Abstract
Statins have been widely advocated for use in COVID-19 based on large favorable observational associations buttressed by theoretical expected benefits. However, past favorable associations of statins to pre-COVID-19 infection outcomes (also buttressed by theoretical benefits) were unsupported in meta-analysis of RCTs, RR = 1.00. Initial RCTs in COVID-19 appear to follow this trajectory. Healthy-user/tolerator effects and indication bias may explain these disparities. Moreover, cholesterol drops in proportion to infection severity, so less severely affected individuals may be selected for statin use, contributing to apparent favorable statin associations to outcomes. Cholesterol transports fat-soluble antioxidants and immune-protective vitamins. Statins impair mitochondrial function in those most reliant on coenzyme Q10 (a mevalonate pathway product also transported on cholesterol)—i.e., those with existing mitochondrial compromise, whom data suggest bear increased risks from both COVID-19 and from statins. Thus, statin risks of adverse outcomes are amplified in those patients at risk of poor COVID-19 outcomes—i.e., those in whom adjunctive statin therapy may most likely be given. High reported rates of rhabdomyolysis in hospitalized COVID-19 patients underscore the notion that statin-related risks as well as benefits must be considered. Advocacy for statins in COVID-19 should be suspended pending clear evidence of RCT benefits, with careful attention to risk modifiers.
1. Introduction
Since the COVID-19 pandemic broke in the U.S. in March 2020, numerous sources have directly advocated statin use for treatment of COVID-19, have provided evidence nominally supporting statin use for COVID-19, or have focused on observational associations of statin use with COVID-19 that might be construed as supporting statin use [1] (Supplement SI). We do not reprise the arguments here, though we address the related observational studies. Points in favor of statins include their anti-inflammatory effects [2] and reduction in cardiovascular outcomes [3], outcomes that can arise in association with COVID-19 [4,5]. (Among additional hypothesized mechanisms of benefit include in silico evidence that some statins may affect the virus itself [5].) Widely disseminated position pieces have advocated while others have appeared to endorse off-label (high-dose) statins for COVID-19 infections by suggesting potential benefits of statins in this setting.
On the other side of the risk–benefit equation, we show evidence that adverse effects (AEs) of statins share mechanisms with AEs of COVID-19, and that many of these same adverse outcomes are present for both. Such AEs include, but are not limited to, muscle pain, muscle weakness, and fatigue. Rhabdomyolysis and autoimmune necrotizing myositis specifically have occurred with COVID-19 [6,7,8,9] as they can occur with statins [10,11,12,13,14,15,16]. Based on these and other considerations, the risk side of the risk–benefit ratio should also be considered seriously in deliberations about statin use. Of further note, key risk factors for adverse statin outcomes are also risk factors for adverse COVID-19 outcomes (e.g., hypertension, diabetes, obesity, and older age) [17], so those at high COVID-19 risk, in whom adjunctive therapy might be advised, are those in whom statins are most likely to produce serious problems.
Here, we review relevant evidence related to the use of statins for COVID-19 and post-COVID syndrome (long-COVID), addressing primarily those issues that have received little attention in the literature to date. We demonstrate that massive “healthy-user/healthy-tolerator” effects underlie favorable statin associations to infection. Although pre-COVID-19 observational studies showed apparent large favorable associations of statins to infection, meta-analysis of randomized controlled trials (RCTs) failed to support even a small causal effect. Observational associations for COVID-19 have included similar favorable associations, also these were not reproduced in randomized trial evidence. We address additional information related to effects of statins and functions of cholesterol that might inform deliberations about statin use in the setting of COVID-19, particularly in high-risk COVID-19 patients. Thus, we address clinical evidence related to statin use in COVID-19 and review underconsidered physiological and epidemiological factors that aid in evaluating and understanding this clinical evidence.
Key considerations extend to long-COVID. Symptoms of long-COVID prominently encompass symptoms that can arise with statins, including muscle weakness, fatigue, fatigue with exertion, and cognitive compromise [18,19,20,21,22,23].
2. Statins
Cholesterol-lowering medications, mostly statins, are used by approximately one in four persons over the age of 40 in the U.S. [24] (with widespread use elsewhere), adding high importance to understanding potential interactions between statin use and COVID-19 infection. Statins are pharmacological inhibitors of HMG-CoA reductase with regulatory effects on mevalonate synthesis. HMG-CoA reductase is ubiquitously expressed in mammalian cells and found in the membrane of the endoplasmic reticulum. Statins inhibit pathways for which mevalonate is a precursor (Figure 1), affecting not only synthesis of cholesterol and its downstream products, but multiple other pathways. Among these are the production of heme A and coenzyme Q10 (CoQ10, also known as ubiquinone), products of the inhibited mevalonate pathway that are required for mitochondrial electron transport which drives ATP synthesis. CoQ10 deficiencies (whether primary or secondary/acquired) may affect multiple organ systems (including heart and skeletal muscle, as well as other organs that can be affected by COVID-19—lungs, central and peripheral nervous systems, liver, and kidney) [25,26]. Inhibiting the mevalonate pathway, statins lead to dose-dependent reduction in CoQ10. CoQ10 is also an important endogenous antioxidant [27,28] that is transported in association with cholesterol [29]. Just as CoQ10 supplementation can effectively bypass mitochondrial “respiratory chain” defects [30], improving cell energy, so too can the lowering of CoQ10 with statins unmask (precipitate) mitochondrial compromise [31]—impairing cell energy and increasing free radical release [32,33]. These effects on mitochondrial energy production and impaired antioxidation contribute to statin AEs, including but not limited to risk of muscle symptoms, new-onset diabetes, and cognitive impairment [20,21,34,35].
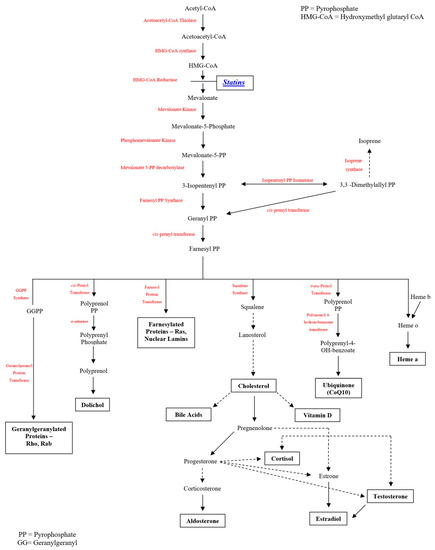
Figure 1.
Mevalonate Pathway Inhibition by Statins (HMG-CoA Reductase Inhibitors).
The mevalonate pathway, for which statins inhibit the rate-limiting step, produces multiple compounds of relevance to statin effects as well as COVID-19 effects. Both coenzyme Q10 and heme A play critical roles in mitochondrial function. Mitochondrial function in turn moderates oxidative stress and resulting apoptosis, inflammation, and coagulation activation, blood flow/endothelial function, and autoimmunity. Testosterone is important for muscle strength and for COVID-19 cell entry. Other examples of these pathways also play roles in the muscle effects of statins, relevant in the COVID-19 setting. Statins have been reported to promote multiple pleiotropic mechanisms, including benefits to endothelial function, inflammation, coagulation activation, as well as antioxidation. However, antioxidation itself may be responsible for much or most of these benefits, since antioxidation supports endothelial function [36,37] and reduces apoptosis [38,39] that in turn promotes inflammation [40] and coagulation activation [40]. (In fact, inflammation and coagulation activation were strong predictors of severe COVID-19 outcomes [41,42], and this may, in part, arise from the fact that these reflect oxidatively mediated cell death.) A full discussion of pleiotropic effects of statins, the factors that underlie these, and the possible contribution of these to effects of statins on COVID-19 is outside the scope of this paper. The absence of randomized trial support for net statin benefit in treating patients with COVID-19 materially diminishes the imperative for detailed exposition of these pleiotropic mechanisms. Our focus is on key documented mechanisms with direct relevance for statins, for COVID-19, and for the interaction between them.
Statins are widely used to reduce low-density lipoprotein cholesterol (LDL) and to lower cardiovascular morbidity and mortality. Cardiovascular morbidity and cardiovascular mortality are reduced with statins in most statin trials, but effects on patient-centered (versus cause-specific) outcomes—those most relevant for preventive medications—show more mixed results, with a number of RCTs showing no trend to improvement in all-cause mortality or, where assessed, a proxy for all-cause serious morbidity (e.g., EXCEL, AFCAPS [43], ALLHAT-LLT [44], PROSPER [45]).
3. COVID-19 (SARS-CoV-2 Infection)
COVID-19 can be asymptomatic, can present with mild illness (e.g., with upper respiratory symptoms, fever, and fatigue), or can manifest severe disease encompassing refractory hypoxemia, pneumonia, and cytokine storm [46]. Severe disease can necessitate intensive care, can spur intubation (an issue depending on COVID-19 strain) and ventilator support, and can progress to death. Protean potential complications of COVID-19 can include (but are not limited to) cardiovascular [4], neurological [47], muscular [47], hepatic [48], and renal [48] compromise.
Of note, clinical predictors of adverse COVID-19 outcomes largely encompass conditions that amplify (or reflect amplified) cell energy impairment: metabolic syndrome features (hypertension, obesity, diabetes), as well as older age [49]. Mitochondrial impairment rises exponentially with age [32] and, although this is underrecognized, metabolic syndrome features are markers of (and adaptations to) impaired cell energy [49]—often including mitochondrial impairment [49]. Other COVID-19 risk factors also reflect and/or contribute to impaired cell energy, including congestive heart failure (CHF) and chronic kidney disease [50] (in turn often tied to diabetes and/or hypertension).
4. Statins and COVID-19 Outcomes: Observational Studies and Randomized Controlled Trials
Favorable associations of statins to respiratory and other infection outcomes before the COVID-19 pandemic, though of large magnitude, were subsequently shown to be chimerical, with no suggestion of favorable effect in meta-analysis of randomized trials [51,52].
Specifically, a pre-COVID-19 meta-analysis of a total of 20 observational studies states, “Meta-analysis for various infection-related outcomes revealed the following pooled odds ratios all in favor of statin use vs. non: 0.61 (95% confidence interval [CI], 0.48–0.73) for 30-day mortality (n = 7), 0.38 (95% CI, 0.13–0.64) for in-hospital mortality (n = 7), 0.63 (95% CI, 0.55–0.71) for pneumonia-related mortality (n = 7), 0.33 (95% CI, 0.09–0.75) for bacteremia-related mortality (n = 4), 0.40 (95% CI, 0.23–0.57) for sepsis-related mortality (n = 4), and 0.50 (95% CI, 0.18–0.83) for mixed infection-related mortality (n = 4)” [51]. These pooled estimates encompass individual studies that report still more favorable associations, which we note in order to point out, in juxtaposition with the RCT meta-analysis to follow, that even apparently massive favorable associations have no necessary implications for causal effect occurrence.
However, a subsequent meta-analysis of RCTs examining the actual causal relation of statin use to such outcomes found no relationship. A meta-analysis showed no effect of statins on the risk of infections or on infection-related deaths: relative risk (95% confidence interval, or CI), 1.00 (0.96–1.05) and 0.97 (0.83–1.13), respectively [52]. Anti-inflammatory benefits of statins did not translate to clinical benefits to infection, when the best quality evidence was considered. The disparity between massive favorable observational relationships (associations) and no relationships (effects) whatsoever on RCTs comports with the often-seen, well-documented large “healthy-user” and “healthy-tolerator” effects for statins (and other preventive medicines) spanning many outcomes [53]. Indeed, the chasm between observational and randomized findings is evident in that observational associations showed apparent “reductions” (favorable associations) in some infection-related outcomes exceeding 60% upon pooled analysis [53]. There may also be a role for “indication bias”, in which statin use serves as a proxy for higher cholesterol (prior to or during COVID-19 infection—see below) [53].
Similar associations are expected for statin use with COVID-19 outcomes, and indeed analogous favorable associations have now been reported [54,55,56]. (See Table 1 for a table summarizing the following results.) In one analysis, use of statins prior to admission was tied to reduced risk of severe COVID-19: adjusted odds ratio (OR) (95% CI), 0.29 (0.11–0.71), p < 0.01 [54]. In another analysis, statin use was associated with favorable outcomes, in a proportional hazards model adjusted for baseline differences, considering statins as a time-varying exposure: adjusted hazard ratio (95% CI), 0.63 (0.48–0.84, p = 0.001). In a mixed-effects Cox model that did not consider statins as a time-varying exposure, statin use was also associated with a decreased incidence of death, OR, 0.58 (0.43–0.80, p = 0.001) [55]. Sequential meta-analyses reported the following (none of which de facto included randomized trials), with the third meta-analysis being a follow-up to the first, incorporating subsequently published studies [57,58,59]: total included number of trials in the three were n = 24, n = 22, and n = 47, respectively. Each of the studies provided data on in-hospital COVID-19 mortality both for pre-hospital statin use and in-hospital statin use. For pre-hospital statin use, the mortality ORs/relative risks (95% CIs) were: 0.77 (0.60–0.98), 0.69 (0.56–0.84), and 1.06 (0.82–1.37) based on n = 18, n = 14, n = 29 studies, respectively. For in-hospital statin use, the respective values for the three meta-analyses were: 0.40 (0.22–0.73), 0.57 (0.54–0.60), and 0.54 (0.50–0.58) based on n = 3, n = 11, and n = 7 included studies, respectively. A further “meta-analysis” published as a letter to the editor (that included two of our four peer-reviewed citations, plus two non-peer-reviewed findings), showed a pooled hazard ratio for “fatal or severe” COVID-19 outcomes: 0.70 (0.53–0.94) [60]. (Subsequent observational studies of statin use in hospitalized COVID-19 patients show analogous favorable associations to severe and fatal COVID-19 outcomes [61,62].) However, in-hospital statin use is expected to be a proxy for higher in-hospital LDL cholesterol, which is expected to be a proxy for better expected outcomes (irrespective of statin use) in two different ways. First, higher pre-infection LDL is correlated with higher in-hospital LDL, other events being equal; as discussed elsewhere, higher pre-hospital cholesterol is tied to lower risk of severe infection outcomes. Second, since acute infection lowers cholesterol, with cholesterol reduction correlated with infection severity [63], higher in-hospital cholesterol (and corresponding comfort with administration of statins) is associated with less severe COVID-19 infection and expectation of lower COVID-19 mortality. Thus, in-hospital statin use—through its presumed relation to higher in-hospital LDL—serves as a proxy for lower prior risk of, and lower current presence of, severe infection. Although the authors do not mention this, a large favorable association of in-hospital statin use to lower mortality has no necessary implications for whether the causal effect of in-hospital statin use on mortality in hospitalized COVID-19 patients is favorable, adverse, or neutral.
Not all observational studies, even those of in-hospital statin use, report favorable associations. An Italian study including 3988 COVID-19 patients showed no independent statin association to mortality upon multivariable analysis: hazard ratio (95% CI), 0.98 (0.81–1.20) [64].
A sizable study of close to 20,000 patients with COVID-19 found that statin use was not associated with improved outcomes (encompassing outcomes like death and ICU stay, according to what was assessed in the encompassed study in a meta-analysis) in unadjusted analysis: OR (95% CI), 1.02 (0.69–1.50, p = 0.94). However, statin use was associated with markedly reduced risk of adverse outcomes with adjusted analysis: OR (95% CI), 0.51 (0.41–0.63, p < 0.0005) [65]. Other observational studies variably report beneficial associations to symptoms (but not serious outcomes) [66] and beneficial associations to severe COVID-19 and faster time to recovery [54]. Other studies showed no significant association or a significantly adverse association to severe COVID-19 and/or mortality. A meta-analysis of nine studies showed no association between statin use and severe outcomes (OR (95% CI), 1.64 (0.51–5.23, p = 0.41)) or mortality (OR, 0.78 (0.50–1.21, p = 0.26)) [67]. A Johns Hopkins study showed no association between statin use and mortality in hospitalized patients (relative risk (95% CI), 1.00 (0.99–1.01, p = 0.928)) and an association to increased severe COVID-19 (relative risk, 1.18 (1.11–1.27, p < 0.001)) [68]. Finally, in a study of COVID-19 mortality in patients with type 2 diabetes with an average age of 70.9, statin use was associated with significantly increased mortality at 7 days (OR (95% CI), 1.74 (1.13–2.65)) and at 28 days (OR, 1.46 (1.08–1.95)) [69].

Table 1.
Observational studies and meta-analyses of statin use in the COVID-19 setting.
Table 1.
Observational studies and meta-analyses of statin use in the COVID-19 setting.
| First Author | N | Population | % on Statins | Outcomes | Measure | Results | 95% CI | p |
|---|---|---|---|---|---|---|---|---|
| Daniels et al. (2020) [54] | 170 | Adult patients hospitalized with COVID-19, at UC San Diego Health | 27%, on admission | Reduced risk of severe COVID-19 | OR | 0.29 | 0.11–0.71 | p < 0.01 |
| Faster time to recovery among those without severe disease | HR | 2.69 | 1.36–5.33 | p < 0.01 | ||||
| Zhang et al. (2020) [55] | 13,981 | Adult hospitalized patients with COVID-19, in Hubei Province, China | 8.7% | Cox time-varying model: All-cause mortality | HR | 0.63 | 0.48–0.84 | p = 0.001 |
| Mixed-effects Cox model: All-cause mortality | 0.58 | 0.43–0.80 | p = 0.001 | |||||
| Grasselli et al. (2020) [64] | 3988 | Adult hospitalized patients with COVID-19, in Italy | 12% | Multivariable Cox proportional hazards regression analysis: mortality | HR | 0.98 | 0.81–1.20 | p = 0.87 |
| Ayeh et al. (2021) [68] | 4447 | Adult hospitalized patients with COVID-19, at John Hopkins Medical Institutions | 13.4% | Mortality | RR | 1.00 | 0.99–1.01 | p = 0.928 |
| Severe Infection | 1.18 | 1.11–1.27 | p < 0.001 | |||||
| Cariou et al. (2021) [69] | 2449 | Adult hospitalized patients with COVID-19 and Type 2 Diabetes, from CORONADO study | 49% | 7-day mortality | OR | 1.74 | 1.13–2.65 | N/A |
| 28-day mortality | 1.46 | 1.08–1.95 | ||||||
| Israel et al. (2020) [56] | 6530 | Adult hospitalized patients with COVID-19, in Israel | 5.0% | Hospitalization | OR | 0.673 | 0.596–0.758 | p < 0.001 |
| Meta-analyses | ||||||||
| Vahedian-Azimi et al. (2021) [57] | 32,715 (24 studies total) | Varies by study | Varies by study | Pre-hospital use of statins: mortality (n = 18 studies) | OR | 0.77 | 0.60–0.98 | N/A |
| In-hospital use of statins: mortality (n = 3 studies) | 0.40 | 0.22–0.73 | ||||||
| Wu et al. (2021) [58] | (22 studies total) | Varies by study | Varies by study | Pre-hospital use of statins: mortality | RR | 0.69 | 0.56–0.84 | p < 0.001 |
| In-hospital use of statins: mortality | 0.57 | 0.54–0.60 | p < 0.001 | |||||
| Vahedian-Azimi et al. (2021)—Follow-up [59] | 3,238,508 (47 studies total) | Varies by study | Varies by study | Pre-hospital use of statins: mortality (n = 29 studies) | OR | 1.06 | 0.82–1.37 | p = 0.670 |
| In-hospital use of statins: mortality (n = 7 studies) | 0.54 | 0.50–0.58 | p < 0.001 | |||||
| Kow et al. (2020) [60] | Four studies | Varies by study | Varies by study | “Fatal or severe” COVID-19 outcomes | HR | 0.70 | 0.53–0.94 | N/A |
| Pal et al. (2021) [65] | 19,988 (14 studies total) | Varies by study | Varies by study | Unadjusted analysis: statin use not associated with improved clinical outcomes | OR | 1.02 | 0.69–1.50 | p = 0.94 |
| Adjusted analysis: statin found to reduce risk of adverse outcomes | 0.51 | 0.41–-0.63 | p < 0.0005 | |||||
| Hariyanto et al. (2020) [67] | 3449 (Nine studies total) | Varies by study | Varies by study | Severe outcomes | OR | 1.64 | 0.51–5.23 | p = 0.41 |
| Mortality | 0.78 | 0.50–1.21 | p = 0.26 | |||||
OR = odds ratio; HR = hazard ratio; RR = relative risk/risk ratio.
Another study examined drugs associated with reduced COVID-19 severity. Associations for rosuvastatin with reduced hospitalization were consistent with aforementioned healthy-user/tolerator effects: OR (95% CI), 0.673 (0.596–0.758, p < 0.001) for COVID-19 hospitalization [56]. Of interest was the far larger favorable association of ubiquinone (coenzyme Q10), which is not expected to have similar healthy-tolerator contributions: OR (95% CI), 0.185 (0.058–0.458). The OR for hospitalization of <0.19 is noteworthy since statins lead to dose-dependent reductions in coenzyme Q10, which is also a product of the mevalonate pathway and is transported with cholesterol [35].
On a relevant note, given the occurrence of acute respiratory distress syndrome (ARDS) in COVID-19 patients, it is of interest that a retrospective analysis of an RCT including 683 subjects with infection-related acute respiratory distress syndrome (ARDS) found that rosuvastatin administration was associated with increased interleukin-18 plasma levels, which were tied to higher mortality [70]. Consistent with this, in mouse models, statin exposure also increased inflammasome activation, interleukin-18 expression, and acute lung injury [71].
Four randomized controlled trials of statin use in the COVID-19 setting were identified [72,73,74,75]. (See Table 2 for a table summarizing the following results.) A search of PubMed was conducted on 18 April 2022 through EndNote using the following search terms as title words: “statin or rosuvastatin or atorvastatin or fluvastatin or simvastatin or lovastatin or pravastatin” and “COVID or SARS-CoV-2” and “randomized”. An expanded search was attempted, allowing “randomized” to be an abstract term, but no additional randomized trials were identified with this approach. A subsequent search (October 2022) identified one additional RCT [75]. These findings are at best mixed, with one apparently unequivocally adverse result, two neutral results, and one result favorable for a single outcome out of a suite of assessed outcomes. Given these findings, existing RCT data do not provide support for statin use in the acute COVID-19 setting. A fifth randomized trial included statin together with colchicine but did not separate the effects of these two agents and so can provide no information on the independent effects of statins [76]. The four identified RCTs have findings as follows: The first RCT, with a sample size of n = 156, randomized hospitalized COVID-19 patients to “standard therapy” (hydroxychloroquine + Kaletra®) or 20 mg of atorvastatin + standard therapy. Statin vs. placebo led to the following reported effects: mean hospitalization days (7.72 days vs. 5.06 days, p = 0.001) and frequency of hospitalization in the ICU (18.4% vs. 1.3%, p = 0.001) were longer and greater in those randomized to atorvastatin compared to the comparison group [72]. The second (n = 587) randomized patients to 20 mg of atorvastatin or placebo and reported that atorvastatin was not associated with a significant reduction in the primary outcome, which was a composite of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or all-cause mortality—OR (95% CI), 0.84 (0.58–1.21) [73]. The third (n = 40) randomized patients to 40 mg of atorvastatin + 400/100 mg of lopinavir/ritonavir or 400/100 mg of lopinavir/ritonavir alone, with outcomes as follows: duration of hospitalization (in days) was longer in the lopinavir/ritonavir group compared to the lopinavir/ritonavir + atorvastatin group (9.75 ± 2.29 vs. 7.95 ± 2.04, p = 0.012); however, there was no significant difference in the secondary outcomes (need for interferon or immunoglobulin and receipt of invasive mechanical ventilation) between the randomized groups [74]. The corresponding centers make use of other COVID-19 treatments and involve different patient populations, so that different interaction effects or sources of effect modification are not excluded. The fourth was a randomized, factorial design, open-label study of hospitalized patients with mild-to-moderate COVID-19 in which participants received atorvastatin 40 mg, aspirin 75 mg, both, or neither (n = 900, with n = 225 in the atorvastatin and both groups, n = 226 in the neither group). The primary outcome was “clinical deterioration to WHO Ordinal Scale for Clinical Improvement ≥ 6”. The rate of this outcome in the standard care group was 3.2%. In the statin group, it was also 3.2%: HR (95% CI), 1.0 (0.41–2.46, p = 0.99) [75]. (In the aspirin group, the rate was 1.4%; in the atorvastatin + aspirin group, it was 3.6%—neither differed significantly from the standard care group.) A 2023 meta-analysis of statin use in adult hospitalized COVID-19 patients identified the same four trials we previously identified and affirmed our conclusion: “There was no significant difference in all-cause mortality (odds ratio [OR] 0.96; 95% confidence interval [95% CI]: 0.61–1.51; p = 0.86…), duration of hospitalization (mean difference [MD] 0.21; 95% CI: −1.74–2.16; p = 0.83…), intensive care unit admission (OR = 3.31; 95% CI: 0.13–87.1; p = 0.47…), need for mechanical ventilation (OR = 1.03; 95% CI: 0.36–2.94; p = 0.95…)…between patients treated with or without statin therapy [77]”. Clearly, available randomized trial data do not provide meaningful support for statin administration for COVID-19 in patients. We underscore again the disparity between the large observational associations of in-hospital statin use to lower COVID-19 mortality upon meta-analysis and the absence of evidence for mortality improvement with in-hospital COVID-19 for patients in RCTs. These findings suggest that favorable observational associations of statins to COVID-19 outcomes (however large)—just as was seen above for favorable statin associations to pre-COVID-19 infection outcomes—have no implications for causal effects.

Table 2.
Randomized controlled trials and meta-analysis of statin use in the COVID-19 setting.
Incidentally, as treatments for COVID-19 emerge, risk–benefit considerations for statins may further alter, in particular, due to potential drug interactions. For instance, nirmatrelvir/ritonavir (“Paxlovid”), by inhibiting the CYP3A4 metabolizing pathway, may heighten risks associated with statins in proportion to the degree to which they are metabolized through that pathway. This especially affects simvastatin and lovastatin, which should not be co-administered, with potential interactions for atorvastatin and rosuvastatin for which close monitoring and potential dose adjustment are advised [78].
5. Cholesterol and Infection
Higher cholesterol has been associated with lower risk of infection and respiratory disease outcomes, including pneumonia/influenza hospitalizations [79], respiratory disease deaths [80], and even HIV [81]. These associations examine long-term risk and are not driven by existing disease. Indeed, higher cholesterol measured even years before the HIV epidemic “broke” were tied to lower risk of death from AIDS later [81]. Similarly, higher cholesterol was tied to lower risk of death from respiratory diseases, even after omitting the first five years of deaths [80]. (That said, serious infection can also lower cholesterol, which may contribute to the lower cholesterol reported in COVID-19 patients in China [82].)
Functions of cholesterol associated with infection protection are beyond the scope of this review but, briefly, include transport of nutrients that are critical to mucosal immunity, to innate and adaptive immunity, to quelling of inflammation in the setting of infection, to support for cell energy (important in COVID-19, where there is low energy supply, e.g., due to hypoxemia and high energy demand), and confer critical protection against oxidative stress (including from reoxygenation injury arising from COVID-19 hypoxemia) which in turn can trigger apoptosis [83] and result in inflammation and coagulation activation [40] (see VI). Nutrients transported by cholesterol—and specifically by LDL—as well as products of these, include vitamins that have been shown in RCTs to protect against viral infection occurrence and mortality. Oxidative stress, against which these nutrients defend, also depresses natural killer cell number and function, amplifying viral infection risk. Cholesterol itself also plays a pivotal role in cell barrier function and in cell energy defense, the latter in part by reducing heavy energy demands of the transmembrane sodium/potassium gradient.
Cholesterol declines more strongly with greater COVID-19 severity, as shown in multiple studies and meta-analyses, including for total cholesterol, LDL, and HDL cholesterol [84,85,86,87,88]. These drops in lipids not only reflect severity of disease, but because both LDL and HDL are involved in transporting critical fat-soluble antioxidants [89,90,91,92,93], and HDL also transports substances like paraoxonase with antioxidant (and anti-SARS-CoV-2 properties [94]) properties [95,96,97], these reductions might not just reflect but may conceivably contribute to worse COVID-19-associated outcomes. Statin use, clinically, is typically tied to/driven by levels of LDL cholesterol, which reproducibly respond to statin therapy [98]. HDL cholesterol is often, on average, neutrally influenced by statin therapy [99]. For these reasons, although HDL is of vital importance as a reflector of and contributor to oxidative stress defense potential, it is not the recipient of equal attention in the discussion here.
What about for COVID-19? An analysis of 5279 COVID-19 patients in New York City found that, in multivariable regression examining risk factors for hospitalization, diagnosed hyperlipidemia appeared protective: OR (95% CI), 0.62 (0.52–0.74, p < 0.001) [50]. (This does not imply that the lipids measured would remain elevated throughout the COVID-19 course, because of the large impact that severe infection can have in depressing cholesterol values [88].)
Long-term rates of community-acquired sepsis are greater specifically with lower LDL [100], suggesting that associations of higher cholesterol to lower infectious complications extend to bacterial conditions, which can complicate COVID-19. Statins can also have antioxidant effects; however, they are reproducibly prooxidant in some, and it is precisely the groups at high risk for COVID-19 outcomes (as below) in which this is expected.
6. Statins in COVID-19 High-Risk Groups
Potentially offsetting the reduction in cholesterol-associated transport of nutrients critical to viral defense, statins are often antioxidant. However, statins are sometimes reproducibly prooxidant [101]. This prooxidant predominance is tied to statin adverse effects [102], tied in turn to older age and metabolic syndrome factors [31]. It is in groups with worse mitochondrial status—e.g., those with older age or metabolic syndrome features—that CoQ10 withdrawal due to statins unmasks mitochondrial impairment [30] and increases free radical release [32,33]—offering prospects for statins’ prooxidant properties to override statins’ antioxidant mechanisms.
Thus, in obese, diabetic, hypertensive, and/or older patients—patients at risk for serious COVID-19 outcomes [17] that might be deemed to most merit adjunctive off-label statin therapy—statins may be adverse not just to oxidative stress but, in consequence, to downstream effects of oxidative stress that may worsen COVID-19 sequelae as well. One relevant effect (and cause) of oxidative stress is mitochondrial dysfunction [33], for which adverse portent is magnified in patients with hypoxemia. Another, as above, is apoptosis [83], with resultant triggering of inflammation [40] and coagulation activation [40], two serious adverse prognostic indicators in COVID-19 [50]. (Though statins are often anti-inflammatory, effects can be bidirectional as with oxidative stress. As citations here show, statins are sometimes pro-inflammatory [103,104].)
The statin AEs to which these COVID-19 high-vulnerability groups are at heightened risk include cardiac, neurological, muscular, hepatic, and renal AEs [31]—shared domains with COVID-19 AEs, affording further reason for caution with statin administration in the setting of COVID-19. However, the best known statin AEs affect muscle [21,31,105].
7. Statins and Muscle: Airways and Heart
Skeletal muscle symptoms are the most commonly reported statin adverse effects [31], and COVID-19 (as well as long-COVID) is also tied to muscle complications [22,106,107,108,109,110]. As above, statin-associated reductions in CoQ10, unmasking mitochondrial compromise, can impair cell energy and increase free radical release [32,33]. A common consequence of impaired cell energy is impaired muscle strength [111,112,113]. COVID-19 also impairs cell energy via sometimes-profound hypoxemia [50], and low CoQ10 is also seen following COVID-19 [114]. Skeletal muscle symptoms, with CK elevation, are reported as a complication of COVID-19 [47], as they are of statins (see later discussion of rhabdomyolysis) [21,31,111]. Cardiac muscle complications, in the form of CHF, have been reported in association with statins [115,116] and are also reported with COVID-19 [117,118].
7.1. Skeletal Muscle (Airway Dilator Muscles and Muscles of Respiration)
Statins can increase muscle-related symptoms [21,31,105] including exertional fatigue [18] and muscle weakness [19,21] (with implications also for upper airway dilator muscles and muscles of respiration, particularly important in severe COVID-19 infection). Statins are particularly apt to produce such muscle compromise in older age [19,31] and presence of metabolic syndrome features [31]. Muscle weakness, from either mitochondrial impairment or hypoxemia, can compromise upper airway dilator muscles and promote airway narrowing especially in the supine position, promoting sleep apnea. For this reason, sleep apnea is common in mitochondrial disorders [119] and in other conditions of muscle weakness [119]. Presumably, it is via these means that statins have been reported to promote sleep apnea in some [120]. Impaired cell energy can compromise airway caliber and worsen hypoxemia, magnifying morbidity and mortality in settings of severe respiratory compromise—just as improved cell energy can contribute to the widening of airways (greater airway dilation), extending to daytime and non-supine positioning [121]. Airway dilator collapse is most prominent in the supine position. Apropos of this are representations of benefit to COVID-19 patients with “proning” and nasal continuous positive airway pressure (CPAP) therapy [122,123]. (Potentially underscoring the importance of airway integrity in COVID-19 outcomes, COVID-19 risk is particularly great in obese patients [124], in whom sleep apnea is singularly common.) Of note, sleep apnea is attended causally by oxidative stress [125,126,127,128] (related to hypoxemia and reoxygenation [126]), with oxidative stress being a potentially serious mechanism in COVID-19 [129,130,131,132,133], contributing to other factors that adversely affect cell energy such as endothelial function compromise [125,129,134]. (Like other oxidative stress-promoting exposures, bidirectional effects of hypoxemia on outcomes may arise in some settings [135], likely via the impact of oxidative preconditioning whereby modest exposure to an oxidative stressor may lead to upregulation of antioxidant defenses [136]—where these are not already overwhelmed—leading to favorable clinical effects. Oxidative preconditioning has been proposed as a potential therapeutic approach for COVID-19 [137].)
An additional consideration is the potential for both statins and COVID-19 to promote autoimmunity. Autoimmune necrotizing myositis [10,11,12,138] is a feared complication of statin use. (Other autoimmune complications of statins are also reported [139,140,141,142,143]—which can affect muscle [144,145,146,147]—and in some cases, these accompany autoimmune myositis [13,14,15,16,148].) Autoimmunity is also a complication of COVID-19 [149,150,151,152] (encompassing, specifically, autoimmune conditions reported with statins [6,7,153,154,155] including necrotizing myositis [8,9]) and is a factor in long-COVID [156,157,158,159]: in one “multi-omics” study of long-COVID, an autoimmune profile represented one of the four key patterns observed [160]. (See Section 11 for further discussion of long-COVID.)
7.2. Cardiac Muscle
Mitochondria comprise about 40% of the volume of cardiac muscle cells [161]. The heart consumes 20 times its weight in ATP per day [162]. Diastolic function, even more than systolic function, is strongly ATP-dependent [115]. Consistent with statin-induced CoQ10 depression [163], clinical findings suggest that statins may worsen diastolic function [115] and may induce statin-associated cardiomyopathy in vulnerable patients [116]. Conversely, in a double-blind RCT, CoQ10 supplementation cut all-cause mortality by 42% (p = 0.036) in heart failure patients [28]—underscoring the importance of CoQ10 alteration to heart failure outcomes. CoQ10 transport is among relevant functions of cholesterol-carrying lipoproteins [91] (in addition to CoQ10 being a product of the mevalonate pathway inhibited by statins). Therefore, risking worsening cardiac function with statin-induced mevalonate inhibition and CoQ10 depletion may be imprudent in those with factors prognostic for adverse COVID-19 outcomes—which as above, double as risk factors for adverse statin outcomes. (Concordant with these reports, atorvastatin exposure impaired the structure and function of cardiac mitochondria, thereby worsening cardiac outcomes in a mouse heart failure model [164].) CHF is an independent risk factor for adverse COVID-19 outcomes [4,50]—as well as a complication of COVID-19 [118]. Hypoxemia from COVID-19—like mitochondrial impairment from statins—can worsen CHF outcomes.
Of note, low levels of serum total cholesterol and LDL are associated with reduced survival in heart failure patients [165]. This so-called “reverse epidemiology”, which occurs in a range of conditions tied to impaired cell energy [49], is evidently applicable irrespective of statin treatment [166].
8. Statins and COVID-19: Effects on Liver
Abundant evidence supports occasional hepatotoxicity with statin use. Although effects are commonly mild, more serious hepatotoxicity can occur [140,142,167,168]. Statin-mediated liver toxicity mechanisms likely include mitochondrial impairment [169] and extend to autoimmune hepatitis [139,141].
Similarly, COVID-19 confers potential for hepatotoxicity, mild or severe [170,171,172]. As with statins, mechanisms of liver toxicity likely include mitochondrial impairment [171] and also extend to autoimmune hepatitis [153,154,173,174].
9. Statin Effects on Testosterone: Implications for Muscle, as Well as Viral Entry to Cells, in Acute COVID-19
Statins lead to significant but highly variable depressions in testosterone (a biochemical product of cholesterol that, like all steroid hormones, is made in the mitochondria [175]). These effects, while significant on average in sufficiently sized samples or meta-analyses [176,177] and/or studies with higher potency statins [178], are highly variable. Since COVID-19 entry into cells is androgen-dependent (believed to contribute to the male preponderance of severe COVID-19) [179,180], this mechanism might confer benefit in relation to acute COVID-19. At the same time, our preliminary data show that the degree of the drop in testosterone in statin users is related to the degree of increase in muscle pain and muscle weakness (in data from a double-blind randomized controlled trial [181]), which could have implications for caliber/patency of the upper airway (through effects on upper airway dilator muscles [121]) and strength of respiratory musculature (and potentially cardiac muscle action).
10. Rhabdomyolysis
Rhabdomyolysis is promoted in settings and with exposures and conditions tied to impaired energy supply and/or increased energy demand—particularly, these risk factors in combination [31,182]. Statin-induced rhabdomyolysis (or, more generally, myopathy) is more common with greater statin potency [31] (which is more likely to impair cell energy) [31,163]. Risk is also greater in older age and in those with conditions like hypertension, obesity, and diabetes [31]. These are settings tied to impaired energy supply [49] and are also risk factors for worse outcomes (e.g., hospitalization, death) with COVID-19 [50,183]—settings where off-label adjunctive therapy might be tried.
Additional risk of rhabdomyolysis from statins in COVID-19 patients may emanate from drug interactions [31,184]. Drug interactions may arise with agents used for comorbidities and hospital-related prophylaxis. These may include recognized or yet-to-be-recognized statin interactions with agents used to address COVID-19 or its complications and interactions with antibiotics that may be given for known or suspected coinfections [185].
Moreover, infection is itself a reported risk factor for rhabdomyolysis [186,187]. The associated increase in energy demand [49,188] adds to risk of rhabdomyolysis with statin use [31] in high-risk COVID-19 patients. COVID-19, specifically, has characteristics that further magnify risk. Hypoxemia is common. Moreover, underlying mechanisms encompass oxidative stress (potentiating endothelial/blood flow impairment and mitochondrial impairment) and mitochondrial dysfunction [189] itself. That is, mechanisms implicated in COVID-19 severity parallel those of statin toxicity, explaining why risk factors are so tightly shared and underscoring the potential for statin use to compound risk of rhabdomyolysis in association with COVID-19.
What is the magnitude of threat of rhabdomyolysis in association with COVID-19? Retrospective cohort studies suggest it is unexpectedly high. Reported fractions of hospitalized COVID-19 patients who met criteria for rhabdomyolysis are as high as 16% and 20% [190,191]. (Rhabdomyolysis commonly involves multiple predisposing factors, so other risk factors besides COVID-19 were evident in a number of such cases.) Moreover, mortality of hospitalized COVID-19 patients with rhabdomyolysis may be extreme, with approximately half (47.1%) of such patients dying, in one series [191].
Beyond the hundreds of cases of COVID-19-associated rhabdomyolysis reflected in published retrospective reports of hospitalized COVID-19 patients, more than 100 individually reported cases of COVID-19-associated rhabdomyolysis have been published as case reports (to date). A good number involve concurrent statin use, consistent with ~30% of hospitalized rhabdomyolysis patients in the above retrospective study [191] taking statins prior to admission. Whether adjunctive use of statins for COVID-19 patients once hospitalized was a factor in any is not stated. Given the striking reported risk of rhabdomyolysis in hospitalized COVID-19 patients and the dire mortality prospects in such patients, the injudiciousness of introducing a further rhabdomyolysis risk factor (statins or other) imposes unwarranted risk.
11. Statin Risk–Benefit in Groups at High Risk for Adverse COVID-19 Outcomes
Above, we examined implications of statins related to COVID-19, for high-risk COVID-19 populations. We then focused on muscle implications. We now expand out, for a broader view of statin risk–benefit in patients at risk for adverse COVID-19 outcomes, addressing risk–benefit implications independent of COVID-19. Statins are less favorable—in terms of risk–benefit to patient-centered outcomes that balance serious risk and benefit, like all-cause mortality—in high-risk COVID-19 groups like the elderly (PROSPER) [45], those with hypertension (ALLHAT-LLT) [44], or other metabolic syndrome factors such as low high-density lipoprotein cholesterol (AFCAPS) [43]. The statin trials mentioned targeting these groups showed no trend to benefit to all-cause mortality or, where assessed, to a proxy for all-cause serious morbidity. This failure of statins to improve patient-centered outcomes in these trials of the elderly and of patients with these metabolic syndrome features, despite their elevated coronary disease risk, merits serious consideration in deliberations regarding use of statins in these groups—even in those without COVID-19 infection and, a fortiori, in those with COVID-19. The importance of this is underscored by the fact that, as above, such patients—precisely because of their high COVID-19 risk—may be prioritized for adjunctive statin therapy.
12. Statins and Long-COVID, Also Known as Post-COVID Syndrome
Statin use has been advocated by physicians in training programs regarding management of long-COVID [192]. Long-COVID involves fatigue and fatigue with exertion as perhaps the most prominent reported symptoms (leading, indeed, to comparisons of long-COVID to chronic fatigue syndrome/myalgic encephalomyelitis—and, indeed, some patients meet criteria for this condition) [23,193,194,195]. However, statins were reported—in the only RCT we are aware of that assessed this—to significantly increase fatigue and fatigue with exertion (compared to placebo) [18]. Muscle weakness is also a significant feature of long-COVID [22] that can also be engendered by statins [19,21] through mitochondrial [31,196], oxidative [102], and autoimmune mechanisms [10,11,12,138,139,140,141,142]—mechanisms shared with COVID-19 [114,197,198,199,200,201,202,203]. Long-COVID also commonly involves reported cognitive compromise [194,195]: this is also a reported adverse effect of statins [20,204] and, indeed, is the subject of an FDA label change [205]. Shortness of breath (dyspnea) arises with long-COVID [23] and can be a feature in statin myopathy: both respiratory muscle myopathy [206] and, potentially, simply impaired mitochondrial respiration [207] may be contributors. The two randomized trials of statin use that assessed cognitive function, focusing on non-elderly and pre-trained participants of cognitive testing (each reducing variance and change variance and enhancing statistical power; there are large and highly variable training effects with cognitive tests [208]) showed significant (adverse) cognitive change when on statins relative to the placebo [209,210]. Weakness is also reported as a long-COVID symptom in some and has been found to be worsened when on statins (versus placebo) in key at-risk groups—including those with any baseline weakness (as may be present with long-COVID) who exercise regularly (as may be advocated with long-COVID) and in older women [19]. These findings were replicated across each of two assessed statins compared to the placebo, so are unlikely to represent chance findings.
Statins lead to dose-dependent reductions in CoQ10 [163,211,212], which plays a crucial role in cellular energy production. Both mitochondrial impairment and oxidative stress are considerations in COVID-19 [114]; chronic fatigue has been tied to mitochondrial impairment [213]; oxidative stress and mitochondrial impairment each can arise with statins and are tied to statin adverse effects [31,101,102]). Of note, long-COVID may be disproportionately common in the setting of diabetes: statins can increase risk of diabetes [214,215] and diabetes also increases risk of statin complications [31].
In the post-COVID-19 setting, benefits of lowered testosterone to viral cell entry (Section 8) may lose relevance, while statin-induced testosterone depressions continue to have adverse implications for fatigue [216,217,218], muscle strength [219,220,221], and cognition [222], among other central features of long-COVID. An exception arises for instances in which there is persistence of COVID-19 virus, which is indeed one of four identified patterns of post-COVID syndrome, also known as long-COVID [159,160].
We suggest that statin use (or at least statin initiation targeted to COVID-19 mitigation) should be avoided in long-COVID unless and until RCT data affirming the benefit become available.
13. Summary
In a comprehensive meta-analysis of randomized trials, there is a conspicuous absence of evidence suggesting that statins reduce infection or infection-related mortality [52,53]. This contradicts the apparently favorable outcomes linked to statin use in observational studies, a discrepancy that can be attributed substantially to the phenomena of “healthy-user” and “healthy-tolerator” effects. Similar spurious associations have also been observed in COVID-19 studies.
Many observational studies report a positive correlation between statin use and improved COVID-19 outcomes. However, these findings are uncorroborated by randomized trials. Severe infections are often accompanied by significant cholesterol reduction, thereby reducing the likelihood of receiving adjunctive statins (confounding by indication). This dynamic could compound the potential for misleadingly favorable observational associations between statin use and better COVID-19 outcomes.
Statins are known for their anti-inflammatory effects [2], which could theoretically provide some benefit in the context of COVID-19. However, statins may also exert adverse effects on COVID-19 incidence and outcomes: LDL cholesterol, a central transporter of key fat-soluble nutrients, plays a vital role in viral defense, antioxidation, and energy support. Such considerations may offset statin mechanisms that many have presumed would confer infection benefit.
Groups that are particularly vulnerable to severe COVID-19 outcomes—including the elderly and individuals with obesity, hypertension, and diabetes—are also at heightened risk for adverse effects from statin use. Although statins have known anti-inflammatory effects and confer protection against cardiovascular outcomes in many settings, the absence of infection protection and the potential promotion of cardiac as well as skeletal muscle compromise, which may worsen COVID-19 outcomes [109], create significant concerns in relation to statin use in COVID-19 (where CHF is both a risk factor and an adverse outcome [223]).
Against these considerations, use of statins for COVID-19 infection has been endorsed in position pieces that have achieved broad circulation. (See Supplement SI.) Indeed, a widely circulated MGH document [1] that endorsed statins for treatment of COVID-19 cited observational studies that had shown an apparent association between statin use and better infection outcomes but had not referenced the meta-analysis of randomized trials that showed no suggestion of a causal relationship of statins to better infection outcomes [52,53]. Recommendations should avoid appearing to promote off-label use of a prescription drug class that has prospects for material harm, in the absence of clear overriding benefit in RCTs.
Perhaps most importantly, hospitalized COVID-19 patients have shown high reported rates of rhabdomyolysis, a well-known complication of statin use. Moreover, such patients have had high reported mortality. Given this, the addition of an agent—like statins—that magnifies risk of rhabdomyolysis may be imprudent, particularly in absence of unequivocal RCT evidence countermanding clear prospects for harm.
Regarding statin use in long-COVID, statins have potential to cause core long-COVID symptoms, including fatigue (increased on average, in RCT evidence) [18], as well as muscle [21,31,196] and cognitive [20,204] problems. Mechanisms of toxicity engaged by both statins and the COVID spike protein include oxidative stress and mitochondrial impairment, which may underlie many or most long-COVID symptoms, and some individuals sensitive to these effects from one of the exposures may be sensitive to the effects from both. At present, there is no evidence that the benefits of statins in the long-COVID setting convincingly trump their harms.
14. Conclusions
Many of the very patient characteristics that magnify risk with COVID-19 and might prompt physicians to consider unproven statin therapy also magnify risks with statins. Problematically, COVID-19 may itself increase risks of serious and fatal adverse outcomes from statins, and statins might themselves increase risks of serious and fatal adverse outcomes from COVID-19. This extends to rhabdomyolysis—for which reported risks have been high in hospitalized COVID-19 patients.
Given the evident risks and the absence of clear benefit of statins to COVID-19, we suggest clinicians use caution in initiating statins for COVID-19 or long-COVID, unless/until RCT data clearly support this intervention.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12144659/s1, Figure S1: Top “COVID-19 and statins” Google Search Result and Recommendations in Associated Document; Table S1: Unauthored MGH recommendations dated 3-17-2020. Table from MGH-generated PDF version 1.0 provided by MGH website (lopinavir/ritonavir and Interferon beta-B1 rows were removed later on, in version 2.12); Table S2: Unauthored MGH recommendations dated 4-20-2020. Table from MGH-generated PDF version 2.12 provided by MGH website (favipiravir and sarilumab rows were added in version 2.12). References [1,224,225] are cited in the supplementary materials.
Author Contributions
Conceptualization, B.A.G., A.E.Z.-H. and P.H.L.; methodology, B.A.G.; project administration, B.A.G., J.H.H. and A.E.Z.-H.; resources, B.A.G.; validation, B.A.G., J.H.H. and E.D.; writing—original draft preparation, B.A.G. and A.E.Z.-H.; writing—review and editing, B.A.G., J.H.H., E.D., A.E.Z.-H. and P.H.L.; supervision, B.A.G. and A.E.Z.-H.; funding acquisition, B.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
Some materials used in the preparation of this manuscript were funded by the NIHLBI, NIH grant# RO1 HL63055-05 for Golomb.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Some materials used in the preparation of this manuscript were funded by the NIHLBI, NIH grant# RO1 HL63055-05 for Golomb. We gratefully acknowledge the assistance of Paloma Chavez in producing the mevalonate pathway figure.
Conflicts of Interest
Zemljic-Harpf reports grants from MSD. Golomb has inherited stock in statin manufacturing companies. Langsjoen, Dinkeloo, and Han have no conflict of interest to declare.
References
- Rationale for Consideration of Statins for COVID-19 Patients. Available online: https://www.massgeneral.org/assets/MGH/pdf/news/coronavirus/covid-19_domID_statin.pdf (accessed on 4 October 2020).
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed]
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389. [Google Scholar]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Reiner, Z.; Hatamipour, M.; Banach, M.; Pirro, M.; Al-Rasadi, K.; Jamialahmadi, T.; Radenkovic, D.; Montecucco, F.; Sahebkar, A. Statins and the COVID-19 main protease: In silico evidence on direct interaction. Arch. Med. Sci. 2020, 16, 490–496. [Google Scholar] [CrossRef]
- Dalakas, M.C. Complement in autoimmune inflammatory myopathies, the role of myositis-associated antibodies, COVID-19 associations, and muscle amyloid deposits. Expert Rev. Clin. Immunol. 2022, 18, 413–423. [Google Scholar] [CrossRef]
- Veyseh, M.; Koyoda, S.; Ayesha, B. COVID-19 IgG-related autoimmune inflammatory necrotizing myositis. BMJ Case Rep. 2021, 14, e239457. [Google Scholar] [CrossRef]
- De Marco, G.; Giryes, S.; Williams, K.; Alcorn, N.; Slade, M.; Fitton, J.; Nizam, S.; Smithson, G.; Iqbal, K.; Tran, G.; et al. A Large Cluster of New Onset Autoimmune Myositis in the Yorkshire Region Following SARS-CoV-2 Vaccination. Vaccines 2022, 10, 1184. [Google Scholar] [CrossRef]
- Loghman, M.; Rahmanian, E.; Alikhani, M.; Saffar, H.; Beikmohamadi Hezaveh, S.; Nekooeian, M.; Shahriarirad, R.; Faezi, S.T. Necrotizing autoimmune myositis following coronavirus disease 2019 infection: A case report. J. Med. Case Rep. 2022, 16, 488. [Google Scholar] [CrossRef]
- Nemati, M.; Srai, M.; Rudrangi, R. Statin-Induced Autoimmune Myopathy. Cureus 2021, 13, e13576. [Google Scholar] [CrossRef]
- Treppo, E.; Infantino, M.; Benucci, M.; Ravagnani, V.; Palterer, B.; Fabris, M.; Tomietto, P.; Manfredi, M.; Giudizi, M.G.; Ligobbi, F.; et al. Efficacy and Safety of High-Dose Immunoglobulin-Based Regimen in Statin-Associated Autoimmune Myopathy: A Multi-Center and Multi-Disciplinary Retrospective Study. J. Clin. Med. 2020, 9, 3454. [Google Scholar] [CrossRef]
- Troyanov, Y.; Landon-Cardinal, O.; Fritzler, M.J.; Ferreira, J.; Targoff, I.N.; Rich, E.; Goulet, M.; Goulet, J.R.; Bourre-Tessier, J.; Robitaille, Y.; et al. Atorvastatin-induced necrotizing autoimmune myositis: An emerging dominant entity in patients with autoimmune myositis presenting with a pure polymyositis phenotype. Medicine 2017, 96, e5694. [Google Scholar] [CrossRef]
- Qasim Agha, O.; Kaur, S.; Vijayavel, N. Statin-induced necrotising autoimmune myopathy and autoimmune hepatitis presenting with dysphagia. BMJ Case Rep. 2020, 13, e232391. [Google Scholar] [CrossRef]
- Ajmal, M.; Singh, A.; Kubba, S.; Hershman, M.; Acharya, T. Statin-Induced Triad of Autoimmune Myocarditis, Myositis, and Transaminitis. Case Rep. Cardiol. 2021, 2021, 6660362. [Google Scholar] [CrossRef]
- Frasson, E.; Simonetto, M.; Bertolasi, L.; Caneve, G.; Vilotti, C.; Ruzza, G.; Perelli, A.; Piccinno, M.G.; Monaco, S. Statin-associated necrotizing autoimmune myopathy with concurrent myasthenia gravis. Clin. Case Rep. 2021, 9, e03925. [Google Scholar] [CrossRef]
- Parikh, P.; Onuorah, N.; Vashisht, P. A rare overlap of statin-induced anti-3-hydroxy-3-methyl-glutaryl-coenzyme A necrotizing autoimmune myositis and dermatomyositis. Rheumatol. Adv. Pract. 2021, 5, rkab064. [Google Scholar] [CrossRef]
- Wolff, D.; Nee, S.; Hickey, N.S.; Marschollek, M. Risk factors for Covid-19 severity and fatality: A structured literature review. Infection 2021, 49, 15–28. [Google Scholar] [CrossRef]
- Golomb, B.A.; Evans, M.A.; Dimsdale, J.E.; White, H.L. Effects of statins on energy and fatigue with exertion: Results from a randomized controlled trial. Arch. Intern. Med. 2012, 172, 1180–1182. [Google Scholar] [CrossRef]
- Golomb, B.A.; Koperski, S. Who becomes weak on statins? Effect modification exposed in a RCT by risk factor compounding. Circulation 2013, 127, AP072. [Google Scholar] [CrossRef]
- Evans, M.A.; Golomb, B.A. Statin-associated adverse cognitive effects: Survey results from 171 patients. Pharmacotherapy 2009, 29, 800–811. [Google Scholar] [CrossRef]
- Cham, S.; Evans, M.A.; Denenberg, J.O.; Golomb, B.A. Statin-associated muscle-related adverse effects: A case series of 354 patients. Pharmacotherapy 2010, 30, 541–553. [Google Scholar] [CrossRef]
- Soares, M.N.; Eggelbusch, M.; Naddaf, E.; Gerrits, K.H.L.; van der Schaaf, M.; van den Borst, B.; Wiersinga, W.J.; van Vugt, M.; Weijs, P.J.M.; Murray, A.J.; et al. Skeletal muscle alterations in patients with acute Covid-19 and post-acute sequelae of Covid-19. J. Cachexia Sarcopenia Muscle 2022, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-de-Las-Penas, C.; Palacios-Cena, D.; Gomez-Mayordomo, V.; Palacios-Cena, M.; Rodriguez-Jimenez, J.; de-la-Llave-Rincon, A.I.; Velasco-Arribas, M.; Fuensalida-Novo, S.; Ambite-Quesada, S.; Guijarro, C.; et al. Fatigue and Dyspnoea as Main Persistent Post-COVID-19 Symptoms in Previously Hospitalized Patients: Related Functional Limitations and Disability. Respiration 2022, 101, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Paulose-Ram, R.; Burt, V.L.; Kit, B.K. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. In NCHS Data Brief; United States National Center for Health Statistics: Hyattsville, MD, USA, 2014; pp. 1–8. [Google Scholar]
- Alcazar-Fabra, M.; Trevisson, E.; Brea-Calvo, G. Clinical syndromes associated with Coenzyme Q10 deficiency. Essays Biochem. 2018, 62, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Navas, P.; Cascajo, M.V.; Alcazar-Fabra, M.; Hernandez-Camacho, J.D.; Sanchez-Cuesta, A.; Rodriguez, A.B.C.; Ballesteros-Simarro, M.; Arroyo-Luque, A.; Rodriguez-Aguilera, J.C.; Fernandez-Ayala, D.J.M.; et al. Secondary CoQ10 deficiency, bioenergetics unbalance in disease and aging. Biofactors 2021, 47, 551–569. [Google Scholar] [CrossRef]
- Littarru, G.P.; Tiano, L. Bioenergetic and antioxidant properties of coenzyme Q10: Recent developments. Mol. Biotechnol. 2007, 37, 31–37. [Google Scholar] [CrossRef]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef]
- Sunesen, V.H.; Weber, C.; Holmer, G. Lipophilic antioxidants and polyunsaturated fatty acids in lipoprotein classes: Distribution and interaction. Eur. J. Clin. Nutr. 2001, 55, 115–123. [Google Scholar] [CrossRef]
- Barbiroli, B.; Frassineti, C.; Martinelli, P.; Iotti, S.; Lodi, R.; Cortelli, P.; Montagna, P. Coenzyme Q10 improves mitochondrial respiration in patients with mitochondrial cytopathies. An in vivo study on brain and skeletal muscle by phosphorous magnetic resonance spectroscopy. Cell Mol. Biol. 1997, 43, 741–749. [Google Scholar]
- Golomb, B.A.; Evans, M.A. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs 2008, 8, 373–418. [Google Scholar] [CrossRef]
- Wei, Y.H. Oxidative stress and mitochondrial DNA mutations in human aging. Proc. Soc. Exp. Biol. Med. 1998, 217, 53–63. [Google Scholar] [CrossRef]
- Lee, H.C.; Wei, Y.H. Role of Mitochondria in Human Aging. J. Biomed. Sci. 1997, 4, 319–326. [Google Scholar] [CrossRef]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Tavana, E.; Fanni, G.; Bo, S.; Banach, M.; Pirro, M.; von Haehling, S.; Jamialahmadi, T.; Sahebkar, A. Effects of statins on mitochondrial pathways. J. Cachexia Sarcopenia Muscle 2021, 12, 237–251. [Google Scholar] [CrossRef]
- Haendeler, J.; Hoffmann, J.; Zeiher, A.M.; Dimmeler, S. Antioxidant effects of statins via S-nitrosylation and activation of thioredoxin in endothelial cells: A novel vasculoprotective function of statins. Circulation 2004, 110, 856–861. [Google Scholar] [CrossRef]
- Graham, D.; Huynh, N.N.; Hamilton, C.A.; Beattie, E.; Smith, R.A.; Cocheme, H.M.; Murphy, M.P.; Dominiczak, A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009, 54, 322–328. [Google Scholar] [CrossRef]
- Alleva, R.; Tomasetti, M.; Andera, L.; Gellert, N.; Borghi, B.; Weber, C.; Murphy, M.P.; Neuzil, J. Coenzyme Q blocks biochemical but not receptor-mediated apoptosis by increasing mitochondrial antioxidant protection. FEBS Lett. 2001, 503, 46–50. [Google Scholar] [CrossRef]
- Dave, M.; Attur, M.; Palmer, G.; Al-Mussawir, H.E.; Kennish, L.; Patel, J.; Abramson, S.B. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008, 58, 2786–2797. [Google Scholar] [CrossRef]
- Reutelingsperger, C.P.; van Heerde, W.L. Annexin V, the regulator of phosphatidylserine-catalyzed inflammation and coagulation during apoptosis. Cell. Mol. Life Sci. 1997, 53, 527–532. [Google Scholar] [CrossRef]
- Carlino, M.V.; Valenti, N.; Cesaro, F.; Costanzo, A.; Cristiano, G.; Guarino, M.; Sforza, A. Predictors of Intensive Care Unit admission in patients with coronavirus disease 2019 (COVID-19). Monaldi. Arch. Chest Dis. 2020, 90, 1410. [Google Scholar] [CrossRef]
- Milenkovic, M.; Hadzibegovic, A.; Kovac, M.; Jovanovic, B.; Stanisavljevic, J.; Djikic, M.; Sijan, D.; Ladjevic, N.; Palibrk, I.; Djukanovic, M.; et al. D-dimer, CRP, PCT, and IL-6 Levels at Admission to ICU Can Predict In-Hospital Mortality in Patients with COVID-19 Pneumonia. Oxid. Med. Cell. Longev. 2022, 2022, 8997709. [Google Scholar] [CrossRef]
- Downs, J.R.; Clearfield, M.; Weis, S.; Whitney, E.; Shapiro, D.R.; Beere, P.A.; Langendorfer, A.; Stein, E.A.; Kruyer, W.; Gotto, A.M., Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. J. Am. Med. Assoc. 1998, 279, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). J. Am. Med. Assoc. 2002, 288, 2998–3007. [CrossRef] [PubMed]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Rismanbaf, A.; Zarei, S. Liver and Kidney Injuries in COVID-19 and Their Effects on Drug Therapy. Arch. Acad. Emerg. Med. 2020, 8, e17. [Google Scholar]
- Golomb, B.A. The starving cell: Metabolic syndrome as an adaptive process. Nat. Preced. 2011. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.F.; Chernyak, Y.; Tobin, K.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv 2020, 369, m1966. [Google Scholar] [CrossRef]
- Janda, S.; Young, A.; Fitzgerald, J.M.; Etminan, M.; Swiston, J. The effect of statins on mortality from severe infections and sepsis: A systematic review and meta-analysis. J. Crit. Care 2010, 25, 656.e7–656.e22. [Google Scholar] [CrossRef]
- van den Hoek, H.L.; Bos, W.J.W.; de Boer, A.; van de Garde, E.M.W. Statins and prevention of infections: Systematic review and meta-analysis of data from large randomised placebo controlled trials. BMJ 2011, 343, 10. [Google Scholar] [CrossRef]
- Golomb, B.A. Do statins reduce the risk of infection? BMJ 2011, 343, d7134. [Google Scholar] [CrossRef]
- Daniels, L.B.; Sitapati, A.M.; Zhang, J.; Zou, J.; Bui, Q.M.; Ren, J.; Longhurst, C.A.; Criqui, M.H.; Messer, K. Relation of Statin Use Prior to Admission to Severity and Recovery Among COVID-19 Inpatients. Am. J. Cardiol. 2020, 136, 149–155. [Google Scholar] [CrossRef]
- Zhang, X.J.; Qin, J.J.; Cheng, X.; Shen, L.; Zhao, Y.C.; Yuan, Y.; Lei, F.; Chen, M.M.; Yang, H.; Bai, L.; et al. In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19. Cell Metab. 2020, 32, 176–187.e174. [Google Scholar] [CrossRef]
- Israel, A.; Schaffer, A.; Cicurel, A.; Feldhamer, I.; Tal, A.; Cheng, K.; Sinha, S.; Schiff, E.; Lavie, G.; Ruppin, E. Large population study identifies drugs associated with reduced COVID-19 severity. medRxiv 2020. [Google Scholar] [CrossRef]
- Vahedian-Azimi, A.; Mohammadi, S.M.; Heidari Beni, F.; Banach, M.; Guest, P.C.; Jamialahmadi, T.; Sahebkar, A. Improved COVID-19 ICU admission and mortality outcomes following treatment with statins: A systematic review and meta-analysis. Arch. Med. Sci. 2021, 17, 579–595. [Google Scholar] [CrossRef]
- Wu, C.C.; Lee, A.J.; Su, C.H.; Huang, C.Y.; Islam, M.M.; Weng, Y.C. Statin Use Is Associated with a Decreased Risk of Mortality among Patients with COVID-19. J. Clin. Med. 2021, 10, 1450. [Google Scholar] [CrossRef]
- Vahedian-Azimi, A.; Mohammadi, S.M.; Banach, M.; Beni, F.H.; Guest, P.C.; Al-Rasadi, K.; Jamialahmadi, T.; Sahebkar, A. Improved COVID-19 Outcomes following Statin Therapy: An Updated Systematic Review and Meta-analysis. Biomed. Res. Int. 2021, 2021, 1901772. [Google Scholar] [CrossRef]
- Kow, C.S.; Hasan, S.S. Meta-analysis of Effect of Statins in Patients with COVID-19. Am. J. Cardiol. 2020, 134, 153–155. [Google Scholar] [CrossRef]
- Kouhpeikar, H.; Khosaravizade Tabasi, H.; Khazir, Z.; Naghipour, A.; Mohammadi Moghadam, H.; Forouzanfar, H.; Abbasifard, M.; Kirichenko, T.V.; Reiner, Z.; Banach, M.; et al. Statin Use in COVID-19 Hospitalized Patients and Outcomes: A Retrospective Study. Front. Cardiovasc. Med. 2022, 9, 820260. [Google Scholar] [CrossRef]
- Memel, Z.N.; Lee, J.J.; Foulkes, A.S.; Chung, R.T.; Thaweethai, T.; Bloom, P.P. Association of Statins and 28-Day Mortality Rates in Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2022, 225, 19–29. [Google Scholar] [CrossRef]
- Feingold, K.R.; Brinton, E.A.; Grunfeld, C. The Effect of Endocrine Disorders on Lipids and Lipoproteins. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Banerjee, M.; Yadav, U.; Bhattacharjee, S. Statin use and clinical outcomes in patients with COVID-19: An updated systematic review and meta-analysis. Postgrad. Med. J. 2021, 98, 354–359. [Google Scholar] [CrossRef] [PubMed]
- De Spiegeleer, A.; Bronselaer, A.; Teo, J.T.; Byttebier, G.; De Tre, G.; Belmans, L.; Dobson, R.; Wynendaele, E.; Van De Wiele, C.; Vandaele, F.; et al. The Effects of ARBs, ACEis, and Statins on Clinical Outcomes of COVID-19 Infection Among Nursing Home Residents. J. Am. Med. Dir. Assoc. 2020, 21, 909–914.e902. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Kurniawan, A. Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab. Syndr. 2020, 14, 1613–1615. [Google Scholar] [CrossRef]
- Ayeh, S.K.; Abbey, E.J.; Khalifa, B.A.A.; Nudotor, R.D.; Osei, A.D.; Chidambaram, V.; Osuji, N.; Khan, S.; Salia, E.L.; Oduwole, M.O.; et al. Statins use and COVID-19 outcomes in hospitalized patients. PLoS ONE 2021, 16, e0256899. [Google Scholar] [CrossRef]
- Cariou, B.; Goronflot, T.; Rimbert, A.; Boullu, S.; Le May, C.; Moulin, P.; Pichelin, M.; Potier, L.; Smati, S.; Sultan, A.; et al. Routine use of statins and increased COVID-19 related mortality in inpatients with type 2 diabetes: Results from the CORONADO study. Diabetes Metab. 2021, 47, 101202. [Google Scholar] [CrossRef]
- Rogers, A.J.; Guan, J.; Trtchounian, A.; Hunninghake, G.M.; Kaimal, R.; Desai, M.; Kozikowski, L.A.; DeSouza, L.; Mogan, S.; Liu, K.D.; et al. Association of Elevated Plasma Interleukin-18 Level With Increased Mortality in a Clinical Trial of Statin Treatment for Acute Respiratory Distress Syndrome. Crit. Care Med. 2019, 47, 1089–1096. [Google Scholar] [CrossRef]
- Xu, J.F.; Washko, G.R.; Nakahira, K.; Hatabu, H.; Patel, A.S.; Fernandez, I.E.; Nishino, M.; Okajima, Y.; Yamashiro, T.; Ross, J.C.; et al. Statins and pulmonary fibrosis: The potential role of NLRP3 inflammasome activation. Am. J. Respir. Crit. Care Med. 2012, 185, 547–556. [Google Scholar] [CrossRef]
- Ghafoori, M.; Saadati, H.; Taghavi, M.; Azimian, A.; Alesheikh, P.; Mohajerzadeh, M.S.; Behnamfar, M.; Pakzad, M.; Rameshrad, M. Survival of the hospitalized patients with COVID-19 receiving atorvastatin: A randomized clinical trial. J. Med. Virol. 2022, 94, 3160–3168. [Google Scholar] [CrossRef]
- Investigators, I.-S. Atorvastatin versus placebo in patients with COVID-19 in intensive care: Randomized controlled trial. BMJ 2022, 376, e068407. [Google Scholar] [CrossRef]
- Davoodi, L.; Jafarpour, H.; Oladi, Z.; Zakariaei, Z.; Tabarestani, M.; Ahmadi, B.M.; Razavi, A.; Hessami, A. Atorvastatin therapy in COVID-19 adult inpatients: A double-blind, randomized controlled trial. Int. J. Cardiol. Heart Vasc. 2021, 36, 100875. [Google Scholar] [CrossRef]
- Ghati, N.; Bhatnagar, S.; Mahendran, M.; Thakur, A.; Prasad, K.; Kumar, D.; Dwivedi, T.; Mani, K.; Tiwari, P.; Gupta, R.; et al. Statin and aspirin as adjuvant therapy in hospitalised patients with SARS-CoV-2 infection: A randomised clinical trial (RESIST trial). BMC Infect. Dis. 2022, 22, 606. [Google Scholar] [CrossRef]
- Gaitan-Duarte, H.G.; Alvarez-Moreno, C.; Rincon-Rodriguez, C.J.; Yomayusa-Gonzalez, N.; Cortes, J.A.; Villar, J.C.; Bravo-Ojeda, J.S.; Garcia-Pena, A.; Adarme-Jaimes, W.; Rodriguez-Romero, V.A.; et al. Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: A pragmatic, open-label randomized trial. EClinicalMedicine 2022, 43, 101242. [Google Scholar] [CrossRef]
- Xavier, D.P.; Chagas, G.C.L.; Gomes, L.G.F.; Ferri-Guerra, J.; Oquet, R.E.H. Effects of statin therapy in hospitalized adult COVID-19 patients: A systematic review and meta-analysis of randomized controlled trials. Einstein 2023, 21, eRW0351. [Google Scholar] [CrossRef]
- Marzolini, C.; Kuritzkes, D.R.; Marra, F.; Boyle, A.; Gibbons, S.; Flexner, C.; Pozniak, A.; Boffito, M.; Waters, L.; Burger, D.; et al. Recommendations for the Management of Drug-Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Paxlovid) and Comedications. Clin. Pharmacol. Ther. 2022, 112, 1191–1200. [Google Scholar] [CrossRef]
- Iribarren, C.; Dwyer, J. Serum total cholesterol, stroke and all-cause mortality: Lessons from the Honolulu heart program. Nutr. Metab. Cardiovasc. Dis. 1997, 7, 169–174. [Google Scholar]
- Jacobs, D.; Blackburn, H.; Higgins, M.; Reed, D.; Iso, H.; McMillan, G.; Neaton, J.; Nelson, J.; Potter, J.; Rifkind, B.; et al. Report of the Conference on Low Blood Cholesterol: Mortality Associations. Circulation 1992, 86, 1046–1060. [Google Scholar] [CrossRef]
- Neaton, J.D.; Wentworth, D.N. Low serum cholesterol and risk of death from AIDS. AIDS 1997, 11, 929–930. [Google Scholar]
- Hu, X.; Chen, D.; Wu, L.; He, G.; Ye, W. Low Serum Cholesterol Level among Patients with COVID-19 Infection in Wenzhou, China. Lancet 2020. [Google Scholar] [CrossRef]
- Takahashi, A.; Masuda, A.; Sun, M.; Centonze, V.E.; Herman, B. Oxidative stress-induced apoptosis is associated with alterations in mitochondrial caspase activity and Bcl-2-dependent alterations in mitochondrial pH (pHm). Brain Res. Bull 2004, 62, 497–504. [Google Scholar] [CrossRef]
- Chidambaram, V.; Shanmugavel Geetha, H.; Kumar, A.; Majella, M.G.; Sivakumar, R.K.; Voruganti, D.; Mehta, J.L.; Karakousis, P.C. Association of Lipid Levels With COVID-19 Infection, Disease Severity and Mortality: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 862999. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Hussien, N.R.; Al-Niemi, M.S.; Fahad, E.H.; Al-Buhadily, A.K.; Al-Gareeb, A.I.; Al-Hamash, S.M.; Tsagkaris, C.; Papadakis, M.; Alexiou, A.; et al. SARS-CoV-2 induced HDL dysfunction may affect the host’s response to and recovery from COVID-19. Immun. Inflamm. Dis. 2023, 11, e861. [Google Scholar] [CrossRef] [PubMed]
- Correa, Y.; Del Giudice, R.; Waldie, S.; Thepaut, M.; Micciula, S.; Gerelli, Y.; Moulin, M.; Delaunay, C.; Fieschi, F.; Pichler, H.; et al. High-Density Lipoprotein function is modulated by the SARS-CoV-2 spike protein in a lipid-type dependent manner. J. Colloid. Interface Sci. 2023, 645, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Parra, S.; Saballs, M.; DiNubile, M.; Feliu, M.; Iftimie, S.; Revuelta, L.; Pavon, R.; Avila, A.; Levinson, S.; Castro, A. Low HDL-c levels at admission are associated with greater severity and worse clinical outcomes in patients with COVID-19 disease. Atheroscler. Plus 2023, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Grunfeld, C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. 2015. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326741/ (accessed on 7 July 2023).
- Bjornson, L.K.; Kayden, H.J.; Miller, E.; Moshell, A.N. The transport of alpha-tocopherol and beta-carotene in human blood. J. Lipid Res. 1976, 17, 343–352. [Google Scholar] [CrossRef]
- Gonzalez, M.J. Serum concentrations and cellular uptake of vitamin E. Med. Hypotheses 1990, 32, 107–110. [Google Scholar] [CrossRef]
- Johansen, K.; Theorell, H.; Karlsson, J.; Diamant, B.; Folkers, K. Coenzyme Q10, alpha-tocopherol and free cholesterol in HDL and LDL fractions. Ann. Med. 1991, 23, 649–656. [Google Scholar] [CrossRef]
- Kokoglu, E.; Ulakoglu, E. The transport of vitamin E in plasma and its correlation to plasma lipoproteins in non-insulin-dependent diabetes mellitus. Diabetes Res. Clin. Pract. 1991, 14, 175–181. [Google Scholar] [CrossRef]
- Littarru, G.P.; Langsjoen, P. Coenzyme Q(10) and statins: Biochemical and clinical implications. Mitochondrion 2007, 7 (Suppl. 1), S168–S174. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, J.R.; Lee, I.C.; Kwon, H.J. Native High-Density Lipoproteins (HDL) with Higher Paraoxonase Exerts a Potent Antiviral Effect against SARS-CoV-2 (COVID-19), While Glycated HDL Lost the Antiviral Activity. Antioxidants 2021, 10, 209. [Google Scholar] [CrossRef]
- Blatter Garin, M.C.; Moren, X.; James, R.W. Paraoxonase-1 and serum concentrations of HDL-cholesterol and apoA-I. J. Lipid Res. 2006, 47, 515–520. [Google Scholar] [CrossRef]
- Jaouad, L.; Milochevitch, C.; Khalil, A. PON1 paraoxonase activity is reduced during HDL oxidation and is an indicator of HDL antioxidant capacity. Free Radic Res. 2003, 37, 77–83. [Google Scholar] [CrossRef]
- Koren-Gluzer, M.; Aviram, M.; Meilin, E.; Hayek, T. The antioxidant HDL-associated paraoxonase-1 (PON1) attenuates diabetes development and stimulates beta-cell insulin release. Atherosclerosis 2011, 219, 510–518. [Google Scholar] [CrossRef]
- Kuhar, M.B. Update on managing hypercholesterolemia. The new NCEP guidelines. AAOHN J. 2002, 50, 360–364. [Google Scholar] [CrossRef]
- Hasvold, P.; Thuresson, M.; Sundström, J.; Hammar, N.; Kjeldsen, S.E.; Johansson, G.; Holme, I.; Bodegård, J. Association Between Paradoxical HDL Cholesterol Decrease and Risk of Major Adverse Cardiovascular Events in Patients Initiated on Statin Treatment in a Primary Care Setting. Clin. Drug Investig. 2016, 36, 225–233. [Google Scholar] [CrossRef]
- Guirgis, F.W.; Donnelly, J.P.; Dodani, S.; Howard, G.; Safford, M.M.; Levitan, E.B.; Wang, H.E. Cholesterol levels and long-term rates of community-acquired sepsis. Crit. Care 2016, 20, 408. [Google Scholar] [CrossRef]
- Sinzinger, H.; Chehne, F.; Lupattelli, G. Oxidation Injury in Patients Receiving HMG-CoA Reductase Inhibitors: Occurrence in Patients Without Enzyme Elevation or Myopathy. Drug Saf. 2002, 25, 877–883. [Google Scholar] [CrossRef]
- Sinzinger, H.; Lupattelli, G.; Chehne, F.; Oguogho, A.; Furberg, C.D. Isoprostane 8-epi-PGF2alpha is frequently increased in patients with muscle pain and/or CK-elevation after HMG-Co-enzyme-A-reductase inhibitor therapy. J. Clin. Pharm. Ther. 2001, 26, 303–310. [Google Scholar] [CrossRef]
- Schalke, B.B.; Schmidt, B.; Toyka, K.; Hartung, H.P. Pravastatin-associated inflammatory myopathy. N. Engl. J. Med. 1992, 327, 649–650. [Google Scholar]
- Kuncova, K.; Sedlackova, M.; Vencovsky, J.; Mann, H.; Tomcik, M.; Wenchich, L.; Zamecnik, J. Inflammatory myopathy associated with statins: Report of three cases. Mod. Rheumatol. 2014, 24, 366–371. [Google Scholar] [CrossRef]
- Hoffman, K.B.; Kraus, C.; Dimbil, M.; Golomb, B.A. A Survey of the FDA’s AERS Database Regarding Muscle and Tendon Adverse Events Linked to the Statin Drug Class. PLoS ONE 2012, 7, e42866. [Google Scholar] [CrossRef] [PubMed]
- Honarmand, A.; Sheybani, F.; Aflatoonian, E.; Saberinia, A. COVID-19 patients at referral to hospital during the first peak of disease: Common clinical findings including myalgia and fatigue. Eur. J. Transl. Myol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kanda, T. Peripheral Neuropathy and Myopathy Associated with COVID-19. Brain Nerve 2022, 74, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Pierucci, F.; Garcia-Gil, M. Skeletal Muscle and COVID-19: The Potential Involvement of Bioactive Sphingolipids. Biomedicines 2022, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Attaway, A.; Welch, N.; Dasarathy, D.; Amaya-Hughley, J.; Bellar, A.; Biehl, M.; Dugar, S.; Engelen, M.; Zein, J.; Dasarathy, S. Acute skeletal muscle loss in SARS-CoV-2 infection contributes to poor clinical outcomes in COVID-19 patients. J. Cachexia Sarcopenia Muscle 2022, 13, 2436–2446. [Google Scholar] [CrossRef]
- Filippetti, L.; Pace, N.; Louis, J.S.; Mandry, D.; Goehringer, F.; Rocher, M.S.; Jay, N.; Selton-Suty, C.; Hossu, G.; Huttin, O.; et al. Long-Lasting Myocardial and Skeletal Muscle Damage Evidenced by Serial CMR During the First Year in COVID-19 Patients From the First Wave. Front. Cardiovasc. Med. 2022, 9, 831580. [Google Scholar] [CrossRef]
- Bouitbir, J.; Sanvee, G.M.; Panajatovic, M.V.; Singh, F.; Krahenbuhl, S. Mechanisms of statin-associated skeletal muscle-associated symptoms. Pharmacol. Res. 2020, 154, 104201. [Google Scholar] [CrossRef]
- Cros, D.; Palliyath, S.; DiMauro, S.; Ramirez, C.; Shamsnia, M.; Wizer, B. Respiratory failure revealing mitochondrial myopathy in adults. Chest 1992, 101, 824–828. [Google Scholar] [CrossRef]
- Arena, I.G.; Pugliese, A.; Volta, S.; Toscano, A.; Musumeci, O. Molecular Genetics Overview of Primary Mitochondrial Myopathies. J. Clin. Med. 2022, 11, 632. [Google Scholar] [CrossRef]
- Sumbalova, Z.; Kucharska, J.; Palacka, P.; Rausova, Z.; Langsjoen, P.H.; Langsjoen, A.M.; Gvozdjakova, A. Platelet mitochondrial function and endogenous coenzyme Q10 levels are reduced in patients after COVID-19. Bratisl. Lek. Listy 2022, 123, 9–15. [Google Scholar] [CrossRef]
- Langsjoen, P.H.; Langsjoen, J.O.; Langsjoen, A.M.; Rosenfeldt, F. Statin-Associated Cardiomyopathy Responds to Statin Withdrawal and Administration of Coenzyme Q10. Perm. J. 2019, 23, 257. [Google Scholar] [CrossRef]
- Silver, M.A.; Langsjoen, P.H.; Szabo, S.; Patil, H.; Zelinger, A. Effect of atorvastatin on left ventricular diastolic function and ability of coenzyme Q10 to reverse that dysfunction. Am. J. Cardiol. 2004, 94, 1306–1310. [Google Scholar] [CrossRef]
- Fried, J.A.; Ramasubbu, K.; Bhatt, R.; Topkara, V.K.; Clerkin, K.J.; Horn, E.; Rabbani, L.; Brodie, D.; Jain, S.S.; Kirtane, A.; et al. The Variety of Cardiovascular Presentations of COVID-19. Circulation 2020, 141, 1930–1936. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Cerveri, I.; Fanfulla, F.; Zoia, M.C.; Manni, R.; Tartara, A. Sleep disorders in neuromuscular diseases. Monaldi. Arch. Chest. Dis. 1993, 48, 318–321. [Google Scholar]
- Cham, S.; Gill, K.; Koperski, S.; Golomb, B.A. Improvement in sleep apnoea associated with switch from simvastatin to pravastatin. BMJ Case Rep. 2009, 2009, bcr05.2009.1875. [Google Scholar] [CrossRef]
- Corda, L.; Redolfi, S.; Montemurro, L.T.; La Piana, G.E.; Bertella, E.; Tantucci, C. Short- and long-term effects of CPAP on upper airway anatomy and collapsibility in OSAH. Sleep Breath 2009, 13, 187–193. [Google Scholar] [CrossRef]
- O-Brien, R. Doctors are Improvising Coronavirus Treatments, Then Quickly Sharing Them. Wall Street Journal, 9 April 2020. [Google Scholar]
- Elharrar, X.; Youssef, T.; Martinez, M.; Maulin, L. Prone Positioning in Spontaneously Breathing Nonintubated COVID-19 Patient: A Pilot Study (ProCov). Available online: https://clinicaltrials.gov/ct2/show/NCT04344106 (accessed on 5 May 2020).
- Bansal, M. Cardiovascular disease and COVID-19. Diabetes Metab. Syndr. 2020, 14, 247–250. [Google Scholar] [CrossRef]
- Lavie, L. Oxidative stress inflammation and endothelial dysfunction in obstructive sleep apnea. Front. Biosci. 2012, 4, 1391–1403. [Google Scholar] [CrossRef]
- Franco, C.M.; Lima, A.M.; Ataide, L., Jr.; Lins, O.G.; Castro, C.M.; Bezerra, A.A.; de Oliveira, M.F.; Oliveira, J.R. Obstructive Sleep Apnea Severity Correlates with Cellular and Plasma Oxidative Stress Parameters and Affective Symptoms. J. Mol. Neurosci. 2012, 47, 300–310. [Google Scholar] [CrossRef]
- Murri, M.; Garcia-Delgado, R.; Alcazar-Ramirez, J.; Fernandez de Rota, L.; Fernandez-Ramos, A.; Cardona, F.; Tinahones, F.J. Continuous positive airway pressure therapy reduces oxidative stress markers and blood pressure in sleep apnea-hypopnea syndrome patients. Biol. Trace Elem. Res. 2011, 143, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.; Alcazar-Ramirez, J.; Garrido-Sanchez, L.; Linde, F.; Alcaide, J.; Cardona, F.; Tinahones, F.J. Oxidative stress and metabolic changes after continuous positive airway pressure treatment according to previous metabolic disorders in sleep apnea-hypopnea syndrome patients. Transl. Res. 2009, 154, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Montiel, V.; Lobysheva, I.; Gerard, L.; Vermeersch, M.; Perez-Morga, D.; Castelein, T.; Mesland, J.B.; Hantson, P.; Collienne, C.; Gruson, D.; et al. Oxidative stress-induced endothelial dysfunction and decreased vascular nitric oxide in COVID-19 patients. EBioMedicine 2022, 77, 103893. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.R.S.; Heimfarth, L.; Monteiro, B.S.; Correa, C.B.; Moura, T.R.; Araujo, A.A.S.; Martins-Filho, P.R.; Quintans-Junior, L.J.; Quintans, J.S.S. Oxidative stress and inflammatory markers in patients with COVID-19: Potential role of RAGE, HMGB1, GFAP and COX-2 in disease severity. Int. Immunopharmacol. 2022, 104, 108502. [Google Scholar] [CrossRef]
- Seyhanli, E.S.; Koyuncu, I.; Yasak, I.H.; Demir, H.A.; Temiz, E. Asprosin and Oxidative Stress Level in COVID-19 Patients. Clin. Lab. 2022, 68, 35–42. [Google Scholar] [CrossRef]
- Skesters, A.; Kustovs, D.; Lece, A.; Moreino, E.; Petrosina, E.; Rainsford, K.D. Selenium, selenoprotein P, and oxidative stress levels in SARS-CoV-2 patients during illness and recovery. Inflammopharmacology 2022, 30, 499–503. [Google Scholar] [CrossRef]
- van Eijk, L.E.; Tami, A.; Hillebrands, J.L.; den Dunnen, W.F.A.; de Borst, M.H.; van der Voort, P.H.J.; Bulthuis, M.L.C.; Veloo, A.C.M.; Wold, K.I.; Vincenti Gonzalez, M.F.; et al. Mild Coronavirus Disease 2019 (COVID-19) Is Marked by Systemic Oxidative Stress: A Pilot Study. Antioxidants 2021, 10, 2022. [Google Scholar] [CrossRef]
- Liu, N.; Long, H.; Sun, J.; Li, H.; He, Y.; Wang, Q.; Pan, K.; Tong, Y.; Wang, B.; Wu, Q.; et al. New laboratory evidence for the association between endothelial dysfunction and COVID-19 disease progression. J. Med. Virol. 2022, 94, 3112–3120. [Google Scholar] [CrossRef]
- Tessema, B.; Sack, U.; Konig, B.; Serebrovska, Z.; Egorov, E. Effects of Intermittent Hypoxia in Training Regimes and in Obstructive Sleep Apnea on Aging Biomarkers and Age-Related Diseases: A Systematic Review. Front. Aging Neurosci. 2022, 14, 878278. [Google Scholar] [CrossRef]
- Zhou, X.; Zhai, X.; Ashraf, M. Direct evidence that initial oxidative stress triggered by preconditioning contributes to second window of protection by endogenous antioxidant enzyme in myocytes. Circulation 1996, 93, 1177–1184. [Google Scholar] [CrossRef]
- Akki, R.; Fath, N.; Mohti, H. COVID-19: Oxidative Preconditioning as a Potential Therapeutic Approach. ACS Chem. Neurosci. 2020, 11, 3732–3740. [Google Scholar] [CrossRef]
- Albayda, J.; Mammen, A.L. Is statin-induced myositis part of the polymyositis disease spectrum? Curr. Rheumatol. Rep. 2014, 16, 433. [Google Scholar] [CrossRef]
- Kawasaki, E.; Fukuyama, T.; Kuriyama, E.; Uchida, A.; Sagara, Y.; Tamai, H.; Nakano, Y.; Tojikubo, M.; Koga, N. Statin-induced autoimmune hepatitis in patients with type 1 diabetes: A report of two cases and literature review. J. Diabetes Investig. 2020, 11, 1673–1676. [Google Scholar] [CrossRef]
- Shah, J.; Lingiah, V.; Pyrsopoulos, N.; Galan, M. Acute Liver Injury in a Patient Treated with Rosuvastatin: A Rare Adverse Effect. Gastroenterol. Res. 2019, 12, 263–266. [Google Scholar] [CrossRef]
- Sanchez, M.; Castiella, A.; Zapata, E.; Zubiaurre, L.; Perez-Yeboles, J.; Mendibil, L.; Iribarren, A. Autoimmune Hepatitis (Immune-Mediated Liver Injury) Induced By Rosuvastatin. Gastroenterol. Hepatol. 2018, 41, 311–313. [Google Scholar] [CrossRef]
- Clarke, A.T.; Johnson, P.C.; Hall, G.C.; Ford, I.; Mills, P.R. High Dose Atorvastatin Associated with Increased Risk of Significant Hepatotoxicity in Comparison to Simvastatin in UK GPRD Cohort. PLoS ONE 2016, 11, e0151587. [Google Scholar] [CrossRef]
- de Jong, H.J.; Tervaert, J.W.; Saldi, S.R.; Vandebriel, R.J.; Souverein, P.C.; Meyboom, R.H.; van Loveren, H.; Klungel, O.H. Association between statin use and lupus-like syndrome using spontaneous reports. Semin. Arthritis Rheum. 2011, 41, 373–381. [Google Scholar] [CrossRef]
- Niklas, K.; Niklas, A.; Puszczewicz, M.; Wolska-Bulach, A.; Tykarski, A. Polymyositis induced by atorvastatin. Kardiol. Pol. 2015, 73, 1336. [Google Scholar] [CrossRef]
- Kanth, R.; Shah, M.S.; Flores, R.M. Statin-associated polymyositis following omeprazole treatment. Clin. Med. Res. 2013, 11, 91–95. [Google Scholar] [CrossRef]
- Gras-Champel, V.; Masmoudi, I.; Batteux, B.; Merle, P.E.; Liabeuf, S.; Masmoudi, K. Statin-associated myasthenia: A case report and literature review. Therapie 2020, 75, 301–309. [Google Scholar] [CrossRef]
- Gale, J.; Danesh-Meyer, H.V. Statins can induce myasthenia gravis. J. Clin. Neurosci. 2014, 21, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Visconti, M.J.; Bashyam, A.M.; Jorizzo, J.L. Statin-induced dermatomyositis for the practicing dermatologist: A review of the literature. Int. J. Dermatol. 2020, 59, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Tutal, E.; Ozaras, R.; Leblebicioglu, H. Systematic review of COVID-19 and autoimmune thyroiditis. Travel Med. Infect. Dis. 2022, 47, 102314. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Caricchio, R.; Casanova, J.L.; Combes, A.J.; Diamond, B.; Fox, S.E.; Hanauer, D.A.; James, J.A.; Kanthi, Y.; Ladd, V.; et al. The intersection of COVID-19 and autoimmunity. J. Clin. Investig. 2021, 131, e154886. [Google Scholar] [CrossRef] [PubMed]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef]
- Gupta, M.; Weaver, D.F. COVID-19 as a Trigger of Brain Autoimmunity. ACS Chem. Neurosci. 2021, 12, 2558–2561. [Google Scholar] [CrossRef]
- Kabacam, G.; Wahlin, S.; Efe, C. Autoimmune hepatitis triggered by COVID-19: A report of two cases. Liver Int. 2021, 41, 2527–2528. [Google Scholar] [CrossRef]
- Monton Rodriguez, C.; Navarro Cortes, P.; Lluch Garcia, P.; Minguez Perez, M. Autoimmune hepatitis triggered by COVID-19. Rev. Esp. Enferm. Dig. 2022, 114, 64–65. [Google Scholar] [CrossRef]
- Dalakas, M.C. Guillain-Barre syndrome: The first documented COVID-19-triggered autoimmune neurologic disease: More to come with myositis in the offing. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e781. [Google Scholar] [CrossRef]
- Rojas, M.; Rodriguez, Y.; Acosta-Ampudia, Y.; Monsalve, D.M.; Zhu, C.; Li, Q.Z.; Ramirez-Santana, C.; Anaya, J.M. Autoimmunity is a hallmark of post-COVID syndrome. J. Transl. Med. 2022, 20, 129. [Google Scholar] [CrossRef]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rojas, M.; Rodriguez, Y.; Zapata, E.; Ramirez-Santana, C.; Anaya, J.M. Persistent Autoimmune Activation and Proinflammatory State in Post-COVID Syndrome. J. Infect. Dis. 2022, 225, 2155–2162. [Google Scholar] [CrossRef]
- Steinestel, K.; Czech, A.; Hackenbroch, C.; Bloch, W.; Gagiannis, D. Clinical, radiological, and histopathological features of pulmonary post-COVID syndrome: A form of autoimmune-mediated interstitial lung disease? Pathologe 2021, 42, 160–164. [Google Scholar] [CrossRef]
- Blagova, O.V.; Kogan, E.A.; Lutokhina, Y.A.; Kukleva, A.D.; Ainetdinova, D.H.; Novosadov, V.M.; Rud, R.S.; Zaitsev, A.Y.; Zaidenov, V.A.; Kupriyanova, A.G.; et al. Subacute and chronic post-covid myoendocarditis: Clinical presentation, role of coronavirus persistence and autoimmune mechanisms. Kardiologiia 2021, 61, 11–27. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Page, E.; McCallister, L.P. Quantitative electron microscopic description of heart muscle cells. Application to normal, hypertrophied and thyroxin-stimulated hearts. Am. J. Cardiol. 1973, 31, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.J. Mechanisms of disease: Is mitochondrial function altered in heart failure? Methodist. Debakey Cardiovasc. J. 2013, 9, 44–48. [Google Scholar] [CrossRef]
- Mortensen, S.A.; Leth, A.; Agner, E.; Rohde, M. Dose-related decrease of serum coenzyme Q10 during treatment with HMG-CoA reductase inhibitors. Mol. Asp. Med. 1997, 18 (Suppl. 1), S137–S144. [Google Scholar] [CrossRef]
- Godoy, J.C.; Niesman, I.R.; Busija, A.R.; Kassan, A.; Schilling, J.M.; Schwarz, A.; Alvarez, E.A.; Dalton, N.D.; Drummond, J.C.; Roth, D.M.; et al. Atorvastatin, but not pravastatin, inhibits cardiac Akt/mTOR signaling and disturbs mitochondrial ultrastructure in cardiac myocytes. FASEB J. 2018, 33, 1209–1225. [Google Scholar] [CrossRef]
- Greene, S.J.; Vaduganathan, M.; Lupi, L.; Ambrosy, A.P.; Mentz, R.J.; Konstam, M.A.; Nodari, S.; Subacius, H.P.; Fonarow, G.C.; Bonow, R.O.; et al. Prognostic significance of serum total cholesterol and triglyceride levels in patients hospitalized for heart failure with reduced ejection fraction (from the EVEREST Trial). Am. J. Cardiol. 2013, 111, 574–581. [Google Scholar] [CrossRef]
- Frohlich, H.; Raman, N.; Tager, T.; Schellberg, D.; Goode, K.M.; Kazmi, S.; Grundtvig, M.; Hole, T.; Cleland, J.G.F.; Katus, H.A.; et al. Statins attenuate but do not eliminate the reverse epidemiology of total serum cholesterol in patients with non-ischemic chronic heart failure. Int. J. Cardiol. 2017, 238, 97–104. [Google Scholar] [CrossRef]
- Dale, K.M.; White, C.M.; Henyan, N.N.; Kluger, J.; Coleman, C.I. Impact of statin dosing intensity on transaminase and creatine kinase. Am. J. Med. 2007, 120, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Averbukh, L.D.; Turshudzhyan, A.; Wu, D.C.; Wu, G.Y. Statin-induced Liver Injury Patterns: A Clinical Review. J. Clin. Transl. Hepatol. 2022, 10, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Carmona, A.; Castell, J.V.; Gomez-Lechon, M.J.; Donato, M.T. High-content screening of drug-induced mitochondrial impairment in hepatic cells: Effects of statins. Arch. Toxicol. 2015, 89, 1847–1860. [Google Scholar] [CrossRef]
- Roshanshad, R.; Roshanshad, A.; Fereidooni, R.; Hosseini-Bensenjan, M. COVID-19 and liver injury: Pathophysiology, risk factors, outcome and management in special populations. World J. Hepatol. 2023, 15, 441–459. [Google Scholar] [CrossRef]
- Akbari, H.; Taghizadeh-Hesary, F. COVID-19 induced liver injury from a new perspective: Mitochondria. Mitochondrion 2023, 70, 103–110. [Google Scholar] [CrossRef]
- Helou, M.; Nasr, J.; El Osta, N.; Jabbour, E.; Husni, R. Liver manifestations in COVID-19 patients: A review article. World J. Clin. Cases 2023, 11, 2189–2200. [Google Scholar] [CrossRef]
- Martini, N.; Singla, P.; Arbuckle, E.; Goyal, G.; Liu, Q.; Santos-Zabala, M.L.; Zainah, H. SARS-CoV-2-Induced Autoimmune Hepatitis. Cureus 2023, 15, e38932. [Google Scholar] [CrossRef]
- Durazzo, M.; Ferro, A. SARS-CoV-2 and autoimmune hepatitis onset: A new association. Minerva Gastroenterol. 2022, 68, 259–260. [Google Scholar] [CrossRef]
- Miller, W.L. Steroid hormone synthesis in mitochondria. Mol. Cell Endocrinol. 2013, 379, 62–73. [Google Scholar] [CrossRef]
- Hyyppa, M.T.; Kronholm, E.; Virtanen, A.; Leino, A.; Jula, A. Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology 2003, 28, 181–194. [Google Scholar] [CrossRef]
- Schooling, C.M.; Au Yeung, S.L.; Freeman, G.; Cowling, B.J. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Dobs, A.S.; Schrott, H.; Davidson, M.H.; Bays, H.; Stein, E.A.; Kush, D.; Wu, M.; Mitchel, Y.; Illingworth, R.D. Effects of high-dose simvastatin on adrenal and gonadal steroidogenesis in men with hypercholesterolemia. Metabolism 2000, 49, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Moulin, T.C.; Schioth, H.B. Sex differences in COVID-19: The role of androgens in disease severity and progression. Endocrine 2021, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Pegiou, S.; Rentzeperi, E.; Koufakis, T.; Metallidis, S.; Kotsa, K. The role of sexual dimorphism in susceptibility to SARS-CoV-2 infection, disease severity, and mortality: Facts, controversies and future perspectives. Microbes Infect. 2021, 23, 104850. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A. Higher LDL and Lesser LDL-Drop Linked to More Muscle Problems in Men on Statins. Circulation 2013, 127, AP073. [Google Scholar]
- Linares, L.; Golomb, B.; Jaojoco, J.; Sikand, H.; Phillips, P.S. The Modern Spectrum of Rhabdomyolysis: Drug Toxicity Revealed by Creatine Kinase Screening. Curr. Drug Saf. 2009, 4, 181–187. [Google Scholar] [CrossRef]
- Wang, F.; Cao, J.; Yu, Y.; Ding, J.; Eshak, E.S.; Liu, K.; Mubarik, S.; Shi, F.; Wen, H.; Zeng, Z.; et al. Epidemiological characteristics of patients with severe COVID-19 infection in Wuhan, China: Evidence from a retrospective observational study. Int. J. Epidemiol. 2020, 49, 1940–1950. [Google Scholar] [CrossRef]
- Wiggins, B.S.; Saseen, J.J.; Page, R.L., 2nd; Reed, B.N.; Sneed, K.; Kostis, J.B.; Lanfear, D.; Virani, S.; Morris, P.B. Recommendations for Management of Clinically Significant Drug-Drug Interactions With Statins and Select Agents Used in Patients With Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e468–e495. [Google Scholar] [CrossRef]
- Jin, M.; Tong, Q. Rhabdomyolysis as potential late complication associated with 2019 novel coronavirus disease. Emerg. Infect. Dis. 2020, 26, 1618–1620. [Google Scholar] [CrossRef]
- Fadila, M.F.; Wool, K.J. Rhabdomyolysis secondary to influenza a infection: A case report and review of the literature. N. Am. J. Med. Sci. 2015, 7, 122–124. [Google Scholar] [CrossRef]
- Singh, U.; Scheld, W.M. Infectious etiologies of rhabdomyolysis: Three case reports and review. Clin. Infect. Dis. 1996, 22, 642–649. [Google Scholar] [CrossRef]
- Chauhan, P.; Saha, B. Metabolic regulation of infection and inflammation. Cytokine 2018, 112, 1–11. [Google Scholar] [CrossRef]
- Ganji, R.; Reddy, P.H. Impact of COVID-19 on Mitochondrial-Based Immunity in Aging and Age-Related Diseases. Front. Aging Neurosci. 2020, 12, 614650. [Google Scholar] [CrossRef]
- Ali, L.; Mohammed, I.; Janjua, I.; Naeem, M.; Adeli, G.; Elalamy, O.; Alhatou, M.; Akhtar, N.; Canibano, B.; Iqrar, A. Acute Myocardial Injury and Rhabdomyolysis in COVID-19 Patients: Incidence and Mortality. Cureus 2021, 13, e18899. [Google Scholar] [CrossRef]
- Haroun, M.W.; Dieiev, V.; Kang, J.; Barbi, M.; Marashi Nia, S.F.; Gabr, M.; Eman, G.; Kajita, G.; Swedish, K. Rhabdomyolysis in COVID-19 Patients: A Retrospective Observational Study. Cureus 2021, 13, e12552. [Google Scholar] [CrossRef]
- Ellis, R.; Graves, J.; Horton, L.E.; Lee, S.J.; Liang, N.-C.; Minassian, A.; Phreaner, N.; Wang, A. COVID-19 for the Long Haul: Symposium on Post COVID Recovery and Care. In Proceedings of the CME Symposium, Online, 26 February 2022. [Google Scholar]
- Twomey, R.; DeMars, J.; Franklin, K.; Culos-Reed, S.N.; Weatherald, J.; Wrightson, J.G. Chronic Fatigue and Postexertional Malaise in People Living with Long COVID: An Observational Study. Phys. Ther. 2022, 102, pzac005. [Google Scholar] [CrossRef]
- Stallmach, A.; Kesselmeier, M.; Bauer, M.; Gramlich, J.; Finke, K.; Fischer, A.; Fleischmann-Struzek, C.; Heutelbeck, A.; Katzer, K.; Mutschke, S.; et al. Comparison of fatigue, cognitive dysfunction and psychological disorders in post-COVID patients and patients after sepsis: Is there a specific constellation? Infection 2022, 50, 661–669. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Phillips, P.S.; Haas, R.H.; Bannykh, S.; Hathaway, S.; Gray, N.L.; Kimura, B.J.; Vladutiu, G.D.; England, J.D. Statin-associated myopathy with normal creatine kinase levels. Ann. Intern. Med. 2002, 137, 581–585. [Google Scholar] [CrossRef]
- Alam, M.S.; Czajkowsky, D.M. SARS-CoV-2 infection and oxidative stress: Pathophysiological insight into thrombosis and therapeutic opportunities. Cytokine Growth Factor. Rev. 2022, 63, 44–57. [Google Scholar] [CrossRef]
- Fodor, A.; Tiperciuc, B.; Login, C.; Orasan, O.H.; Lazar, A.L.; Buchman, C.; Hanghicel, P.; Sitar-Taut, A.; Suharoschi, R.; Vulturar, R.; et al. Endothelial Dysfunction, Inflammation, and Oxidative Stress in COVID-19-Mechanisms and Therapeutic Targets. Oxid. Med. Cell. Longev. 2021, 2021, 8671713. [Google Scholar] [CrossRef] [PubMed]
- Forcados, G.E.; Muhammad, A.; Oladipo, O.O.; Makama, S.; Meseko, C.A. Metabolic Implications of Oxidative Stress and Inflammatory Process in SARS-CoV-2 Pathogenesis: Therapeutic Potential of Natural Antioxidants. Front. Cell Infect. Microbiol. 2021, 11, 654813. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, B.V.; Popova, E.N.; Prikhodko, A.S.; Grebenchikov, O.A.; Zinovkina, L.A.; Zinovkin, R.A. COVID-19 and Oxidative Stress. Biochemistry 2020, 85, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Pliss, A.; Kuzmin, A.N.; Prasad, P.N.; Mahajan, S.D. Mitochondrial Dysfunction: A Prelude to Neuropathogenesis of SARS-CoV-2. ACS Chem. Neurosci. 2022, 13, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Ajaz, S.; McPhail, M.J.; Singh, K.K.; Mujib, S.; Trovato, F.M.; Napoli, S.; Agarwal, K. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am. J. Physiol. Cell Physiol. 2021, 320, C57–C65. [Google Scholar] [CrossRef]
- Wieczfinska, J.; Kleniewska, P.; Pawliczak, R. Oxidative Stress-Related Mechanisms in SARS-CoV-2 Infections. Oxid. Med. Cell. Longev 2022, 2022, 5589089. [Google Scholar] [CrossRef]
- Wagstaff, L.R.; Mitton, M.W.; Arvik, B.M.; Doraiswamy, P.M. Statin-associated memory loss: Analysis of 60 case reports and review of the literature. Pharmacotherapy 2003, 23, 871–880. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Drug Safety Communication: Important Safety Label Changes to Cholesterol-Lowering Statin Drugs; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2012.
- Chatham, K.; Gelder, C.M.; Lines, T.A.; Cahalin, L.P. Suspected statin-induced respiratory muscle myopathy during long-term inspiratory muscle training in a patient with diaphragmatic paralysis. Phys. Ther. 2009, 89, 257–266. [Google Scholar] [CrossRef]
- Heinicke, K.; Taivassalo, T.; Wyrick, P.; Wood, H.; Babb, T.G.; Haller, R.G. Exertional dyspnea in mitochondrial myopathy: Clinical features and physiological mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R873–R884. [Google Scholar] [CrossRef]
- Scharfen, J.; Peters, J.M.; Holling, H. Retest effects in cognitive ability tests: A meta-analysis. Intelligence 2018, 67, 44–66. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Barger, S.D.; Ryan, C.M.; Flory, J.D.; Lehoczky, J.P.; Matthews, K.A.; Manuck, S.B. Effects of lovastatin on cognitive function and psychological well-being. Am. J. Med. 2000, 108, 538–546. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Ryan, C.M.; Sereika, S.M.; Flory, J.D.; Manuck, S.B. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am. J. Med. 2004, 117, 823–829. [Google Scholar] [CrossRef]
- Mortensen, E.M.; Restrepo, M.I.; Copeland, L.A.; Pugh, M.J.; Anzueto, A. Statins and outcomes in patients with pneumonia: Not only healthy user bias. BMJ 2006, 333, 1123–1124. [Google Scholar] [CrossRef]
- Rundek, T.; Naini, A.; Sacco, R.; Coates, K.; DiMauro, S. Atorvastatin decreases the coenzyme Q10 level in the blood of patients at risk for cardiovascular disease and stroke. Arch. Neurol. 2004, 61, 889–892. [Google Scholar] [CrossRef]
- Myhill, S.; Booth, N.E.; McLaren-Howard, J. Chronic fatigue syndrome and mitochondrial dysfunction. Int. J. Clin. Exp. Med. 2009, 2, 1–16. [Google Scholar]
- Preiss, D.; Seshasai, S.R.; Welsh, P.; Murphy, S.A.; Ho, J.E.; Waters, D.D.; DeMicco, D.A.; Barter, P.; Cannon, C.P.; Sabatine, M.S.; et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. J. Am. Med. Assoc. 2011, 305, 2556–2564. [Google Scholar] [CrossRef]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef]
- de Almeida Ferreira, M.; Mendonca, J.A. Long-term testosterone replacement therapy reduces fatigue in men with hypogonadism. Drugs Context 2022, 11, 1–6. [Google Scholar] [CrossRef]
- Bercea, R.M.; Mihaescu, T.; Cojocaru, C.; Bjorvatn, B. Fatigue and serum testosterone in obstructive sleep apnea patients. Clin. Respir. J. 2015, 9, 342–349. [Google Scholar] [CrossRef]
- Yu, G.; Traish, A.M. Induced testosterone deficiency: From clinical presentation of fatigue, erectile dysfunction and muscle atrophy to insulin resistance and diabetes. Horm. Mol. Biol. Clin. Investig. 2011, 8, 425–430. [Google Scholar] [CrossRef]
- Srinivas-Shankar, U.; Roberts, S.A.; Connolly, M.J.; O’Connell, M.D.; Adams, J.E.; Oldham, J.A.; Wu, F.C. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: A randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2010, 95, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Soelaiman, I.N.; Naina Mohamed, I.; Shahar, S.; Teng, N.I.; Suhana Mohd Ramli, E.; Ahmad, F.; Aminuddin, A.; Zurinah Wan Ngah, W. Testosterone is associated with age-related changes in bone health status, muscle strength and body composition in men. Aging Male 2012, 15, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, J.; Semenova, E.A.; Borisov, O.V.; Larin, A.K.; Moreland, E.; Generozov, E.V.; Ahmetov, I.I. Genomic predictors of testosterone levels are associated with muscle fiber size and strength. Eur. J. Appl. Physiol. 2022, 122, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O. Testosterone and cognitive function: Current clinical evidence of a relationship. Eur. J. Endocrinol. 2006, 155, 773–781. [Google Scholar] [CrossRef]
- Italia, L.; Tomasoni, D.; Bisegna, S.; Pancaldi, E.; Stretti, L.; Adamo, M.; Metra, M. COVID-19 and Heart Failure: From Epidemiology During the Pandemic to Myocardial Injury, Myocarditis, and Heart Failure Sequelae. Front. Cardiovasc. Med. 2021, 8, 713560. [Google Scholar] [CrossRef]
- Marik, P. EVMS Critical Care COVID-19 Management Protocol. Available online: https://www.evms.edu/media/evms_public/departments/internal_medicine/EVMS_Critical_Care_COVID-19_Protocol.pdf (accessed on 5 August 2020).
- Castiglione, V.; Chiriaco, M.; Emdin, M.; Taddei, S.; Vergaro, G. Statin therapy in COVID-19 infection. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 258–259. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).