Abstract
Background: Arteriovenous fistula dysfunction is a widely disputed subject in the scientific literature on end-stage kidney disease (ESKD). The main cause of mortality and morbidity in these patients is the non-maturation or dysfunction of the arteriovenous fistula. Despite the many complications, the native arteriovenous fistula remains the gold standard in the treatment of these patients requiring renal replacement. This study aims to discuss the predictive role of some systemic inflammatory biomarkers (NLR, PLR, SII, IL-6), intimal hyperplasia, and neoangiogenesis (characterized by intimal-media CD31-positive relative surface) in arteriovenous fistula maturation failure. Methods: The present study was designed as an observational, analytical, and prospective study which included patients diagnosed with ESKD with indications of radio-cephalic arteriovenous fistula (RCAVF). Demographic data, comorbidities, preoperative laboratory data and histological/digital morphometry analysis results were processed. The patients included were divided into two groups based on their AVF maturation status at 8 weeks: “Maturation” (Group 1) and “Failed Maturation” (Group 2). Results: There was no difference in the demographic data. In terms of comorbidities, the second group had a greater incidence of heart failure (p = 0.03), diabetes (p = 0.04), peripheral artery disease (p = 0.002), and obesity (p = 0.01). Additionally, regarding the laboratory findings, these patients had higher levels of serum uric acid (p = 0.0005), phosphates (p < 0.0001), and creatinine (p = 0.02), as well as lower levels of total calcium (p = 0.0002), monocytes (p = 0.008), and lymphocytes (p < 0.0001). Moreover, all inflammatory markers (p = 0.001; p < 0.0001; p = 0.006, and p = 0.03) and Ca-P product (p < 0.0001) had higher baseline values in Group 2. Upon immunohistochemical analysis, regarding the density of neoformed vessels, there was a higher incidence of CD31-positive surfaces (p = 0.006) and CD31-positive relative surfaces (p = 0.001); the NLR (r = 0.323; p = 0.03), PLR (r = 0.381; p = 0.04), SII (r = 0.376; p = 0.03), and IL-6 (r = 0.611; p < 0.001) are all significantly correlated with vascular density, as evidenced by CD31. Conclusions: Heart failure, peripheral artery disease, obesity, and diabetes, as well as the systemic inflammatory markers (NLR, PLR, SII, IL-6), intimal hyperplasia, and CD31-positive relative surfaces are predictors of arteriovenous fistula maturation failures.
1. Introduction
Non-maturation of the arteriovenous fistula or its dysfunction remains the leading cause of morbidity and mortality in patients with stage 5 chronic kidney disease (CKD). Despite that, the Cimino fistula remains the gold standard in the treatment of ESKD in comparison with catheters and grafts [1].
The current literature suggests that the arteriovenous fistula (AVF) is the optimal vascular access route for dialysis in patients with end-stage kidney disease (ESKD) [2,3], with patency rates at one year ranging from 52–71% for the radiocephalic AVF (RCAVF) [4,5,6,7,8,9,10,11], 75–89% for the brachiocephalic AVF (BCAVF) [12,13,14,15,16,17,18], and 64–89% for the brachiobasilic AVF [12,16,19,20,21,22,23]. According to the above-mentioned publications, early failure occurs in between 5 and 37% of patients in the case of RCAVF (4–11), 8–16% in the case of BCAVF [12,13,14,15,16,17,18], and 2–23% in the case of BBAVF, all within one month after access creation [12,16,19,20,21,22,23].
Significant arterial atherosclerotic damage, juxta-anastomotic stenosis of the venous segment, or the presence of intimal hyperplasia (IH) with or without penetrating capillaries within neointima-media at the venous segment level, which limits the appropriate dilation and development, are the most frequent causes of failure of AVF maturation [24,25,26]. Wali et al. [27,28] were the first to draw attention to the presence of IH in patients with chronic renal disease. More recently, numerous publications have analyzed maximal intimal thickness [29,30,31,32,33] and its associations with AVF outcomes from a morphometric standpoint, but with inconsistent results. Some mechanical factors can also influence the success of the procedure before the fistula is performed. Preoperative punction of the vein, long-term hospitalization of the patient with intravenous treatment, and dehydration can have a negative impact on the quality of the vein, and can lead to venous intimal hyperplasia.
In comparison to the results reported by Tabbara et al. [29] (p = 0.2), Martinez et al. [30] (p = 0.70), and Allon et al. [31] (p = 0.49), only Lee et al. [26] revealed that the presence of neo-intimal hyperplasia is associated with maturation failure (p = 0.03). Furthermore, the mechanism through which CKD influences the development of IH is not fully understood. Some immunohistochemical studies [26,32,34,35] have identified a low number of CD3+ or CD68+ inflammatory cells in the venous wall. Wasse et al. [34] also discovered the presence of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) expression. IL-6 and TNF-α were initially shown to have cytotoxic effects on the endothelium, causing inflammation [36]. The primary goals of this study are to examine the association between the systemic inflammatory state of patients and intimal thickness and its degree of neovascularization at the venous segment level of AVF, assessing them as possible risk factors for AVF maturation failure.
2. Materials and Methods
2.1. Study Design
The current study included 42 patients with ESRD, hospitalized at the Vascular Surgery Clinic of Targu Mures Emergency County Hospital in Romania from January 2020 to December 2021, with indications of RCAVF. ESRD patients who had previously had an AVF, sepsis, hematological disorders, active tumoral status, a personal history of major surgery in the previous six months, or autoimmune diseases were all excluded. Furthermore, patients with no palpable thrill at the level of anastomosis immediately after AVF construction and patients with no sign of permeability at the level of AVF at 4 and 8 weeks of follow-up were also excluded.
The patients included were divided into two groups based on their AVF maturation status at 8 weeks: “Maturation” (Group 1) and “Failed Maturation” (Group 2).
2.2. Data Collection
The sex, age, and comorbidities of the patients were retrieved from the hospital’s computerized database. The same surgeon performed all RCAVFs and AVFs. An ultrasound assessment of the cephalic vein quality was performed initially, followed by a clinically perceptible pulse at the radial artery level. Regarding the laboratory parameters, we included in this study the laboratory data from samples taken pre-operatively. In terms of systemic inflammatory markers, we measured the IL-6 level and hematological ratios (neutrophil–lymphocyte ratios (NLR), platelet–lymphocyte ratios (PLR), and the systemic inflammatory index (SII)) [3,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. The patients provided written informed consent for the publication of any data.
Inflammatory markers and Ca-P product were calculated according to the following formulas:
- –
- NLR = the ratio between the total number of neutrophils and the total number of lymphocytes;
- –
- PLR = the ratio between the total number of platelets and the total number of lymphocytes;
- –
- SII = the total number of neutrophils multiplied by the total number of platelets, divided by the total number of lymphocytes;
- –
- Ca-P product = calcium level multiplied by the phosphate level.
In terms of maturation requirements, we employed the “rule of 6” in our clinic, which is based on the following guidelines: a minimum flow of 600 mL/min, a vein diameter of 6 mm, a punctionable length of the venous segment more than 6 cm, and a maximum depth of 6 mm [1].
2.3. The Histopathology and Immunohistochemistry of Veins Obtained during the Creation of Arteriovenous Fistula
Samples of 5–10 mm length circumferential venous segments were collected during of surgical creation of AVF fixed in 10% neutral buffered formalin, sent for histological analyses, and embedded in paraffin. The histological features of tissue samples were examined in 4–5 µm hematoxylin and eosin (H&E)- and Elastic van Gieson-stained sections (Verhoeff Van Gieson/EVG Stain’ kit, Abcam, ab150667) by a senior pathologist (EH) who was blinded to the patient’s characteristics. The microscopic examination followed the most important vascular remodeling changes: focal or diffuse intimal hyperplasia, media hypertrophy, intima/media neovascularization, mononuclear inflammatory infiltrate around the vessels in the adventitia, disruption of the internal and external elastic lamina by loss of elastic fibers, foci of microcalcification, and the presence of intraluminal thrombi. The endothelial layers of neoformed vessels at the intima and media thickness were visualized via immunohistochemistry using anti-CD31 mouse monoclonal antibody; clone 1A10 (Bio SB, US). EnVision FLEX/HRP (Agilent, Dako) was used as secondary antibody in combination with 3,3′-diaminobenzidine chromogen (DAB) substrate, in order to give the reaction product a brown color. Nuclei were counterstained with hematoxylin. For the negative control, normal serum was substituted for the primary antibody.
2.4. Quantitative Digital Image Analysis: Digital Morphometry
(a) A morphometric analysis of intimal thickness was performed on Elastin van Gieson-stained slides. Microphotographs were taken using a microscope mounted to a Zeiss AxioCam digital camera from representative regions containing the whole wall thickness at 40× magnification. The resulting images were imported into ImageJ software (National Institute of Health, Bethesda, ML, USA). The internal elastic lamina (IEL) was traced, and on it, ten points with an equal distance from each other were considered. From these points, calibrated linear measurements were effectuated perpendicularly to the IEL, measuring the intimal thickness (from the IEL to the intimal surface) and the medial thickness (from the IEL to the external elastic lamina), respectively. The average of the ten measurements represented the average intimal and medial thickness (Figure 1a). The intimal media thickness (IMT) represents the sum of the thicknesses of the media and intima.
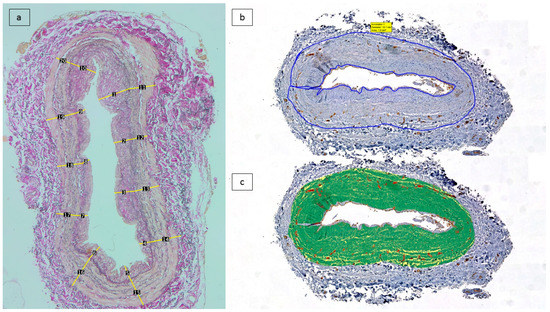
Figure 1.
Scanned tissue areas corresponding to the intima and media were considered for analysis. (a) Morphometric analysis of average intimal and medial thickness by 10 measurements: Elastin van Gieson-stained sections). (b) Tissue segmentation on anti-CD31/DAB immunolabelled tissue sections, followed by annotation of region of interest (intima and media). (c) Application of trained algorithm on the region of interest measurment of the thresholded area.
(b) A study of vascular density was carried out. Analysis of CD31 immunostained sections was performed at the 1st Department of Pathology and Experimental Cancer Research, Semmelweis University. The Panoramic Scan System (3D Histech Ltd., Budapest, Hungary) was used to digitize these 42 slides. Using the Panoramic Viewer program, tissue sections corresponding to the vascular well were manually annotated while excluding the endothelial lining of the intima and the vasa vasorum containing adventitia. Within the selected annotations, the Pattern Quant module of the Quant Center software package (3D Histech) was trained to provide automated differentiation between CD31-stained vascular structures, the background, and counterstained tissues (Figure 1b–c). The segmented areas were measured in µm2, and the positive surface area was estimated from the results.
2.5. Study Outcomes
The primary outcome was maturation failure at 8 weeks. The secondary endpoint was the existence of IH during morphometrical analysis, which was a shared endpoint of the presence of IH and maturation failure.
2.6. Statistical Analysis
SPSS for Mac OS version 28.0.1.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Chi-square tests were used to examine associations between inflammatory markers derived from total neutrophil, platelet, and lymphocyte counts, as well as IL-6, serum calcium, serum phosphate, Ca-P product, and intimal hyperplasia, while Student’s t tests or Mann-Whitney U tests were used to evaluate differences between continuous variables. To determine the association between systemic inflammatory markers and CD31-positive relative surfaces, we used the Spearman correlation. A multivariate logistic regression analysis of variables with p < 0.1 was used to identify independent predictors of vascular access maturation failure.
3. Results
During the study period, all of the included patients met all of the inclusion and exclusion criteria, with 12 patients (28.57%) having failed AVF maturation at 8 weeks. There was no difference in the demographic data. In terms of comorbidities, the second group had a greater incidence of heart failure (66.67% vs. 30%, p = 0.03), diabetes (91.67% vs. 53.33%, p = 0.04), peripheral artery disease (83.33% vs. 26.67%, p = 0.002), and obesity (83.33% vs. 40%, p = 0.01) (Table 1).

Table 1.
Demographic data, comorbidities, risk factors, morphometric and immunohistochemical analysis of all patients.
Additionally, regarding the laboratory findings, the patients with failure of maturation AVF had higher levels of serum uric acid (8.6 mg/dL vs. 6.09 mg/dL, p = 0.0005), serum phosphate (7.56 vs. 4.12, p < 0.0001), and creatinine (7.76 mg/dL vs. 5.92 mg/dL, p = 0.02), as well as lower levels of total calcium (1.9 mmol/L vs. 2.27 mmol/L, p = 0.0002), monocytes (0.66 vs. 0.84, p = 0.008), and lymphocytes (1.14 vs. 1.85, p < 0.0001). Moreover, all inflammatory markers (p = 0.001; p < 0.0001; p = 0.006, and p = 0.03) and Ca-P product (56.52 vs. 38.83, p < 0.0001) had higher baseline values in the patients with failure of AVF maturation. Upon immunohistochemical analysis, regarding the presence of CD31 immunolabelling, there was a higher incidence of CD31-positive surfaces (p = 0.006) and CD31-positive relative surfaces (p = 0.001) (Table 1).
As shown in Figure 2, increased baseline levels of CD31-positive relative surfaces (p < 0.0001) and all inflammatory markers (PLR (p = 0.01), SII (p = 0.02), and IL-6 (p = 0.0001)) except for NLR (p = 0.09) are associated with IH.
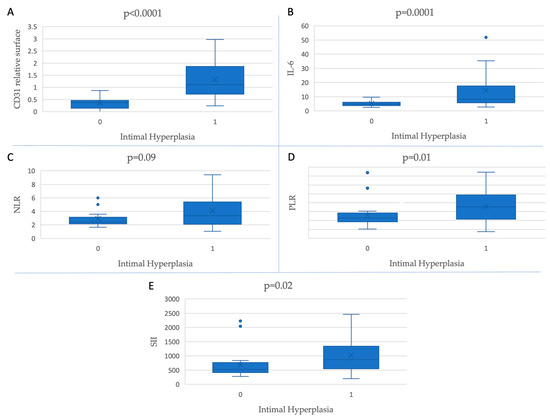
Figure 2.
Inflammatory markers and CD31-positive relative surfaces’ association with the presence of intimal hyperplasia.
For a more in-depth analysis of the interrelationships between the systemic inflammatory status and CD31-positive surfaces in the thickened intima, we also investigated whether a higher value of inflammatory markers was correlated with a higher presence of CD31-positive microvessel density in the neointimal surface. We found that the NLR (r = 0.323; p = 0.03), PLR (r = 0.381; p = 0.04), SII (r = 0.376; p = 0.03), and IL-6 (r = 0.611; p < 0.001) were all positively significantly correlated with CD31 density (Figure 3).
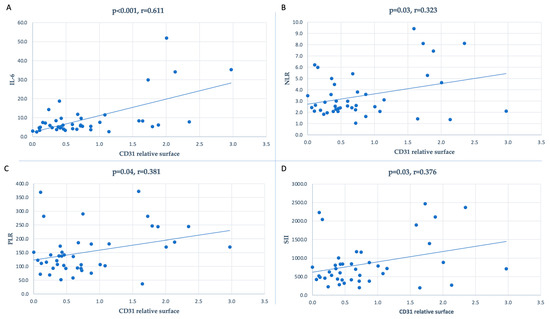
Figure 3.
Correlation between the inflammatory markers and CD31-positive relative surfaces: (A) for IL-6 (r = 0.611, p < 0.001), (B) for NLR (r = 0.323, p = 0.03), (C) for PLR (r = 0.381, p = 0.04), and (D) for SII (r = 0.376, p = 0.03).
A multivariate logistic regression analysis showed that the presence of heart failure (OR:3.71; p = 0.008), peripheral arterial disease (OR:3.82; p = 0.006), obesity (OR:2.28; p = 0.02), pre-operative intimal hyperplasia (OR:5.18; p = 0.03), CD31-positive relative surfaces (OR:10.65, p = 0.001), all high baseline values of systemic inflammatory markers (for all p < 0.05), high baseline values of serum phosphate (OR:7.43, p = 0.004), and Ca-P product (OR:4.51, p = 0.006) are predictors of vascular access maturation failure (Table 2). Additionally, the high baseline level of total serum calcium acts as protective factor against vascular access maturation failure (OR:0.17, p = 0.002) (Table 2).

Table 2.
Predictors of vascular access maturation failure.
4. Discussion
Arteriovenous fistula maturation, patency, and causes of non-maturation and dysfunction are the main topics discussed in the literature regarding end-stage kidney disease. The predictive value of systemic inflammatory markers in AVF dysfunction has been discussed in many papers, but the impact of CKD in intimal hyperplasia at the level of the venous component remains a complex mechanism not yet elucidated.
In our study, we used histopathological data as well as laboratory biomarkers to demonstrate the role of systemic inflammation in arteriovenous fistula dysfunction, the main finding being the correlation between systemic inflammatory markers and microvascular density in intimal hyperplasia. High values of inflammatory markers, the presence of IH, and intima neovascularization (as CD31-positive surface) are predictive factors of AVF dysfunction. Additionally, heart failure, peripheral arterial disease, and obesity are strong predictors of 8-week AVF maturation failure.
The association between vascular remodeling, intimal hyperplasia, and AVF maturation failure has been discussed in recent articles published in the literature, but with inconsistent results. Few studies have investigated the occurrence of neointimal hyperplasia at the venous component and its influence on maturation failure [26,34,53,54,55]. Duque et al. [53], who studied the impact of vasa vasorum density and vasa vasorum area in all layer vein samples taken at the time of AVF with maturation failure using anti-CD31 immunohistochemistry, found a correlation between lower vascularization of the media and an increase in postoperative intimal thickness (r = 0.53, p = 0.003 and r = 0.37, p = 0.045).
Based on comprehensive immunohistochemical research, Shehadeh et al. [54] demonstrated early remodeling 7 days after AVF creation, which needed ligation due to steal syndrome. Because of enhanced intramural oxygenation, the authors observed increased wall neovascularization and the presence of CD163+ macrophages.
In terms of systemic inflammation, Pasqui et al. [56] discovered that high NLR levels (HR: 2.53, p = 0.01) and the presence of diabetes mellitus (HR: 1.41; p = 0.04) are independent predictors of a malfunctioning AVF in a study of 178 patients with AVF. Usman et al. [57] and Yilmaz et al. [58] found that high NLR levels are related to vascular access dysfunction and the occurrence of AVF stenosis. Sarioglu et al. [59] demonstrated that high PLR values correlate with AVF stenosis and thrombosis, and in the recent work published by our group of researchers [3], we demonstrated that high values of NLR, PLR, and SII, as well as the presence of heart failure (OR:4.38, p < 0.001) and diabetes mellitus (OR:5.63, p < 0.001) are predictors of AVF maturation failure in the case of 125 patients.
The serum levels of calcium, phosphate, and Ca-P product have an important role in the homeostasis and optimal functioning of the cardiovascular system [3,60,61]. According to the results of our study, the low level of serum calcium (p = 0.0002), the increased level of serum phosphate (p < 0.0001), and the increased values of Ca-P product (p < 0.0001) are associated with AVF maturation failure. Moreover, as can be seen in Table 2, a high baseline value of serum phosphate (OR:7.43, p = 0.004) and Ca-P product (OR:4.51, p = 0.006) are predictors of vascular access maturation failure. Additionally, the high baseline level of total serum calcium acts as protective factor against vascular access maturation failure (OR:0.17, p = 0.002). Similar to our results, Tuysuz et al. [60] demonstrated in a group of 79 patients that serum levels of phosphate (OR: 1.85, p = 0.05) and Ca-P product (OR: 1.11, p = 0.03) were predictive factors of AVF re-operation. Moreover, Unver et al. [61] demonstrated that high values of Ca-P product are associated with lower blood flow rates (p = 0.03).
Additionally, the results of this study follow those of the previously published study by Kaller et al. [3], in which we demonstrated that high values of systemic inflammatory markers and Ca-P product are predictors of maturation failure (all p < 0.05).
The main reasons for AVF maturation failure and long-term AVF dysfunction are vascular remodeling and intimal hyperplasia [62,63]. Wong et al. [62] discovered that the density of CD31-positive cells increased, beginning on day 7, in a new mouse model of AVF failure. Furthermore, Cai et al. [63] discovered, in an animal model experimental investigation, that at day 14 post-operatively, there was a significant increase in the CD31 index in the angioplasty-treated vessel (p < 0.0001).
Among the study’s strengths are the quantitative measurement of intimal and media thickness and the vascular density (based on anti-CD31 immunolabelling), that is, the examination of inflammatory markers that play a role in the prediction of AVF maturation failure. This study, on the other hand, has a number of shortcomings. First and foremost, the small number of patients involved in this study originate from a single center. Secondly, because we quantitatively rather than qualitatively (immunohistochemically) assessed the local inflammatory infiltrate, we were unable to correlate these two markers of systemic inflammation. Also, due to the retrospective nature of the study, we do not have data on the patients’ chronic medications and cannot determine if these medications affect the inflammatory markers.
Questions regarding the correlation between the inflammatory markers and fistula dysfunction look like they are on their way to being clarified. Because of the low number of patients included in this study, further research must be conducted to demonstrate these results in a larger number of patients, so that they gain true value.
5. Conclusions
According to our findings, some comorbidities, including heart failure, peripheral artery disease, diabetes, and obesity, had a higher incidence in group 2 patients (the failed maturation group); regarding the laboratory findings, these patients had higher levels of serum uric acid and creatinine, as well as lower levels of total calcium, total bilirubin, monocytes, and lymphocytes.
The systemic inflammatory biomarkers (NLR, PLR, SII, and IL-6) showed an association with intimal hyperplasia. During morphometrical analysis, we demonstrated that CD31-positive relative surfaces have a higher baseline value in group 2 patients, and are also associated with intimal hyperplasia. The correlation test between a higher baseline value of the systemic inflammatory markers and a higher CD31-positive relative surface in the intimal hyperplasia area showed a positive result.
Concluding all these findings, we can declare that the mentioned comorbidities, the systemic inflammatory markers (NLR, PLR, SII, IL-6), intimal hyperplasia, and CD31-positive relative surfaces are predictors of arteriovenous fistula maturation failure. By determining these parameters before planning the procedure, we can ensure preoperative risk group stratification and better patient management.
Author Contributions
Conceptualization, R.K., E.H. and E.M.A. (Emil Marian Arbănași); methodology and software, M.J. and E.M.A. (Emil Marian Arbănași); validation, all authors.; formal analysis, investigation and resources, M.J., B.A.S. and I.H.; data curation, A.V.M. and E.R.; writing—original draft preparation, R.K.; writing—review and editing, E.M.A. (Eliza Mihaela Arbănași); visualization, supervision, project administration and funding acquisition, B.A.S., L.D. and E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Mures County Emergency Hospital, 540136, Targu Mures, Romania (protocol code 29290, on 10 November 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Krenács Tibor (1st Department of Pathology and Experimental Cancer Research Semmelweis University, Hungary) for his help in digital image analysis with the Panoramic Scan System (3D Histech Ltd., Budapest, Hungary). This paper was published with the support of the George Emil Palade University of Medicine, Pharmacy, Science and Technology of Targu Mures, 540139, Targu Mures, Romania. This paper is part of a Ph.D. thesis from the Doctoral School of Medicine and Pharmacy within the George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Targu Mures, with the title “Clinical, biological and histopathological aspects in vascular access dysfunction of hemodialysis”, which will be presented by Réka Kaller, with the approval of all authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schmidli, J.; Widmer, M.K.; Basile, C.; de Donato, G.; Gallieni, M.; Gibbons, C.P.; Haage, P.; Hamilton, G.; Hedin, U.; Kamper, L.; et al. Editor’s Choice—Vascular Access: 2018 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2018, 55, 757–818. [Google Scholar] [CrossRef] [PubMed]
- Kaller, R.; Mureșan, A.V.; Arbănași, E.M.; Arbănași, E.M.; Kovács, I.; Horváth, E.; Suciu, B.A.; Hosu, I.; Russu, E. Uncommon Surgical Management by AVF between the Great Saphenous Vein and Anterior Tibial Artery for Old Radiocephalic AVF Failure. Life 2022, 12, 529. [Google Scholar] [CrossRef] [PubMed]
- Kaller, R.; Arbănași, E.M.; Mureșan, A.V.; Voidăzan, S.; Arbănași, E.M.; Horváth, E.; Suciu, B.A.; Hosu, I.; Halmaciu, I.; Brinzaniuc, K.; et al. The Predictive Value of Systemic Inflammatory Markers, the Prognostic Nutritional Index, and Measured Vessels’ Diameters in Arteriovenous Fistula Maturation Failure. Life 2022, 12, 1447. [Google Scholar] [CrossRef] [PubMed]
- Huijbregts, H.J.T.; Bots, M.L.; Wittens, C.H.A.; Schrama, Y.C.; Moll, F.L.; Blankestijn, P.J.; CIMINO Study Group. Hemodialysis Arteriovenous Fistula Patency Revisited: Results of a Prospective, Multicenter Initiative. Clin. J. Am. Soc. Nephrol. CJASN 2008, 3, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Smith, C.J.; Emery, J.; Farrington, K.; Thompson, H.H. Outcome of Primary Radiocephalic Fistula for Haemodialysis. Br. J. Surg. 1999, 86, 211–216. [Google Scholar] [CrossRef]
- Wolowczyk, L.; Williams, A.J.; Donovan, K.L.; Gibbons, C.P. The Snuffbox Arteriovenous Fistula for Vascular Access. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2000, 19, 70–76. [Google Scholar] [CrossRef]
- Gibson, K.D.; Gillen, D.L.; Caps, M.T.; Kohler, T.R.; Sherrard, D.J.; Stehman-Breen, C.O. Vascular Access Survival and Incidence of Revisions: A Comparison of Prosthetic Grafts, Simple Autogenous Fistulas, and Venous Transposition Fistulas from the United States Renal Data System Dialysis Morbidity and Mortality Study. J. Vasc. Surg. 2001, 34, 694–700. [Google Scholar] [CrossRef]
- Dixon, B.S.; Novak, L.; Fangman, J. Hemodialysis Vascular Access Survival: Upper-Arm Native Arteriovenous Fistula. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2002, 39, 92–101. [Google Scholar] [CrossRef]
- Ravani, P.; Marcelli, D.; Malberti, F. Vascular Access Surgery Managed by Renal Physicians: The Choice of Native Arteriovenous Fistulas for Hemodialysis. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2002, 40, 1264–1276. [Google Scholar] [CrossRef]
- Rooijens, P.P.G.M.; Burgmans, J.P.J.; Yo, T.I.; Hop, W.C.J.; de Smet, A.A.E.A.; van den Dorpel, M.A.; Fritschy, W.M.; de Groot, H.G.W.; Burger, H.; Tordoir, J.H.M. Autogenous Radial-Cephalic or Prosthetic Brachial-Antecubital Forearm Loop AVF in Patients with Compromised Vessels? A Randomized, Multicenter Study of the Patency of Primary Hemodialysis Access. J. Vasc. Surg. 2005, 42, 481–486, discussions 487. [Google Scholar] [CrossRef]
- Biuckians, A.; Scott, E.C.; Meier, G.H.; Panneton, J.M.; Glickman, M.H. The Natural History of Autologous Fistulas as First-Time Dialysis Access in the KDOQI Era. J. Vasc. Surg. 2008, 47, 415–421, discussion 420–421. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.J.; Saunders, R.; Metcalfe, M.; Nicholson, M.L. Elbow Fistulas Using Autogeneous Vein: Patency Rates and Results of Revision. Postgrad. Med. J. 2002, 78, 483–486. [Google Scholar] [CrossRef]
- Zeebregts, C.J.; Tielliu, I.F.J.; Hulsebos, R.G.; de Bruin, C.; Verhoeven, E.L.G.; Huisman, R.M.; van den Dungen, J.J.A.M. Determinants of Failure of Brachiocephalic Elbow Fistulas for Haemodialysis. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2005, 30, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.E.; Oliver, M.J.; Su, J.; Bhola, C.; Hannigan, N.; Jassal, S.V. Arteriovenous Fistula Outcomes in the Era of the Elderly Dialysis Population. Kidney Int. 2005, 67, 2462–2469. [Google Scholar] [CrossRef]
- Woo, K.; Farber, A.; Doros, G.; Killeen, K.; Kohanzadeh, S. Evaluation of the Efficacy of the Transposed Upper Arm Arteriovenous Fistula: A Single Institutional Review of 190 Basilic and Cephalic Vein Transposition Procedures. J. Vasc. Surg. 2007, 46, 94–99, discussion 100. [Google Scholar] [CrossRef]
- Koksoy, C.; Demirci, R.K.; Balci, D.; Solak, T.; Köse, S.K. Brachiobasilic versus Brachiocephalic Arteriovenous Fistula: A Prospective Randomized Study. J. Vasc. Surg. 2009, 49, 171–177.e5. [Google Scholar] [CrossRef] [PubMed]
- Palmes, D.; Kebschull, L.; Schaefer, R.M.; Pelster, F.; Konner, K. Perforating Vein Fistula Is Superior to Forearm Fistula in Elderly Haemodialysis Patients with Diabetes and Arterial Hypertension. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2011, 26, 3309–3314. [Google Scholar] [CrossRef] [PubMed]
- Ayez, N.; van Houten, V.A.; de Smet, A.A.; van Well, A.M.; Akkersdijk, G.P.; van de Ven, P.J.; Fioole, B. The Basilic Vein and the Cephalic Vein Perform Equally in Upper Arm Arteriovenous Fistulae. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Segal, J.H.; Kayler, L.K.; Henke, P.; Merion, R.M.; Leavey, S.; Campbell, D.A. Vascular Access Outcomes Using the Transposed Basilic Vein Arteriovenous Fistula. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2003, 42, 151–157. [Google Scholar] [CrossRef]
- Wolford, H.Y.; Hsu, J.; Rhodes, J.M.; Shortell, C.K.; Davies, M.G.; Bakhru, A.; Illig, K.A. Outcome after Autogenous Brachial-Basilic Upper Arm Transpositions in the Post-National Kidney Foundation Dialysis Outcomes Quality Initiative Era. J. Vasc. Surg. 2005, 42, 951–956. [Google Scholar] [CrossRef]
- Arroyo, M.R.; Sideman, M.J.; Spergel, L.; Jennings, W.C. Primary and Staged Transposition Arteriovenous Fistulas. J. Vasc. Surg. 2008, 47, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Keuter, X.H.A.; De Smet, A.A.E.A.; Kessels, A.G.H.; van der Sande, F.M.; Welten, R.J.T.J.; Tordoir, J.H.M. A Randomized Multicenter Study of the Outcome of Brachial-Basilic Arteriovenous Fistula and Prosthetic Brachial-Antecubital Forearm Loop as Vascular Access for Hemodialysis. J. Vasc. Surg. 2008, 47, 395–401. [Google Scholar] [CrossRef]
- Field, M.; Van Dellen, D.; Mak, D.; Winter, H.; Hamsho, A.; Mellor, S.; Inston, N. The Brachiobasilic Arteriovenous Fistula: Effect of Patient Variables. J. Vasc. Access 2011, 12, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Beathard, G.A.; Arnold, P.; Jackson, J.; Litchfield, T.; Physician Operators Forum of RMS Lifeline. Aggressive Treatment of Early Fistula Failure. Kidney Int. 2003, 64, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Rothuizen, T.C.; Wong, C.; Quax, P.H.A.; van Zonneveld, A.J.; Rabelink, T.J.; Rotmans, J.I. Arteriovenous Access Failure: More than Just Intimal Hyperplasia? Nephrol. Dial. Transplant. 2013, 28, 1085–1092. [Google Scholar] [CrossRef]
- Lee, T.; Chauhan, V.; Krishnamoorthy, M.; Wang, Y.; Arend, L.; Mistry, M.J.; El-Khatib, M.; Banerjee, R.; Munda, R.; Roy-Chaudhury, P. Severe Venous Neointimal Hyperplasia Prior to Dialysis Access Surgery. Nephrol. Dial. Transplant. 2011, 26, 2264–2270. [Google Scholar] [CrossRef]
- Wali, M.A.; Eid, R.A.; Dewan, M.; Al-Homrany, M.A. Intimal Changes in the Cephalic Vein of Renal Failure Patients before Arterio-Venous Fistula (AVF) Construction. J. Smooth Muscle Res. 2003, 39, 95–105. [Google Scholar] [CrossRef]
- Wali, M.A.; Eid, R.A.; Dewan, M.; Al-Homrany, M.A. Pre-Existing Histopathological Changes in the Cephalic Vein of Renal Failure Patients before Arterio-Venous Fistula (AVF) Construction. Ann. Thorac. Cardiovasc. Surg. Off. J. Assoc. Thorac. Cardiovasc. Surg. Asia 2006, 12, 341–348. [Google Scholar]
- Tabbara, M.; Duque, J.C.; Martinez, L.; Escobar, L.A.; Wu, W.; Pan, Y.; Fernandez, N.; Velazquez, O.C.; Jaimes, E.A.; Salman, L.H.; et al. Pre-Existing and Postoperative Intimal Hyperplasia and Arteriovenous Fistula Outcomes. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2016, 68, 455–464. [Google Scholar] [CrossRef]
- Martinez, L.; Duque, J.C.; Tabbara, M.; Paez, A.; Selman, G.; Hernandez, D.R.; Sundberg, C.A.; Tey, J.C.S.; Shiu, Y.-T.; Cheung, A.K.; et al. Fibrotic Venous Remodeling and Nonmaturation of Arteriovenous Fistulas. J. Am. Soc. Nephrol. 2018, 29, 1030–1040. [Google Scholar] [CrossRef]
- Allon, M.; Robbin, M.L.; Young, C.J.; Deierhoi, M.H.; Goodman, J.; Hanaway, M.; Lockhart, M.E.; Litovsky, S. Preoperative Venous Intimal Hyperplasia, Postoperative Arteriovenous Fistula Stenosis, and Clinical Fistula Outcomes. Clin. J. Am. Soc. Nephrol. CJASN 2013, 8, 1750–1755. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Imrey, P.B.; Alpers, C.E.; Robbin, M.L.; Radeva, M.; Larive, B.; Shiu, Y.-T.; Allon, M.; Dember, L.M.; Greene, T.; et al. Intimal Hyperplasia, Stenosis, and Arteriovenous Fistula Maturation Failure in the Hemodialysis Fistula Maturation Study. J. Am. Soc. Nephrol. JASN 2017, 28, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Allon, M.; Litovsky, S.H.; Tey, J.C.S.; Sundberg, C.A.; Zhang, Y.; Chen, Z.; Fang, Y.; Cheung, A.K.; Shiu, Y.-T. Abnormalities of Vascular Histology and Collagen Fiber Configuration in Patients with Advanced Chronic Kidney Disease. J. Vasc. Access 2019, 20, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wasse, H.; Huang, R.; Naqvi, N.; Smith, E.; Wang, D.; Husain, A. Inflammation, Oxidation and Venous Neointimal Hyperplasia Precede Vascular Injury from AVF Creation in CKD Patients. J. Vasc. Access 2012, 13, 168–174. [Google Scholar] [CrossRef]
- Halmaciu, I.; Arbănași, E.M.; Kaller, R.; Mureșan, A.V.; Arbănași, E.M.; Bacalbasa, N.; Suciu, B.A.; Cojocaru, I.I.; Runcan, A.I.; Grosu, F.; et al. Chest CT Severity Score and Systemic Inflammatory Biomarkers as Predictors of the Need for Invasive Mechanical Ventilation and of COVID-19 Patients’ Mortality. Diagnostics 2022, 12, 2089. [Google Scholar] [CrossRef]
- Arbănași, E.M.; Halmaciu, I.; Kaller, R.; Mureșan, A.V.; Arbănași, E.M.; Suciu, B.A.; Coșarcă, C.M.; Cojocaru, I.I.; Melinte, R.M.; Russu, E. Systemic Inflammatory Biomarkers and Chest CT Findings as Predictors of Acute Limb Ischemia Risk, Intensive Care Unit Admission, and Mortality in COVID-19 Patients. Diagnostics 2022, 12, 2379. [Google Scholar] [CrossRef]
- Mureșan, A.V.; Hălmaciu, I.; Arbănași, E.M.; Kaller, R.; Arbănași, E.M.; Budișcă, O.A.; Melinte, R.M.; Vunvulea, V.; Filep, R.C.; Mărginean, L.; et al. Prognostic Nutritional Index, Controlling Nutritional Status (CONUT) Score, and Inflammatory Biomarkers as Predictors of Deep Vein Thrombosis, Acute Pulmonary Embolism, and Mortality in COVID-19 Patients. Diagnostics 2022, 12, 2757. [Google Scholar] [CrossRef]
- Arbănași, E.M.; Mureșan, A.V.; Coșarcă, C.M.; Kaller, R.; Bud, T.I.; Hosu, I.; Voidăzan, S.T.; Arbănași, E.M.; Russu, E. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio Impact on Predicting Outcomes in Patients with Acute Limb Ischemia. Life 2022, 12, 822. [Google Scholar] [CrossRef]
- Melinte, R.M.; Arbănași, E.M.; Blesneac, A.; Zolog, D.N.; Kaller, R.; Mureșan, A.V.; Arbănași, E.M.; Melinte, I.M.; Niculescu, R.; Russu, E. Inflammatory Biomarkers as Prognostic Factors of Acute Deep Vein Thrombosis Following the Total Knee Arthroplasty. Medicina 2022, 58, 1502. [Google Scholar] [CrossRef]
- Russu, E.; Mureșan, A.V.; Arbănași, E.M.; Kaller, R.; Hosu, I.; Voidăzan, S.; Arbănași, E.M.; Coșarcă, C.M. The Predictive Role of NLR and PLR in Outcome and Patency of Lower Limb Revascularization in Patients with Femoropopliteal Disease. J. Clin. Med. 2022, 11, 2620. [Google Scholar] [CrossRef]
- Arbănași, E.M.; Mureșan, A.V.; Arbănași, E.M.; Kaller, R.; Cojocaru, I.I.; Coșarcă, C.M.; Russu, E. The Neutrophil-to-Lymphocyte Ratio’s Predictive Utility in Acute Pulmonary Embolism: Systematic Review. J. Cardiovasc. Emerg. 2022, 8, 25–30. [Google Scholar] [CrossRef]
- Niculescu, R.; Russu, E.; Arbănași, E.M.; Kaller, R.; Arbănași, E.M.; Melinte, R.M.; Coșarcă, C.M.; Cocuz, I.G.; Sabău, A.H.; Tinca, A.C.; et al. Carotid Plaque Features and Inflammatory Biomarkers as Predictors of Restenosis and Mortality Following Carotid Endarterectomy. Int. J. Environ. Res. Public Health 2022, 19, 13934. [Google Scholar] [CrossRef] [PubMed]
- Mureșan, A.V.; Russu, E.; Arbănași, E.M.; Kaller, R.; Hosu, I.; Arbănași, E.M.; Voidăzan, S.T. The Predictive Value of NLR, MLR, and PLR in the Outcome of End-Stage Kidney Disease Patients. Biomedicines 2022, 10, 1272. [Google Scholar] [CrossRef]
- Vunvulea, V.; Budișcă, O.A.; Arbănași, E.M.; Mureșan, A.V.; Arbănași, E.M.; Brînzaniuc, K.; Niculescu, R.; Cocuz, I.G.; Ivănescu, A.D.; Hălmaciu, I.; et al. The Predictive Role of Systemic Inflammatory Markers in the Development of Acute Kidney Failure and Mortality in Patients with Abdominal Trauma. J. Pers. Med. 2022, 12, 2045. [Google Scholar] [CrossRef] [PubMed]
- Derșidan, A.A.; Ciucanu, C.C.; Ilioniu, A.M.; Bodiu, I.G.; Covalcic, C.D.; Szanto, L.A.; Mureșan, A.V. Neutrophil-to-Lymphocyte Ratio and Systemic Inflammation Index as Predictors of Poor Outcome in Patients with Critical Limb Ischemia Treated with Remote Endarterectomy. J. Cardiovasc. Emerg. 2022, 8, 67–74. [Google Scholar] [CrossRef]
- Yaprak, M.; Turan, M.N.; Dayanan, R.; Akın, S.; Değirmen, E.; Yıldırım, M.; Turgut, F. Platelet-to-Lymphocyte Ratio Predicts Mortality Better than Neutrophil-to-Lymphocyte Ratio in Hemodialysis Patients. Int. Urol. Nephrol. 2016, 48, 1343–1348. [Google Scholar] [CrossRef]
- Zhu, F.; Yao, Y.; Ci, H.; Shawuti, A. Predictive Value of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio for Primary Patency of Percutaneous Transluminal Angioplasty in Hemodialysis Arteriovenous Fistula Stenosis. Vascular 2022, 30, 920–927. [Google Scholar] [CrossRef]
- Umeres-Francia, G.; Rojas-Fernández, M.; Añazco, P.H.; Benites-Zapata, V. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as a Risk Factor for Mortality in Peruvian Adults with Chronic Kidney Disease. Ren. Replace. Ther. 2021, 8, 30. [Google Scholar] [CrossRef]
- Duan, S.; Sun, L.; Zhang, C.; Wu, L.; Nie, G.; Huang, Z.; Xing, C.; Zhang, B.; Yuan, Y. Association of Platelet-to-Lymphocyte Ratio with Kidney Clinicopathologic Features and Renal Outcomes in Patients with Diabetic Kidney Disease. Int. Immunopharmacol. 2021, 93, 107413. [Google Scholar] [CrossRef]
- Brito, G.M.C.; Fontenele, A.M.M.; Carneiro, E.C.R.L.; Nogueira, I.A.L.; Cavalcante, T.B.; Vale, A.A.M.; Monteiro, S.C.M.; Salgado Filho, N. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Nondialysis Chronic Kidney Patients. Int. J. Inflamm. 2021, 2021, e6678960. [Google Scholar] [CrossRef]
- Catabay, C.; Obi, Y.; Streja, E.; Soohoo, M.; Park, C.; Rhee, C.M.; Kovesdy, C.P.; Hamano, T.; Kalantar-Zadeh, K. Lymphocyte Cell Ratios and Mortality among Incident Hemodialysis Patients. Am. J. Nephrol. 2017, 46, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, R.; Nakayama, M.; Sakoh, T.; Fukui, A.; Katafuchi, E.; Seki, M.; Tsuda, S.; Nakano, T.; Tsuruya, K.; Kitazono, T. High Neutrophil/Lymphocyte Ratio Is Associated with Poor Renal Outcomes in Japanese Patients with Chronic Kidney Disease. Ren. Fail. 2019, 41, 238–243. [Google Scholar] [CrossRef]
- Duque, J.C.; Martinez, L.; Tabbara, M.; Parikh, P.; Paez, A.; Selman, G.; Salman, L.H.; Velazquez, O.C.; Vazquez-Padron, R.I. Vascularization of the Arteriovenous Fistula Wall and Association with Maturation Outcomes. J. Vasc. Access 2020, 21, 161–168. [Google Scholar] [CrossRef]
- Shehadeh, S.A.; Tabbara, M.; Martinez, L.; Vazquez-Padron, R.I. A Snapshot of Early Venous Remodeling in a 7-Day-Old Arteriovenous Fistula. J. Vasc. Access 2022, 11297298221091756. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, A.; Remuzzi, A.; Franzoni, M.; Misra, S. The Molecular Mechanisms of Hemodialysis Vascular Access Failure. Kidney Int. 2016, 89, 303–316. [Google Scholar] [CrossRef]
- Pasqui, E.; de Donato, G.; Lazzeri, E.; Molino, C.; Galzerano, G.; Giubbolini, M.; Palasciano, G. High Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios Are Associated with a Higher Risk of Hemodialysis Vascular Access Failure. Biomedicines 2022, 10, 2218. [Google Scholar] [CrossRef]
- Usman, R.; Jamil, M.; Naveed, M. High Preoperative Neutrophil-Lymphocyte Ratio (NLR) and Red Blood Cell Distribution Width (RDW) as Independent Predictors of Native Arteriovenous Fistula Failure. Ann. Vasc. Dis. 2017, 10, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Bozkurt, A.; Cakmak, M.; Celik, H.T.; Bilgic, M.A.; Bavbek, N.; Akcay, A. Relationship between Late Arteriovenous Fistula (AVF) Stenosis and Neutrophil-Lymphocyte Ratio (NLR) in Chronic Hemodialysis Patients. Ren. Fail. 2014, 36, 1390–1394. [Google Scholar] [CrossRef]
- Sarioglu, O.; Capar, A.E.; Belet, U. Relationship of Arteriovenous Fistula Stenosis and Thrombosis with the Platelet–Lymphocyte Ratio in Hemodialysis Patients. J. Vasc. Access 2020, 21, 630–635. [Google Scholar] [CrossRef]
- Tüysüz, M.E.; Dedemoğlu, M. Calcium Phosphate Product Level as a Predictor for Arteriovenous Fistula Re-Operations in Patients with Chronic Renal Failure. Vascular 2019, 27, 284–290. [Google Scholar] [CrossRef]
- Unver, S.; Atasoyu, E.M.; Evrenkaya, T.R. Effects of Comorbidity from AV Fistula Insufficiency on Fistula Blood Flow Rate in Hemodialysis Patients. Dial. Transplant. 2006, 35, 682–688. [Google Scholar] [CrossRef]
- Wong, C.-Y.; de Vries, M.R.; Wang, Y.; van der Vorst, J.R.; Vahrmeijer, A.L.; van Zonneveld, A.J.; Roy-Chaudhury, P.; Rabelink, T.J.; Quax, P.H.A.; Rotmans, J.I. Vascular Remodeling and Intimal Hyperplasia in a Novel Murine Model of Arteriovenous Fistula Failure. J. Vasc. Surg. 2014, 59, 192–201.e1. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhao, C.; Kilari, S.; Sharma, A.; Singh, A.K.; Simeon, M.L.; Misra, A.; Li, Y.; Misra, S. Experimental Murine Arteriovenous Fistula Model to Study Restenosis after Transluminal Angioplasty. Lab Anim. 2020, 49, 320–334. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).