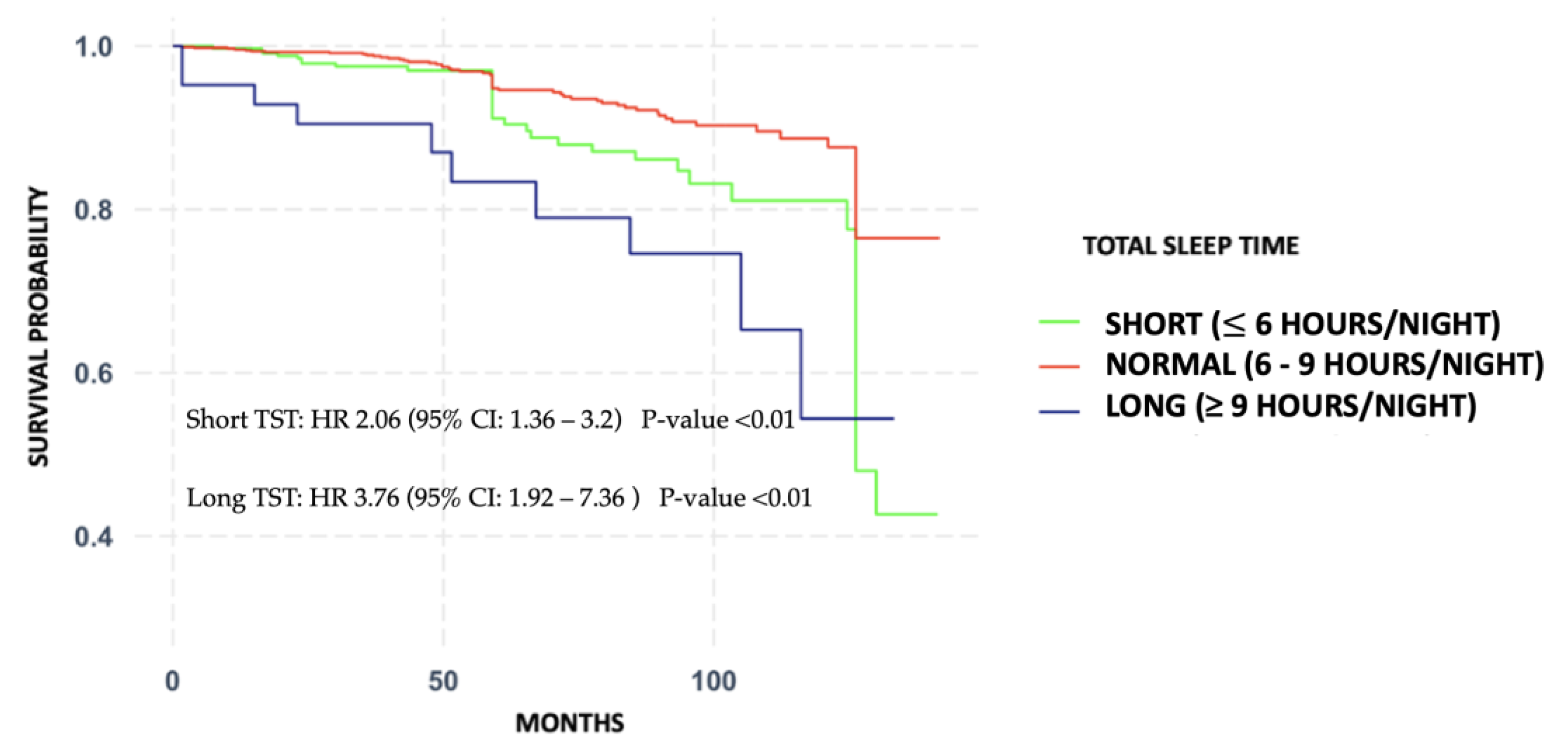
3.1. Association between Total Sleep Time and All-Cause Mortality
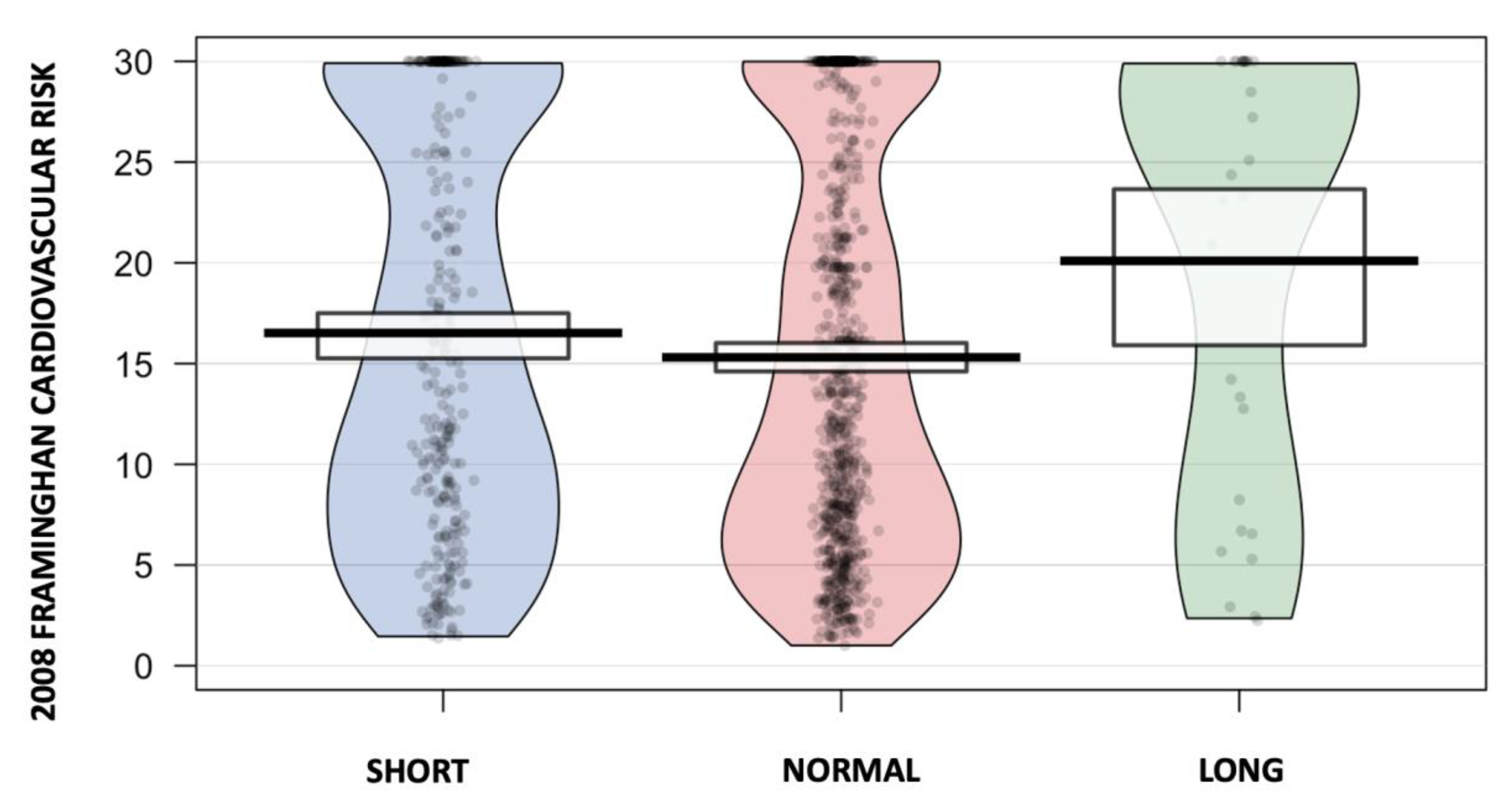
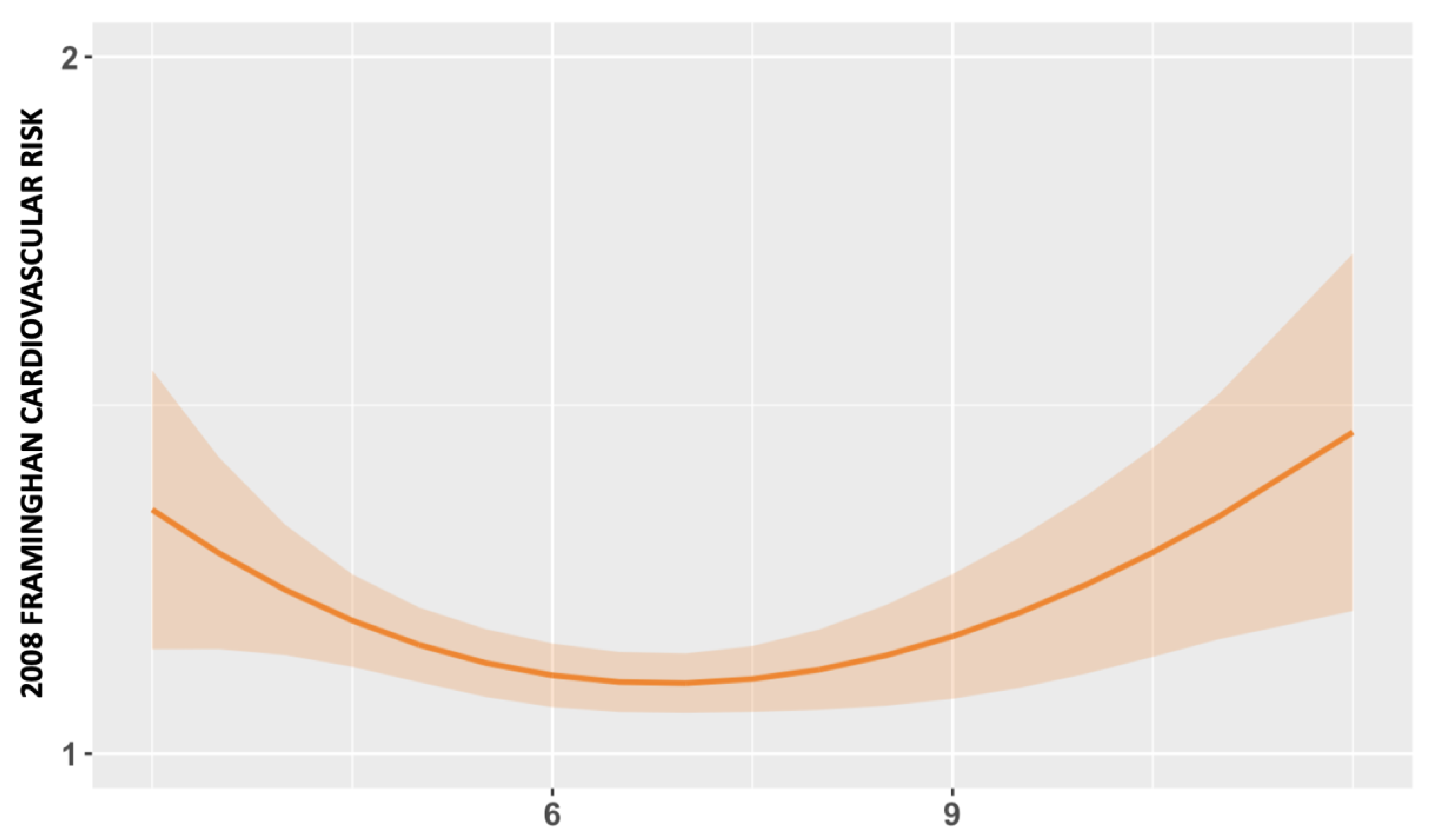
3.2. Association between Total Sleep Time and CVD Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wieczorek, T.; Wieckiewicz, M.; Smardz, J.; Wojakowska, A.; Michalek-Zrabkowska, M.; Mazur, G.; Martynowicz, H. Sleep structure in sleep bruxism: A polysomnographic study including bruxism activity phenotypes across sleep stages. J. Sleep Res. 2020, 29, e13028. [Google Scholar] [CrossRef] [PubMed]
- Michalek-Zrabkowska, M.; Wieckiewicz, M.; Smardz, J.; Gac, P.; Poreba, R.; Wojakowska, A.; Mazur, G.; Martynowicz, H. Determination of Inflammatory Markers, Hormonal Disturbances, and Sleepiness Associated with Sleep Bruxism among Adults. Nat. Sci. Sleep 2020, 12, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Gać, P.; Urbanik, D.; Macek, P.; Martynowicz, H.; Mazur, G.; Poręba, R. Coexistence of cardiovascular risk factors and obstructive sleep apnoea in polysomnography. Respir. Physiol. Neurobiol. 2022, 295, 103782. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.M.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation 2022, 146, e18–e43. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association. Life’s Essential 8. 2023. Available online: https://www.heart.org/en/healthy-living/healthy-lifestyle/lifes-essential-8 (accessed on 8 May 2023).
- Zhang, Y.-B.; Pan, X.-F.; Chen, J.; Cao, A.; Xia, L.; Zhang, Y.; Wang, J.; Li, H.; Liu, G.; Pan, A. Combined lifestyle factors, all-cause mortality and cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. J. Epidemiol. Community Health 2021, 75, 92–99. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Lee, C.-C.; Liu, S.-C.; Tseng, P.-J.; Chien, K.-L. Combined healthy lifestyle factors are more beneficial in reducing cardiovascular disease in younger adults: A meta-analysis of prospective cohort studies. Sci. Rep. 2020, 10, 18165. [Google Scholar] [CrossRef] [PubMed]
- Karam, G.; Agarwal, A.; Sadeghirad, B.; Jalink, M.; Hitchcock, C.L.; Ge, L.; Kiflen, R.; Ahmed, W.; Zea, A.M.; Milenkovic, J.; et al. Comparison of seven popular structured dietary programmes and risk of mortality and major cardiovascular events in patients at increased cardiovascular risk: Systematic review and network meta-analysis. BMJ 2023, 380, e072003. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, M.; Wang, C.-Z.; Yuan, Y.; Liang, J.-H. Association between sedentary behavior, physical activity, and cardiovascular disease-related outcomes in adults-A meta-analysis and systematic review. Front. Public Health 2022, 10, 1018460. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ahmed, W.L.; Zhuo, L.; Yuan, H.; Wang, S.; Zhou, F. Association of Sleep Patterns with Type 2 Diabetes Mellitus: A Cross-Sectional Study Based on Latent Class Analysis. Int. J. Environ. Res. Public Health 2022, 20, 393. [Google Scholar] [CrossRef]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Drummond, S.P.A. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med. Rev. 2007, 11, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Consensus Conference, P.; Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. J. Clin. Sleep Med. 2015, 11, 931–952. [Google Scholar] [CrossRef]
- Salari, N.; Khazaie, H.; Abolfathi, M.; Ghasemi, H.; Shabani, S.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. The effect of obstructive sleep apnea on the increased risk of cardiovascular disease: A systematic review and meta-analysis. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2022, 43, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Yu, Q.; Li, Y.; Du, J.; Qiu, J. Obstructive sleep apnea and cardiovascular events in acute coronary syndrome: A meta-analysis. Coron. Artery Dis. 2023, 34, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.; Wang, Z.; Zhao, G.; Liu, L.; Bi, Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2013, 169, 207–214. [Google Scholar] [CrossRef]
- Mochol, J.; Gawrys, J.; Gajecki, D.; Szahidewicz-Krupska, E.; Martynowicz, H.; Doroszko, A. Cardiovascular Disorders Triggered by Obstructive Sleep Apnea-A Focus on Endothelium and Blood Components. Int. J. Mol. Sci. 2021, 22, 5139. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, 153–639. [Google Scholar] [CrossRef]
- Abbott, S.M.; Weng, J.; Reid, K.J.; Daviglus, M.L.; Gallo, L.C.; Loredo, J.S.; Nyenhuis, S.M.; Ramos, A.R.; Shah, N.A.; Sotres-Alvarez, D.; et al. Sleep Timing, Stability, and BP in the Sueño Ancillary Study of the Hispanic Community Health Study/Study of Latinos. Chest 2019, 155, 60–68. [Google Scholar] [CrossRef]
- Fritz, J.; Phillips, A.J.K.; Hunt, L.C.; Imam, A.; Reid, K.J.; Perreira, K.M.; Mossavar-Rahmani, Y.; Daviglus, M.L.; Sotres-Alvarez, D.; Zee, P.C.; et al. Cross-sectional and prospective associations between sleep regularity and metabolic health in the Hispanic Community Health Study/Study of Latinos. Sleep 2021, 44, zsaa218. [Google Scholar] [CrossRef]
- Maghsoudipour, M.; Allison, M.A.; Patel, S.R.; Talavera, G.A.; Daviglus, M.; Zee, P.C.; Reid, K.J.; Makarem, N.; Malhotra, A. Associations of chronotype and sleep patterns with metabolic syndrome in the Hispanic community health study/study of Latinos. Chronobiol. Int. 2022, 39, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Wu, D.; Patel, S.R.; Loredo, J.S.; Redline, S.; Cai, J.; Gallo, L.C.; Mossavar-Rahmani, Y.; Ramos, A.R.; Teng, Y.; et al. Association Between Sleep Timing, Obesity, Diabetes: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Cohort Study. Sleep 2017, 40, zsx014. [Google Scholar] [CrossRef]
- Labarca, G.; Dreyse, J.; Salas, C.; Letelier, F.; Jorquera, J. A Validation Study of Four Different Cluster Analyses of OSA and the Incidence of Cardiovascular Mortality in a Hispanic Population. Chest 2021, 160, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Labarca, G.; Jorquera, J.; Dreyse, J.; Salas, C.; Letelier, F. Hypoxemic features of obstructive sleep apnea and the risk of mortality: A cluster analysis. Sleep Breath. 2021, 25, 95–103. [Google Scholar] [CrossRef]
- Labarca, G.; Dreyse, J.; Salas, C.; Schmidt, A.; Rivera, F.; Letelier, F.; Jorquera, J.; Barbe, F. A clinic-based cluster analysis in patients with moderate-severe obstructive sleep apnea (OSA) in Chile. Sleep Med. 2020, 73, 16–22. [Google Scholar] [CrossRef]
- D’Agostino, R.B.S.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Li, Y.; Bhupathiraju, S.N.; Huang, T.; Drouin-Chartier, J.-P.; Manson, J.E.; Sun, Q.; Rimm, E.B.; Rexrode, K.M.; Willett, W.C.; et al. Healthy Lifestyle Score Including Sleep Duration and Cardiovascular Disease Risk. Am. J. Prev. Med. 2022, 63, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Petersen, K.S.; Després, J.P.; Anderson, C.A.M.; Deedwania, P.; Furie, K.L.; Lear, S.; Lichtenstein, A.H.; Lobelo, F.; Morris, P.B.; et al. Strategies for Promotion of a Healthy Lifestyle in Clinical Settings: Pillars of Ideal Cardiovascular Health: A Science Advisory From the American Heart Association. Circulation 2021, 144, e495–e514. [Google Scholar] [CrossRef]
- Zhong, Q.; Qin, Z.; Wang, X.; Lan, J.; Zhu, T.; Xiao, X.; Su, L.; Pei, P.; Long, J.; Zhou, L. Healthy sleep pattern reduce the risk of cardiovascular disease: A 10-year prospective cohort study. Sleep Med. 2023, 105, 53–60. [Google Scholar] [CrossRef]
- Ford, E.S. Habitual sleep duration and predicted 10-year cardiovascular risk using the pooled cohort risk equations among US adults. J. Am. Heart Assoc. 2014, 3, e001454. [Google Scholar] [CrossRef]
- Fan, M.; Sun, D.; Zhou, T.; Heianza, Y.; Lv, J.; Li, L.; Qi, L. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: A prospective study of 385 292 UK biobank participants. Eur. Heart J. 2020, 41, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Akinseye, O.A.; Ojike, N.I.; Akinseye, L.I.; Dhandapany, P.S.; Pandi-Perumal, S.R. Association of Sleep Duration with Stroke in Diabetic Patients: Analysis of the National Health Interview Survey. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2016, 25, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Svensson, T.; Saito, E.; Svensson, A.K.; Melander, O.; Orho-Melander, M.; Mimura, M.; Rahman, S.; Sawada, N.; Koh, W.-P.; Shu, X.-O.; et al. Association of Sleep Duration With All- and Major-Cause Mortality Among Adults in Japan, China, Singapore, and Korea. JAMA Netw. Open 2021, 4, e2122837. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Keadle, S.K.; Hollenbeck, A.R.; Matthews, C.E. Sleep duration and total and cause-specific mortality in a large US cohort: Interrelationships with physical activity, sedentary behavior, and body mass index. Am. J. Epidemiol. 2014, 180, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.; Ma, S.H.; Park, S.K.; Chang, S.-H.; Shin, H.-R.; Kang, D.; Yoo, K.-Y. A prospective cohort study on the relationship of sleep duration with all-cause and disease-specific mortality in the Korean Multi-center Cancer Cohort study. J. Prev. Med. Public Health 2013, 46, 271–281. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J.; He, F.; Calhoun, S.L.; Vgontzas, A.N.; Liao, D.; Bixler, E.O. Objective short sleep duration increases the risk of all-cause mortality associated with possible vascular cognitive impairment. Sleep Health 2020, 6, 71–78. [Google Scholar] [CrossRef]
- Patel, S.R.; Ayas, N.T.; Malhotra, M.R.; White, D.P.; Schernhammer, E.S.; Speizer, F.E.; Stampfer, M.J.; Hu, F.B. A prospective study of sleep duration and mortality risk in women. Sleep 2004, 27, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Atanasov, S. Long Sleep Duration and Stroke-Highly Linked, Poorly Understood. Neurol Int. 2023, 15, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Río Vázquez, V.; Anías Calderón, J. Mecanismos fisiopatológicos de las alteraciones cardiovasculares en el Síndrome de apnea obstructiva del sueño. Rev. Cuba. Investig. Biomed. 2009, 28, 1–8. [Google Scholar]
- Kalaydzhiev, P.; Poroyliev, N.; Somleva, D.; Ilieva, R.; Markov, D.; Kinova, E.; Goudev, A. Sleep apnea in patients with exacerbated heart failure and overweight. Sleep Med. X 2023, 5, 100065. [Google Scholar] [CrossRef] [PubMed]
- Risso, T.T.; Poyares, D.; Rizzi, C.F.; Pulz, C.; Guilleminault, C.; Tufik, S.; de Paola, A.A.V.; Cintra, F. The impact of sleep duration in obstructive sleep apnea patients. Sleep Breath. 2013, 17, 837–843. [Google Scholar] [CrossRef]
- Priou, P.; Le Vaillant, M.; Meslier, N.; Paris, A.; Pigeanne, T.; Nguyen, X.-L.; Alizon, C.; Bizieux-Thaminy, A.; Leclair-Visonneau, L.; Humeau, M.-P.; et al. Cumulative association of obstructive sleep apnea severity and short sleep duration with the risk for hypertension. PLoS ONE 2014, 9, e115666. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.; Oga, T.; Takahashi, K.; Takegami, M.; Nakayama-Ashida, Y.; Wakamura, T.; Sumi, K.; Nakamura, T.; Horita, S.; Oka, Y.; et al. Associations between obstructive sleep apnea, metabolic syndrome, and sleep duration, as measured with an actigraph, in an urban male working population in Japan. Sleep 2010, 33, 89–95. [Google Scholar] [CrossRef]
- Kim, N.H.; Lee, S.K.; Eun, C.R.; Seo, J.A.; Kim, S.G.; Choi, K.M.; Baik, S.H.; Choi, D.S.; Yun, C.-H.; Kim, N.H.; et al. Short sleep duration combined with obstructive sleep apnea is associated with visceral obesity in Korean adults. Sleep 2013, 36, 723–729. [Google Scholar] [CrossRef]
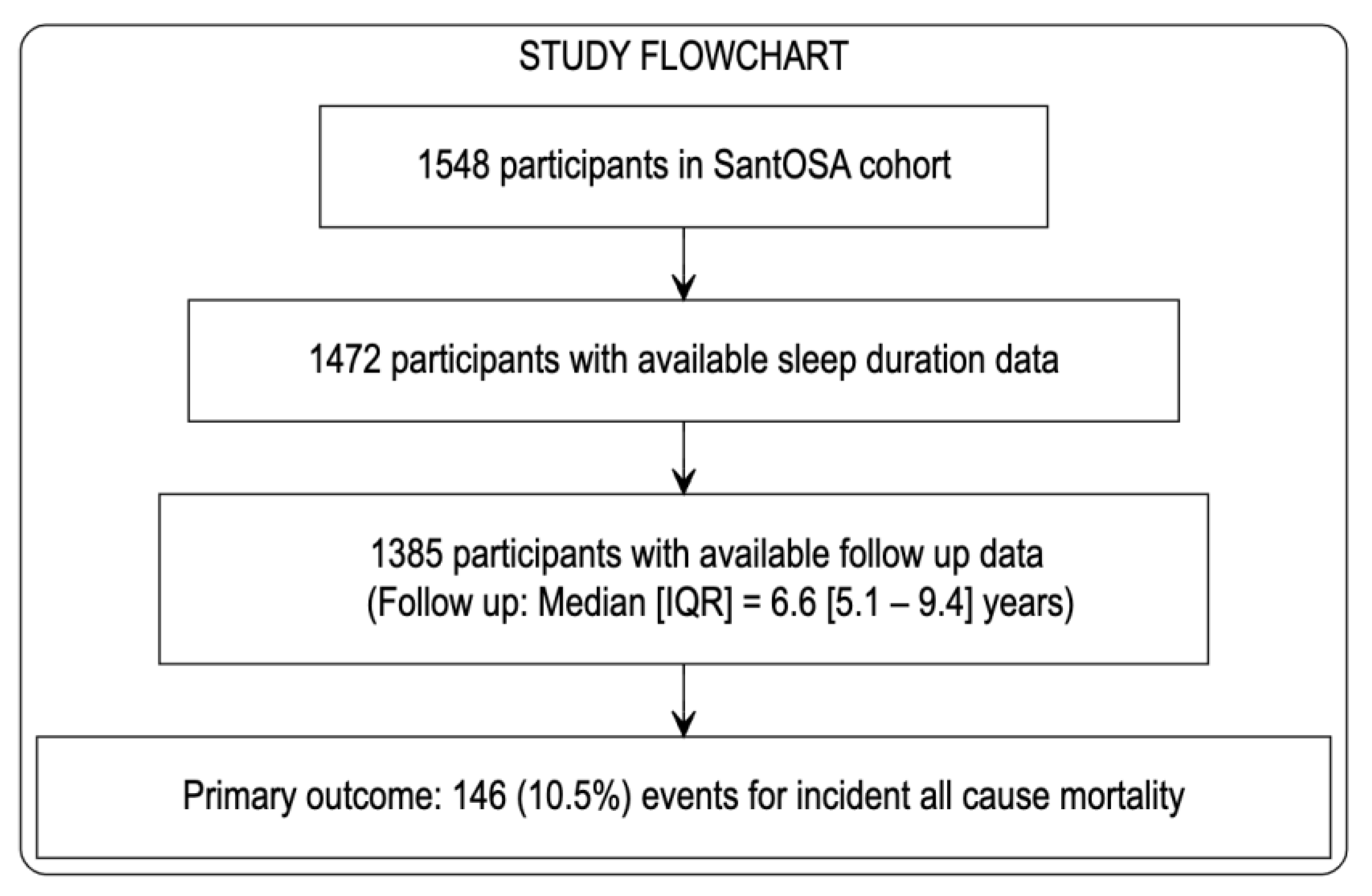
| Short TST (n = 632) | Normal TST (n = 639) | Long TST (n = 114) | p-Value | |
|---|---|---|---|---|
| Sociodemographic Data | ||||
| Sex | <0.001 | |||
| Male, n (%) | 515 (81.5%) | 495 (77.5%) | 72 (63.2%) | |
| Female, n (%) | 117 (18.5%) | 144 (22.5%) | 42 (36.8%) | |
| Age (Years) | 53.0 [43.0;63.0] | 52.0 [41.0;63.0] | 63.0 [43.0;73.8] | <0.001 |
| BMI (Kg/m2) | 29.5 [26.7;32.9] | 29.5 [26.7;33.1] | 30.2 [26.7;33.8] | 0.607 |
| Comorbidities | ||||
| Smoking Status | 0.659 | |||
| Non-Smoker (%) | 290 (46.0%) | 311 (48.7%) | 55 (48.2%) | |
| Current (%) | 197 (31.3%) | 191 (29.9%) | 38 (33.3%) | |
| Former (%) | 143 (22.7%) | 136 (21.4%) | 21 (18.4%) | |
| Systolic Pressure (mmHg) | 120 [110;130] | 120 [110;130] | 124 [116;140] | 0.087 |
| Hypertension, n (%) | 208 (37.5%) | 181 (33.5%) | 45 (51.1%) | 0.005 |
| Diabetes Mellitus, n (%) | 172 (27.2%) | 153 (24.0%) | 34 (29.8%) | 0.259 |
| Framingham 2008 10-year CVD Risk, n (%) | 14.7 [7.44;27.1] | 11.8 [6.26;25.1] | 23.4 [8.11;30.0] | 0.023 |
| Death, n (%) | 59 (9.34%) | 56 (8.76%) | 31 (27.2%) | <0.001 |
| Sleep Apnea | ||||
| Severity of OSA | 0.284 | |||
| Non-OSA, n (%) | 93 (14.7%) | 90 (14.1%) | 15 (13.2%) | |
| Mild OSA, n (%) | 182 (28.8%) | 169 (26.4%) | 28 (24.6%) | |
| Moderate OSA, n (%) | 167 (26.4%) | 174 (27.2%) | 23 (20.2%) | |
| Severe OSA, n (%) | 190 (30.1%) | 206 (32.2%) | 48 (42.1%) |
| Model 1 (HR, 95% CI) | Model 2 (HR, 95% CI) | Model 3 (HR, 95% CI) | |
|---|---|---|---|
| Short TST | 2.06 (1.36–3.2) | 2.28 (1.36–3.84) | 2.51 (1.48–4.25) |
| Normal TST (*) | --- | --- | --- |
| Long TST | 3.76 (1.92–7.36) | 3.69 (1.43–9.51) | 3.97 (1.53–10.29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henríquez-Beltrán, M.; Dreyse, J.; Jorquera, J.; Jorquera-Diaz, J.; Salas, C.; Fernandez-Bussy, I.; Labarca, G. The U-Shaped Association between Sleep Duration, All-Cause Mortality and Cardiovascular Risk in a Hispanic/Latino Clinically Based Cohort. J. Clin. Med. 2023, 12, 4961. https://doi.org/10.3390/jcm12154961
Henríquez-Beltrán M, Dreyse J, Jorquera J, Jorquera-Diaz J, Salas C, Fernandez-Bussy I, Labarca G. The U-Shaped Association between Sleep Duration, All-Cause Mortality and Cardiovascular Risk in a Hispanic/Latino Clinically Based Cohort. Journal of Clinical Medicine. 2023; 12(15):4961. https://doi.org/10.3390/jcm12154961
Chicago/Turabian StyleHenríquez-Beltrán, Mario, Jorge Dreyse, Jorge Jorquera, Jorge Jorquera-Diaz, Constanza Salas, Isabel Fernandez-Bussy, and Gonzalo Labarca. 2023. "The U-Shaped Association between Sleep Duration, All-Cause Mortality and Cardiovascular Risk in a Hispanic/Latino Clinically Based Cohort" Journal of Clinical Medicine 12, no. 15: 4961. https://doi.org/10.3390/jcm12154961
APA StyleHenríquez-Beltrán, M., Dreyse, J., Jorquera, J., Jorquera-Diaz, J., Salas, C., Fernandez-Bussy, I., & Labarca, G. (2023). The U-Shaped Association between Sleep Duration, All-Cause Mortality and Cardiovascular Risk in a Hispanic/Latino Clinically Based Cohort. Journal of Clinical Medicine, 12(15), 4961. https://doi.org/10.3390/jcm12154961