PET/CT and SPECT/CT Imaging of HER2-Positive Breast Cancer
Abstract
1. Introduction
2. Overview: HER2 Diagnostics and Therapeutics
2.1. HER2 Targeted Therapy
2.2. HER2 In Vitro Diagnostic Testing
2.3. HER2 In Vivo Diagnostic Testing with Imaging
3. HER2-Specific Imaging
3.1. Monoclonal Antibodies
3.2. Affibodies and Nanobodies
3.3. SPECT/CT Imaging
4. HER2 Radionucleotide Therapy
5. HER2 Imaging for Staging and Prognostication
6. HER2 Imaging for Response Assessment
7. HER2 Imaging for Surveillance
8. Future Directions
8.1. HER2-Low Cancers
8.2. Artificial Intelligence and Radiomics
8.3. Multimodality Imaging for Response Assessment
8.4. HER2 Intratumoral Heterogeneity
8.5. HER2 beyond Breast Cancer
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalil, D.; Lotfalla, A.; Girard, A.; Ha, R.; Dercle, L.; Seban, R.-D. Advances in PET/CT Imaging for Breast Cancer Patients and Beyond. J. Clin. Med. 2023, 12, 651. [Google Scholar] [CrossRef]
- Baradaran, A.; Derakhshan, M.; Raeisi, S.; Neshat, S.; Raeisi, S. Multicentricity in Different Molecular Subtypes of Breast Cancer: A Cross-Sectional Study in Isfahan. Adv. Biomed. Res. 2023, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- King, C.R.; Kraus, M.H.; Aaronson, S.A. Amplification of a Novel V-ErbB-Related Gene in a Human Mammary Carcinoma. Science 1985, 229, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurti, U.; Silverman, J.F. HER2 in Breast Cancer: A Review and Update. Adv. Anat. Pathol. 2014, 21, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Hrynchak, I.; Santos, L.; Falcão, A.; Gomes, C.M.; Abrunhosa, A.J. Nanobody-Based Theranostic Agents for HER2-Positive Breast Cancer: Radiolabeling Strategies. Int. J. Mol. Sci. 2021, 22, 10745. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Bianchi, S.; Caini, S.; Paglierani, M.; Saieva, C.; Vezzosi, V.; Baroni, G.; Simoni, A.; Palli, D. Tuscany Breast Cancer Study Group Accuracy and Reproducibility of HER2 Status in Breast Cancer Using Immunohistochemistry: A Quality Control Study in Tuscany Evaluating the Impact of Updated 2013 ASCO/CAP Recommendations. Pathol. Oncol. Res. 2015, 21, 477–485. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Arch. Pathol. Lab. Med. 2007, 131, 18–43. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Wang, Z.; Shi, J.; Hu, Z.; Gao, S.; Miao, W.; Ma, Q.; Dong, C.; Wang, F. Imaging and Monitoring HER2 Expression in Breast Cancer during Trastuzumab Therapy with a Peptide Probe 99mTc-HYNIC-H10F. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2613–2623. [Google Scholar] [CrossRef]
- Grinda, T.; Joyon, N.; Lusque, A.; Lefèvre, S.; Arnould, L.; Penault-Llorca, F.; Macgrogan, G.; Treilleux, I.; Vincent-Salomon, A.; Haudebourg, J.; et al. Phenotypic Discordance between Primary and Metastatic Breast Cancer in the Large-Scale Real-Life Multicenter French ESME Cohort. NPJ Breast Cancer 2021, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Specht, J.M.; Tam, S.L.; Kurland, B.F.; Gralow, J.R.; Livingston, R.B.; Linden, H.M.; Ellis, G.K.; Schubert, E.K.; Dunnwald, L.K.; Mankoff, D.A. Serial 2-[18F] Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography (FDG-PET) to Monitor Treatment of Bone-Dominant Metastatic Breast Cancer Predicts Time to Progression (TTP). Breast Cancer Res. Treat. 2007, 105, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Avril, N.; Menzel, M.; Dose, J.; Schelling, M.; Weber, W.; Jänicke, F.; Nathrath, W.; Schwaiger, M. Glucose Metabolism of Breast Cancer Assessed by 18F-FDG PET: Histologic and Immunohistochemical Tissue Analysis. J. Nucl. Med. 2001, 42, 9–16. [Google Scholar] [PubMed]
- Ulaner, G.A.; Riedl, C.C.; Dickler, M.N.; Jhaveri, K.; Pandit-Taskar, N.; Weber, W. Molecular Imaging of Biomarkers in Breast Cancer. J. Nucl. Med. 2016, 57 (Suppl. S1), 53S–59S. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.E.; Ulaner, G.A.; Lewis, J.S. Human Epidermal Growth Factor Receptor 2-Targeted PET/Single- Photon Emission Computed Tomography Imaging of Breast Cancer: Noninvasive Measurement of a Biomarker Integral to Tumor Treatment and Prognosis. PET Clin. 2017, 12, 269–288. [Google Scholar] [CrossRef]
- Gaykema, S.B.M.; Schröder, C.P.; Vitfell-Rasmussen, J.; Chua, S.; Oude Munnink, T.H.; Brouwers, A.H.; Bongaerts, A.H.H.; Akimov, M.; Fernandez-Ibarra, C.; Lub-de Hooge, M.N.; et al. 89Zr-Trastuzumab and 89Zr-Bevacizumab PET to Evaluate the Effect of the HSP90 Inhibitor NVP-AUY922 in Metastatic Breast Cancer Patients. Clin. Cancer Res. 2014, 20, 3945–3954. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Hyman, D.M.; Lyashchenko, S.K.; Lewis, J.S.; Carrasquillo, J.A. 89Zr-Trastuzumab PET/CT for Detection of Human Epidermal Growth Factor Receptor 2-Positive Metastases in Patients With Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. Clin. Nucl. Med. 2017, 42, 912–917. [Google Scholar] [CrossRef]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schröder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-Trastuzumab and PET Imaging of HER2-Positive Lesions in Patients with Metastatic Breast Cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Hyman, D.M.; Ross, D.S.; Corben, A.; Chandarlapaty, S.; Goldfarb, S.; McArthur, H.; Erinjeri, J.P.; Solomon, S.B.; Kolb, H.; et al. Detection of HER2-Positive Metastases in Patients with HER2-Negative Primary Breast Cancer Using 89Zr-Trastuzumab PET/CT. J. Nucl. Med. 2016, 57, 1523–1528. [Google Scholar] [CrossRef]
- Bensch, F.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Jong, J.R.; van der Vegt, B.; Sleijfer, S.; de Vries, E.G.E.; Schröder, C.P. 89Zr-Trastuzumab PET Supports Clinical Decision Making in Breast Cancer Patients, When HER2 Status Cannot Be Determined by Standard Work Up. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, F.; Wu, N.; Bose, R.; Naughton, M.J.; Ma, C.X.; Marquez-Nostra, B.V.; Diebolder, P.; Mpoy, C.; Rogers, B.E.; Lapi, S.E.; et al. Evaluation of [89Zr]Trastuzumab-PET/CT in Differentiating HER2-Positive from HER2-Negative Breast Cancer. Breast Cancer Res. Treat. 2018, 169, 523–530. [Google Scholar] [CrossRef]
- Laforest, R.; Lapi, S.E.; Oyama, R.; Bose, R.; Tabchy, A.; Marquez-Nostra, B.V.; Burkemper, J.; Wright, B.D.; Frye, J.; Frye, S.; et al. [89Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol. Imaging Biol. 2016, 18, 952–959. [Google Scholar] [CrossRef]
- Jauw, Y.W.S.; O’Donoghue, J.A.; Zijlstra, J.M.; Hoekstra, O.S.; Menke-van der Houven van Oordt, C.W.; Morschhauser, F.; Carrasquillo, J.A.; Zweegman, S.; Pandit-Taskar, N.; Lammertsma, A.A.; et al. 89Zr-Immuno-PET: Toward a Noninvasive Clinical Tool to Measure Target Engagement of Therapeutic Antibodies In Vivo. J. Nucl. Med. 2019, 60, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. 89Zr-Atezolizumab Imaging as a Non-Invasive Approach to Assess Clinical Response to PD-L1 Blockade in Cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; Postow, M.A.; Hellmann, M.D.; Harding, J.J.; Barker, C.A.; O’Donoghue, J.A.; Ziolkowska, M.; Ruan, S.; Lyashchenko, S.K.; Tsai, F.; et al. First-in-Humans Imaging with 89Zr-Df-IAB22M2C Anti-CD8 Minibody in Patients with Solid Malignancies: Preliminary Pharmacokinetics, Biodistribution, and Lesion Targeting. J. Nucl. Med. 2020, 61, 512–519. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Carrasquillo, J.A.; Riedl, C.C.; Yeh, R.; Hatzoglou, V.; Ross, D.S.; Jhaveri, K.; Chandarlapaty, S.; Hyman, D.M.; Zeglis, B.M.; et al. Identification of HER2-Positive Metastases in Patients with HER2-Negative Primary Breast Cancer by Using HER2-Targeted 89Zr-Pertuzumab PET/CT. Radiology 2020, 296, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Knorr, K.; Schlapschy, M.; Robu, S.; Morath, V.; Mendler, C.; Yen, H.-Y.; Steiger, K.; Kiechle, M.; Weber, W.; et al. First In-Human Medical Imaging with a PASylated 89Zr-Labeled Anti-HER2 Fab-Fragment in a Patient with Metastatic Breast Cancer. Nucl. Med. Mol. Imaging 2020, 54, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Guo, X.; Yang, M.; Zhu, H.; Yang, Z. 68Ga-ZHER2 PET/CT Reveals HER2-Positive Metastatic Gastric Cancer With Better Image Quality Than 18F-FDG. Clin. Nucl. Med. 2020, 45, e101–e102. [Google Scholar] [CrossRef]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring HER2-Receptor Expression In Metastatic Breast Cancer Using [68Ga]ABY-025 Affibody PET/CT. Theranostics 2016, 6, 262–271. [Google Scholar] [CrossRef]
- Miao, H.; Sun, Y.; Jin, Y.; Hu, X.; Song, S.; Zhang, J. Application of a Novel 68Ga-HER2 Affibody PET/CT Imaging in Breast Cancer Patients. Front. Oncol. 2022, 12, 894767. [Google Scholar] [CrossRef]
- Sandström, M.; Lindskog, K.; Velikyan, I.; Wennborg, A.; Feldwisch, J.; Sandberg, D.; Tolmachev, V.; Orlova, A.; Sörensen, J.; Carlsson, J.; et al. Biodistribution and Radiation Dosimetry of the Anti-HER2 Affibody Molecule 68Ga-ABY-025 in Breast Cancer Patients. J. Nucl. Med. 2016, 57, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-Trastuzumab PET Imaging in Patients with HER2-Positive Breast Cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Sasada, S.; Kurihara, H.; Kinoshita, T.; Yoshida, M.; Honda, N.; Shimoi, T.; Shimomura, A.; Yunokawa, M.; Yonemori, K.; Shimizu, C.; et al. 64Cu-DOTA-Trastuzumab PET Imaging for HER2-Specific Primary Lesions of Breast Cancer. Ann. Oncol. 2017, 28, 2028–2029. [Google Scholar] [CrossRef]
- Carrasquillo, J.A.; Morris, P.G.; Humm, J.L.; Smith-Jones, P.M.; Beylergil, V.; Akhurst, T.; O’donoghue, J.A.; Ruan, S.; Modi, S.; Hudis, C.A.; et al. Copper-64 Trastuzumab PET Imaging: A Reproducibility Study. Q. J. Nucl. Med. Mol. Imaging 2019, 63, 191–198. [Google Scholar] [CrossRef]
- Kurihara, H.; Hamada, A.; Yoshida, M.; Shimma, S.; Hashimoto, J.; Yonemori, K.; Tani, H.; Miyakita, Y.; Kanayama, Y.; Wada, Y.; et al. (64)Cu-DOTA-Trastuzumab PET Imaging and HER2 Specificity of Brain Metastases in HER2-Positive Breast Cancer Patients. EJNMMI Res. 2015, 5, 8. [Google Scholar] [CrossRef]
- Mortimer, J.E.; Bading, J.R.; Colcher, D.M.; Conti, P.S.; Frankel, P.H.; Carroll, M.I.; Tong, S.; Poku, E.; Miles, J.K.; Shively, J.E.; et al. Functional Imaging of Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer Using (64)Cu-DOTA-Trastuzumab PET. J. Nucl. Med. 2014, 55, 23–29. [Google Scholar] [CrossRef]
- Mortimer, J.E.; Bading, J.R.; Park, J.M.; Frankel, P.H.; Carroll, M.I.; Tran, T.T.; Poku, E.K.; Rockne, R.C.; Raubitschek, A.A.; Shively, J.E.; et al. Tumor Uptake of 64Cu-DOTA-Trastuzumab in Patients with Metastatic Breast Cancer. J. Nucl. Med. 2018, 59, 38–43. [Google Scholar] [CrossRef]
- Lee, I.; Lim, I.; Byun, B.H.; Kim, B.I.; Choi, C.W.; Woo, S.-K.; Kim, K.I.; Lee, K.C.; Kang, J.H.; Seong, M.-K.; et al. A Preliminary Clinical Trial to Evaluate 64Cu-NOTA-Trastuzumab as a Positron Emission Tomography Imaging Agent in Patients with Breast Cancer. EJNMMI Res. 2021, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Lim, I.; Byun, B.H.; Kim, B.I.; Choi, C.W.; Lee, K.C.; Kang, C.M.; Seong, M.-K.; Kim, H.-A.; Noh, W.C.; et al. The Prediction of HER2-Targeted Treatment Response Using 64Cu-Tetra-Azacyclododecanetetra-Acetic Acid (DOTA)-Trastuzumab PET/CT in Metastatic Breast Cancer: A Case Report. J. Breast Cancer 2022, 25, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Sasada, S.; Kurihara, H.; Kinoshita, T.; Yoshida, M.; Honda, N.; Shimoi, T.; Shimomura, A.; Yonemori, K.; Shimizu, C.; Hamada, A.; et al. Visualization of HER2-Specific Breast Cancer Intratumoral Heterogeneity Using 64Cu-DOTA-Trastuzumab PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2146–2147. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Sheehan-Dare, G.; Nguyen, A.; Ho, B.; Liu, V.; Lee, J.; Brown, L.; Dear, R.; Chan, L.; Sharma, S.; et al. 64Cu-SAR-Bombesin PET-CT Imaging in the Staging of Estrogen/Progesterone Receptor Positive, HER2 Negative Metastatic Breast Cancer Patients: Safety, Dosimetry and Feasibility in a Phase I Trial. Pharmaceuticals 2022, 15, 772. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, A.; Jha, A.K.; Mithun, S.; Shetye, B.; Kameswaran, M.; Shah, S.; Rangarajan, V.; Gupta, S. Analysis of Absorbed Dose in Radioimmunotherapy with 177Lu-Trastuzumab Using Two Different Imaging Scenarios: A Pilot Study. Nucl. Med. Commun. 2021, 42, 1382–1395. [Google Scholar] [CrossRef]
- Baum, R.P.; Prasad, V.; Müller, D.; Schuchardt, C.; Orlova, A.; Wennborg, A.; Tolmachev, V.; Feldwisch, J. Molecular Imaging of HER2-Expressing Malignant Tumors in Breast Cancer Patients Using Synthetic 111In- or 68Ga-Labeled Affibody Molecules. J. Nucl. Med. 2010, 51, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, D.; Tolmachev, V.; Velikyan, I.; Olofsson, H.; Wennborg, A.; Feldwisch, J.; Carlsson, J.; Lindman, H.; Sörensen, J. Intra-Image Referencing for Simplified Assessment of HER2-Expression in Breast Cancer Metastases Using the Affibody Molecule ABY-025 with PET and SPECT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1337–1346. [Google Scholar] [CrossRef]
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J.; et al. First-in-Human Molecular Imaging of HER2 Expression in Breast Cancer Metastases Using the 111In-ABY-025 Affibody Molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET Imaging with 89Zr: From Radiochemistry to the Clinic. Nucl. Med. Biol. 2013, 40, 3–14. [Google Scholar] [CrossRef]
- Kumar, K.; Seetharam, K.; Poonam, F.; Gulati, A.; Sadiq, A.; Shetty, V. The Role of Cardiac Imaging in the Evaluation of Cardiac Involvement in Systemic Diseases. Cureus 2021, 13, e20708. [Google Scholar] [CrossRef]
- Persson, M.; Tolmachev, V.; Andersson, K.; Gedda, L.; Sandström, M.; Carlsson, J. [(177)Lu]Pertuzumab: Experimental Studies on Targeting of HER-2 Positive Tumour Cells. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1457–1462. [Google Scholar] [CrossRef]
- Yang, E.Y.; Shah, K. Nanobodies: Next Generation of Cancer Diagnostics and Therapeutics. Front. Oncol. 2020, 10, 1182. [Google Scholar] [CrossRef]
- Xavier, C.; Blykers, A.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Lahoutte, T.; Devoogdt, N.; Caveliers, V. (18)F-Nanobody for PET Imaging of HER2 Overexpressing Tumors. Nucl. Med. Biol. 2016, 43, 247–252. [Google Scholar] [CrossRef]
- Simanek, M.; Koranda, P. SPECT/CT Imaging in Breast Cancer-Current Status and Challenges. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2016, 160, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, G.; Flamen, P.; Vries, E.G.E.D.; Jhaveri, K.; Wimana, Z. Imaging Diagnostic and Therapeutic Targets: Human Epidermal Growth Factor Receptor 2. J. Nucl. Med. 2016, 57, 81S–88S. [Google Scholar] [CrossRef]
- Li, L.; Wu, Y.; Wang, Z.; Jia, B.; Hu, Z.; Dong, C.; Wang, F. SPECT/CT Imaging of the Novel HER2-Targeted Peptide Probe 99mTc-HYNIC-H6F in Breast Cancer Mouse Models. J. Nucl. Med. 2017, 58, 821–826. [Google Scholar] [CrossRef]
- Artigas, C.; Mileva, M.; Flamen, P.; Karfis, I. Targeted Radionuclide Therapy: An Emerging Field in Solid Tumours. Curr. Opin. Oncol. 2021, 33, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Massicano, A.V.F.; Marquez-Nostra, B.V.; Lapi, S.E. Targeting HER2 in Nuclear Medicine for Imaging and Therapy. Mol. Imaging 2018, 17, 1536012117745386. [Google Scholar] [CrossRef]
- Kameswaran, M.; Gota, V.; Ambade, R.; Gupta, S.; Dash, A. Preparation and Preclinical Evaluation of 131 I-Trastuzumab for Breast Cancer. J. Labelled. Comp. Radiopharm. 2017, 60, 12–19. [Google Scholar] [CrossRef]
- Rasaneh, S.; Rajabi, H.; Babaei, M.H.; Daha, F.J.; Salouti, M. Radiolabeling of Trastuzumab with 177Lu via DOTA, a New Radiopharmaceutical for Radioimmunotherapy of Breast Cancer. Nucl. Med. Biol. 2009, 36, 363–369. [Google Scholar] [CrossRef]
- Buchegger, F.; Perillo-Adamer, F.; Dupertuis, Y.M.; Delaloye, A.B. Auger Radiation Targeted into DNA: A Therapy Perspective. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1352–1363. [Google Scholar] [CrossRef]
- Costantini, D.L.; Chan, C.; Cai, Z.; Vallis, K.A.; Reilly, R.M. (111)In-Labeled Trastuzumab (Herceptin) Modified with Nuclear Localization Sequences (NLS): An Auger Electron-Emitting Radiotherapeutic Agent for HER2/Neu-Amplified Breast Cancer. J. Nucl. Med. 2007, 48, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Yi, M.; Mittendorf, E.A.; Cormier, J.N.; Buchholz, T.A.; Bilimoria, K.; Sahin, A.A.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M.; Luo, S.; Buzdar, A.U.; et al. Novel Staging System for Predicting Disease-Specific Survival in Patients with Breast Cancer Treated with Surgery as the First Intervention: Time to Modify the Current American Joint Committee on Cancer Staging System. J. Clin. Oncol. 2011, 29, 4654–4661. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Chavez-MacGregor, M.; Lichtensztajn, D.Y.; Yi, M.; Tadros, A.; Hortobagyi, G.N.; Giordano, S.H.; Hunt, K.K.; Mittendorf, E.A. Validation Study of the American Joint Committee on Cancer Eighth Edition Prognostic Stage Compared With the Anatomic Stage in Breast Cancer. JAMA Oncol. 2018, 4, 203–209. [Google Scholar] [CrossRef]
- Seban, R.-D.; Champion, L.; Bellesoeur, A.; Vincent-Salomon, A.; Bidard, F.-C. Clinical Potential of HER2 PET as a Predictive Biomarker to Guide the Use of Trastuzumab Deruxtecan in Breast Cancer Patients. J. Nucl. Med. 2023, 64, 1164–1165. [Google Scholar] [CrossRef]
- Nuciforo, P.; Thyparambil, S.; Aura, C.; Garrido-Castro, A.; Vilaro, M.; Peg, V.; Jimenez, J.; Vicario, R.; Cecchi, F.; Hoos, W.; et al. High HER2 Protein Levels Correlate with Increased Survival in Breast Cancer Patients Treated with Anti-HER2 Therapy. Mol. Oncol. 2016, 10, 138–147. [Google Scholar] [CrossRef]
- Hanker, A.B.; Garrett, J.T.; Estrada, M.V.; Moore, P.D.; Ericsson, P.G.; Koch, J.P.; Langley, E.; Singh, S.; Kim, P.S.; Frampton, G.M.; et al. HER2-Overexpressing Breast Cancers Amplify FGFR Signaling upon Acquisition of Resistance to Dual Therapeutic Blockade of HER2. Clin. Cancer Res. 2017, 23, 4323–4334. [Google Scholar] [CrossRef]
- Van Poznak, C.; Somerfield, M.R.; Bast, R.C.; Cristofanilli, M.; Goetz, M.P.; Gonzalez-Angulo, A.M.; Hicks, D.G.; Hill, E.G.; Liu, M.C.; Lucas, W.; et al. Use of Biomarkers to Guide Decisions on Systemic Therapy for Women With Metastatic Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2015, 33, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Curigliano, G.; Tolaney, S.M. Navigating the HER2-Low Paradigm in Breast Oncology: New Standards, Future Horizons. Cancer Discov. 2022, 12, 2026–2030. [Google Scholar] [CrossRef]
- Edmonds, C.E.; O’Brien, S.R.; Mankoff, D.A.; Pantel, A.R. Novel Applications of Molecular Imaging to Guide Breast Cancer Therapy. Cancer Imaging 2022, 22, 31. [Google Scholar] [CrossRef]
- Urso, L.; Manco, L.; Castello, A.; Evangelista, L.; Guidi, G.; Castellani, M.; Florimonte, L.; Cittanti, C.; Turra, A.; Panareo, S. PET-Derived Radiomics and Artificial Intelligence in Breast Cancer: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 13409. [Google Scholar] [CrossRef]
- Castello, A.; Castellani, M.; Florimonte, L.; Urso, L.; Mansi, L.; Lopci, E. The Role of Radiomics in the Era of Immune Checkpoint Inhibitors: A New Protagonist in the Jungle of Response Criteria. J. Clin. Med. 2022, 11, 1740. [Google Scholar] [CrossRef]
- Manco, L.; Maffei, N.; Strolin, S.; Vichi, S.; Bottazzi, L.; Strigari, L. Basic of Machine Learning and Deep Learning in Imaging for Medical Physicists. Phys. Med. 2021, 83, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bian, H.; Zhang, Y.; Gao, Y.; Yin, G.; Wang, Z.; Li, X.; Ma, W.; Xu, W. Molecular Subtype Classification of Breast Cancer Using Established Radiomic Signature Models Based on 18F-FDG PET/CT Images. Front. Biosci. 2021, 26, 475–484. [Google Scholar] [CrossRef]
- Dercle, L.; McGale, J.; Sun, S.; Marabelle, A.; Yeh, R.; Deutsch, E.; Mokrane, F.-Z.; Farwell, M.; Ammari, S.; Schoder, H.; et al. Artificial Intelligence and Radiomics: Fundamentals, Applications, and Challenges in Immunotherapy. J. Immunother. Cancer 2022, 10, e005292. [Google Scholar] [CrossRef]
- Portnow, L.H.; Kochkodan-Self, J.M.; Maduram, A.; Barrios, M.; Onken, A.M.; Hong, X.; Mittendorf, E.A.; Giess, C.S.; Chikarmane, S.A. Multimodality Imaging Review of HER2-Positive Breast Cancer and Response to Neoadjuvant Chemotherapy. RadioGraphics 2023, 43, e220103. [Google Scholar] [CrossRef]
- Fowler, A.M.; Mankoff, D.A.; Joe, B.N. Imaging Neoadjuvant Therapy Response in Breast Cancer. Radiology 2017, 285, 358–375. [Google Scholar] [CrossRef]
- Di Cosimo, S.; Campbell, C.; Azim, H.A.; Galli, G.; Bregni, G.; Curigliano, G.; Criscitiello, C.; Izquierdo, M.; de la Pena, L.; Fumagalli, D.; et al. The Use of Breast Imaging for Predicting Response to Neoadjuvant Lapatinib, Trastuzumab and Their Combination in HER2-Positive Breast Cancer: Results from Neo-ALTTO. Eur. J. Cancer 2018, 89, 42–48. [Google Scholar] [CrossRef] [PubMed]
- McGuire, K.P.; Toro-Burguete, J.; Dang, H.; Young, J.; Soran, A.; Zuley, M.; Bhargava, R.; Bonaventura, M.; Johnson, R.; Ahrendt, G. MRI Staging After Neoadjuvant Chemotherapy for Breast Cancer: Does Tumor Biology Affect Accuracy? Ann. Surg. Oncol. 2011, 18, 3149–3154. [Google Scholar] [CrossRef]
- van Ramshorst, M.S.; Loo, C.E.; Groen, E.J.; Winter-Warnars, G.H.; Wesseling, J.; van Duijnhoven, F.; Peeters, M.-J.T.V.; Sonke, G.S. MRI Predicts Pathologic Complete Response in HER2-Positive Breast Cancer after Neoadjuvant Chemotherapy. Breast Cancer Res. Treat. 2017, 164, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Hayward, J.H.; Woodard, G.A.; Ray, K.M.; Starr, C.J.; Hylton, N.M.; Joe, B.N.; Lee, A.Y. Complete Breast MRI Response to Neoadjuvant Chemotherapy and Prediction of Pathologic Complete Response. J. Breast Imaging 2019, 1, 217–222. [Google Scholar] [CrossRef]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular Imaging as a Tool to Investigate Heterogeneity of Advanced HER2-Positive Breast Cancer and to Predict Patient Outcome under Trastuzumab Emtansine (T-DM1): The ZEPHIR Trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef]
- Hou, Y.; Nitta, H.; Li, Z. HER2 Intratumoral Heterogeneity in Breast Cancer, an Evolving Concept. Cancers 2023, 15, 2664. [Google Scholar] [CrossRef] [PubMed]
- Filho, O.M.; Viale, G.; Stein, S.; Trippa, L.; Yardley, D.A.; Mayer, I.A.; Abramson, V.G.; Arteaga, C.L.; Spring, L.M.; Waks, A.G.; et al. Impact of HER2 Heterogeneity on Treatment Response of Early-Stage HER2-Positive Breast Cancer: Phase II Neoadjuvant Clinical Trial of T-DM1 Combined with Pertuzumab. Cancer Discov. 2021, 11, 2474–2487. [Google Scholar] [CrossRef]
- Hosonaga, M.; Arima, Y.; Sampetrean, O.; Komura, D.; Koya, I.; Sasaki, T.; Sato, E.; Okano, H.; Kudoh, J.; Ishikawa, S.; et al. HER2 Heterogeneity Is Associated with Poor Survival in HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2018, 19, 2158. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Bang, Y.-J. HER2-Targeted Therapies—A Role beyond Breast Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Stoss, O.; Shi, D.; Büttner, R.; van de Vijver, M.; Kim, W.; Ochiai, A.; Rüschoff, J.; Henkel, T. Assessment of a HER2 Scoring System for Gastric Cancer: Results from a Validation Study. Histopathology 2008, 52, 797–805. [Google Scholar] [CrossRef]
- Rüschoff, J.; Hanna, W.; Bilous, M.; Hofmann, M.; Osamura, R.Y.; Penault-Llorca, F.; van de Vijver, M.; Viale, G. HER2 Testing in Gastric Cancer: A Practical Approach. Mod. Pathol. 2012, 25, 637–650. [Google Scholar] [CrossRef]
- Ferris, R.L.; Jaffee, E.M.; Ferrone, S. Tumor Antigen-Targeted, Monoclonal Antibody-Based Immunotherapy: Clinical Response, Cellular Immunity, and Immunoescape. J. Clin. Oncol. 2010, 28, 4390–4399. [Google Scholar] [CrossRef]
- Ferraro, E.; Drago, J.Z.; Modi, S. Implementing Antibody-Drug Conjugates (ADCs) in HER2-Positive Breast Cancer: State of the Art and Future Directions. Breast Cancer Res. 2021, 23, 84. [Google Scholar] [CrossRef]
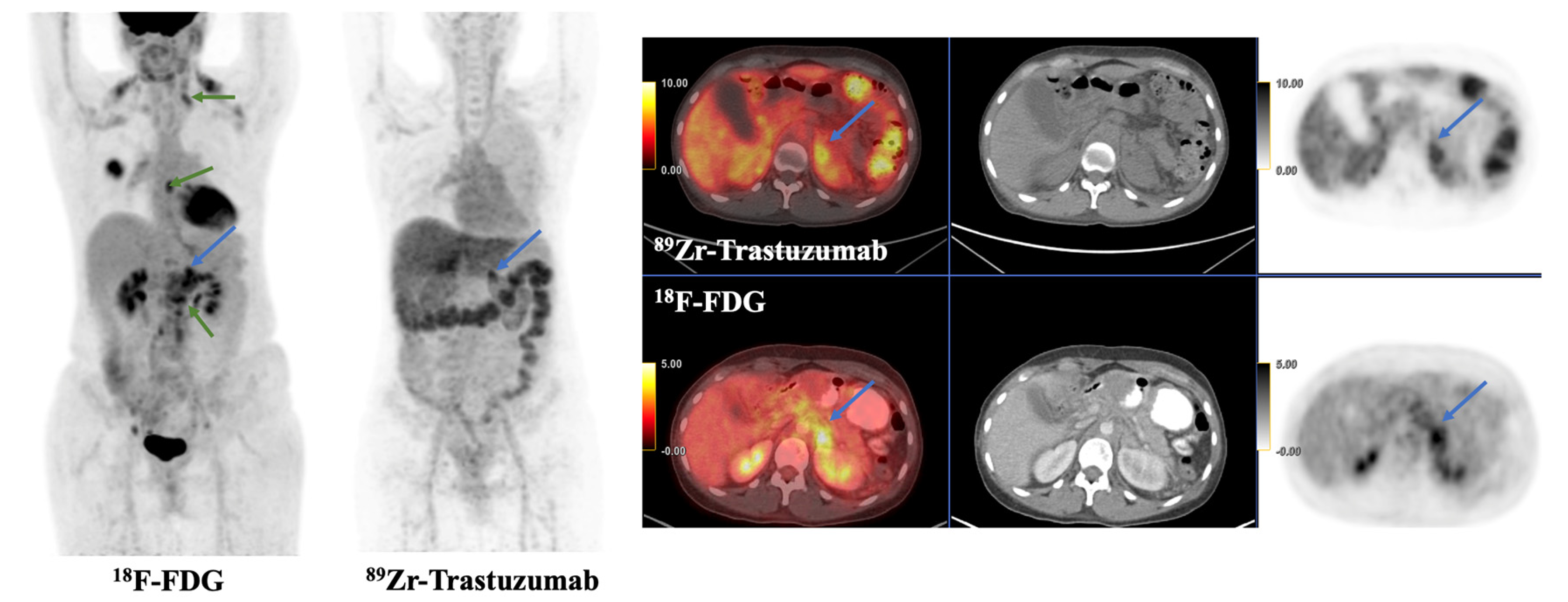
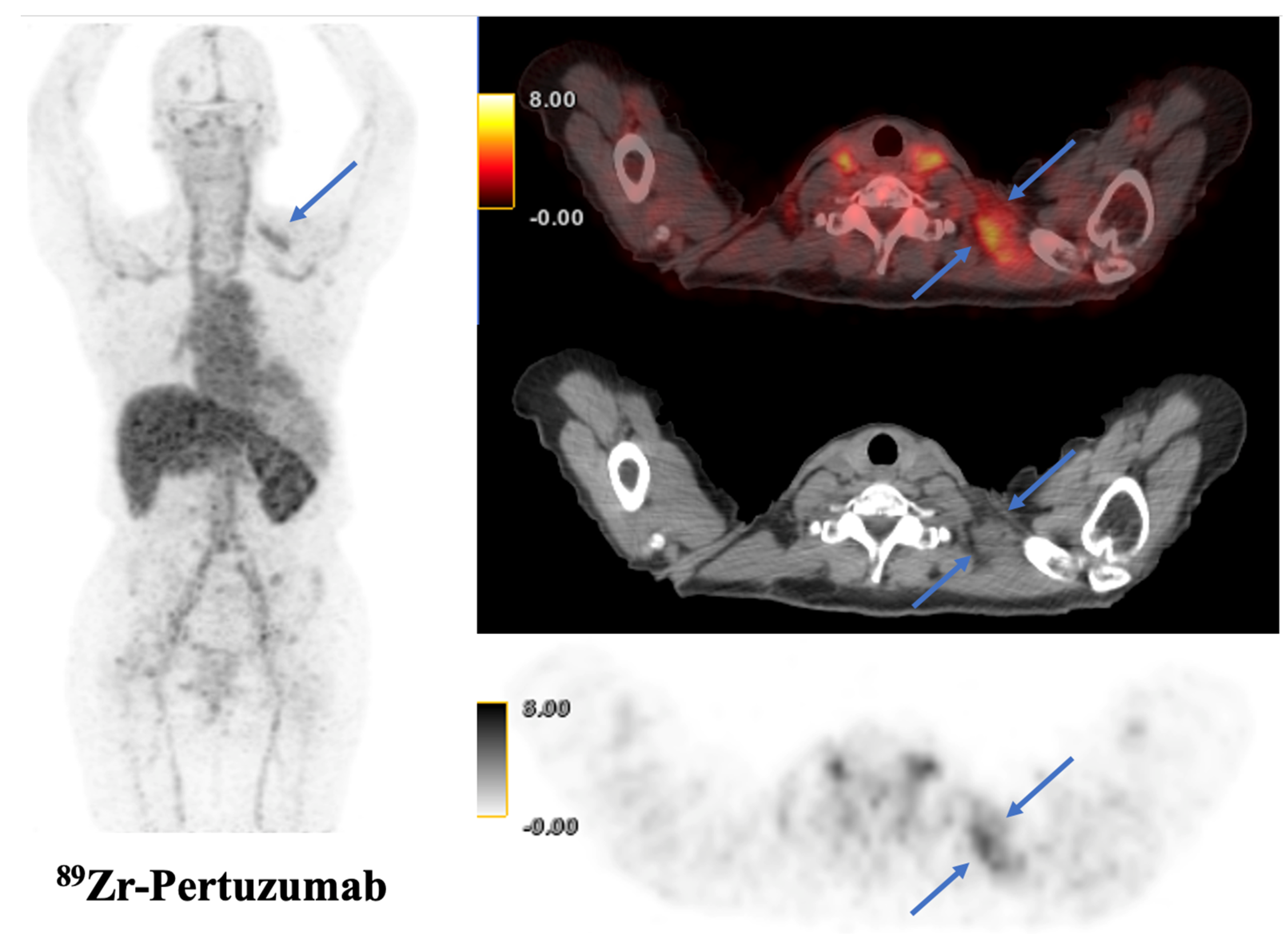
| Imaging Modality | n Patients | Radiotracer | Primary Objective | Year | First Author | PMID | Citation |
|---|---|---|---|---|---|---|---|
| PET/CT | 16 | 89Zr-Trastuzumab | Treatment response | 2014 | Gaykema | 25085789 | [15] |
| 11 | Detection of HER2+ metastases | 2017 | Ulaner | 28872549 | [16] | ||
| 14 | Feasibility study | 2010 | Dijkers | 20357763 | [17] | ||
| 9 | Detection of HER2+ metastases | 2016 | Ulaner | 27151988 | [18] | ||
| 20 | HER2 status determination | 2018 | Bensch | 30058029 | [19] | ||
| 34 | HER2 status determination | 2018 | Dehdashti | 29442264 | [20] | ||
| 12 | Feasibility study | 2016 | Laforest | 27146421 | [21] | ||
| 10 | HER2 status determination | 2019 | Jauw | 31147401 | [22] | ||
| PET/CT | 22 | 89Zr-Atezolizumab | Feasibility study | 2018 | Bensch | 30478423 | [23] |
| PET/CT | 6 | 89Zr-Pertuzumab | Feasibility study | 2017 | Ulaner | 29146695 | [24] |
| 24 | 89Zr-Pertuzumab | Detection of HER2+ metastases | 2020 | Ulaner | 32515679 | [25] | |
| PET/CT | 1 | 89Zr-Fab * | Feasibility study | 2020 | Richter, Knorr | 32377263 | [26] |
| PET/CT | 1 | 68Ga-ZHER2 | Detection of HER2+ metastases | 2020 | Zhou | 31833926 | [27] |
| PET/CT | 20 | 68Ga-nanobody | Feasibility study | 2015 | Keyaerts | 26449837 | [28] |
| PET/CT | 16 | 68Ga-affibody | Feasibility study | 2016 | Sorensen | 26877784 | [29] |
| 24 | HER2 status determination | 2022 | Miao | 35712499 | [30] | ||
| 8 | Feasibility study | 2016 | Sandstrom | 26912439 | [31] | ||
| PET/CT | 6 | 64Cu-Trastuzumab | Feasibility study | 2013 | Tamura | 24029656 | [32] |
| 38 | HER2 status determination | 2017 | Sasada | 28505219 | [33] | ||
| 8 | Feasibility study | 2016 | Carrasquillo | 27171605 | [34] | ||
| 5 | Detection of HER2+ metastases | 2015 | Kurihara | 25853014 | [35] | ||
| 8 | HER2 status determination | 2014 | Mortimer | 24337604 | [36] | ||
| 11 | HER2 status determination | 2018 | Mortimer | 28637802 | [37] | ||
| 7 | Feasibility study | 2021 | Lee | 33475899 | [38] | ||
| 1 | HER2 status determination | 2022 | Lee | 35133094 | [39] | ||
| 1 | HER2 status determination | 2017 | Sasada | 28770275 | [40] | ||
| PET/CT | 7 | 64Cu-SAR † | Feasibility study | 2022 | Wong | 35890071 | [41] |
| PET/CT | 11 | 177Lu-Trastuzumab | Feasibility study | 2021 | Nautiyal | 34406146 | [42] |
| PET/CT | 3 | 111In/68Ga-affibody | HER2 status determination | 2010 | Baum | 20484419 | [43] |
| SPECT/CT | 23 | Feasibility study | 2017 | Sandberg | 28261749 | [44] | |
| SPECT/CT | 7 | 111In-affibody | Feasibility study | 2014 | Sörensen | 24665085 | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGale, J.; Khurana, S.; Huang, A.; Roa, T.; Yeh, R.; Shirini, D.; Doshi, P.; Nakhla, A.; Bebawy, M.; Khalil, D.; et al. PET/CT and SPECT/CT Imaging of HER2-Positive Breast Cancer. J. Clin. Med. 2023, 12, 4882. https://doi.org/10.3390/jcm12154882
McGale J, Khurana S, Huang A, Roa T, Yeh R, Shirini D, Doshi P, Nakhla A, Bebawy M, Khalil D, et al. PET/CT and SPECT/CT Imaging of HER2-Positive Breast Cancer. Journal of Clinical Medicine. 2023; 12(15):4882. https://doi.org/10.3390/jcm12154882
Chicago/Turabian StyleMcGale, Jeremy, Sakshi Khurana, Alice Huang, Tina Roa, Randy Yeh, Dorsa Shirini, Parth Doshi, Abanoub Nakhla, Maria Bebawy, David Khalil, and et al. 2023. "PET/CT and SPECT/CT Imaging of HER2-Positive Breast Cancer" Journal of Clinical Medicine 12, no. 15: 4882. https://doi.org/10.3390/jcm12154882
APA StyleMcGale, J., Khurana, S., Huang, A., Roa, T., Yeh, R., Shirini, D., Doshi, P., Nakhla, A., Bebawy, M., Khalil, D., Lotfalla, A., Higgins, H., Gulati, A., Girard, A., Bidard, F.-C., Champion, L., Duong, P., Dercle, L., & Seban, R.-D. (2023). PET/CT and SPECT/CT Imaging of HER2-Positive Breast Cancer. Journal of Clinical Medicine, 12(15), 4882. https://doi.org/10.3390/jcm12154882








