Risk Factors, Management, and Avoidance of Conduction System Disease after Transcatheter Aortic Valve Replacement
Abstract
1. Introduction
2. Incidence and Risk Factors for Advanced Conduction System Disease after TAVR
2.1. Incidence of Conduction System Disease with TAVR
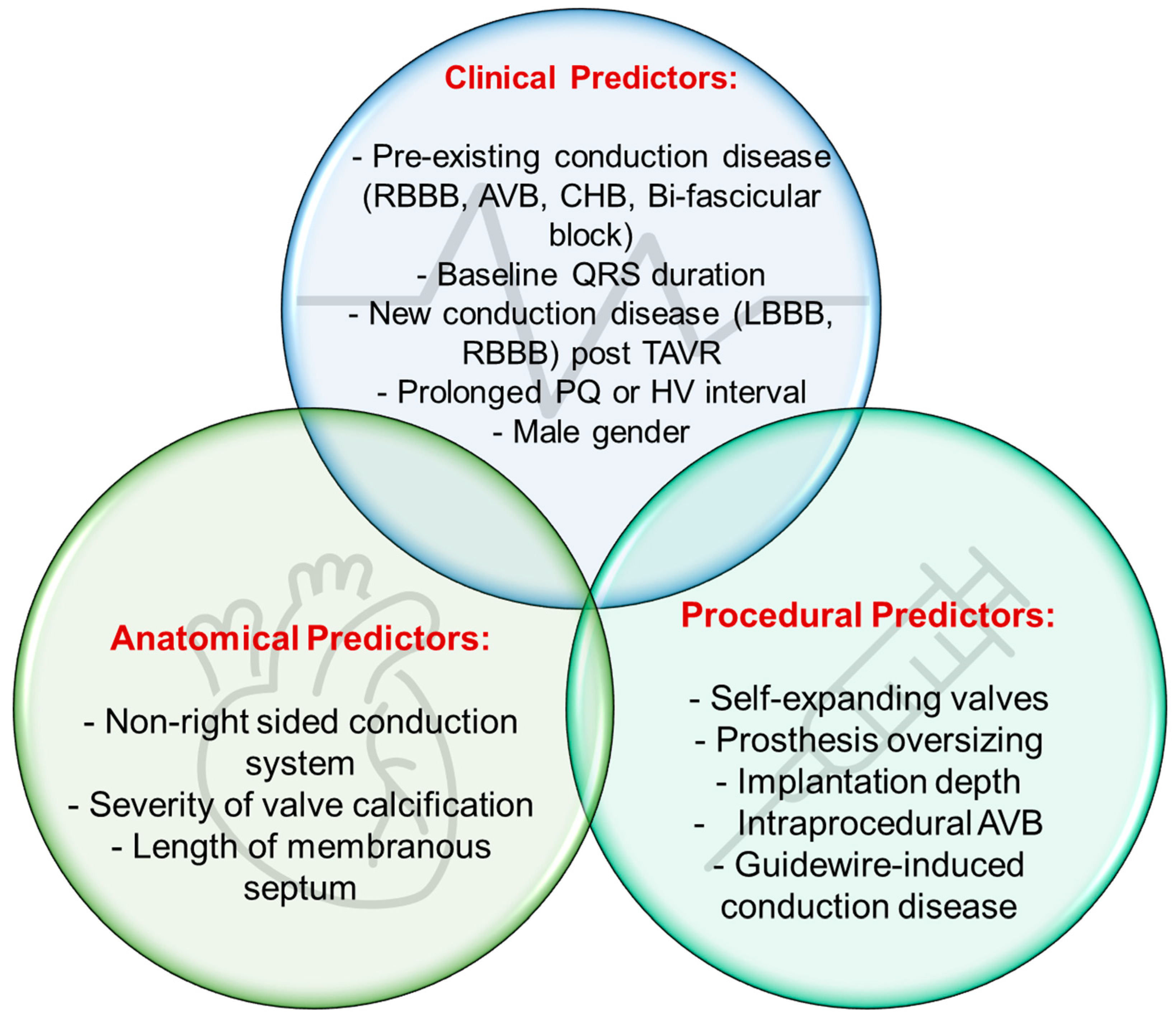
2.2. Risk Factors for Conduction System Disease
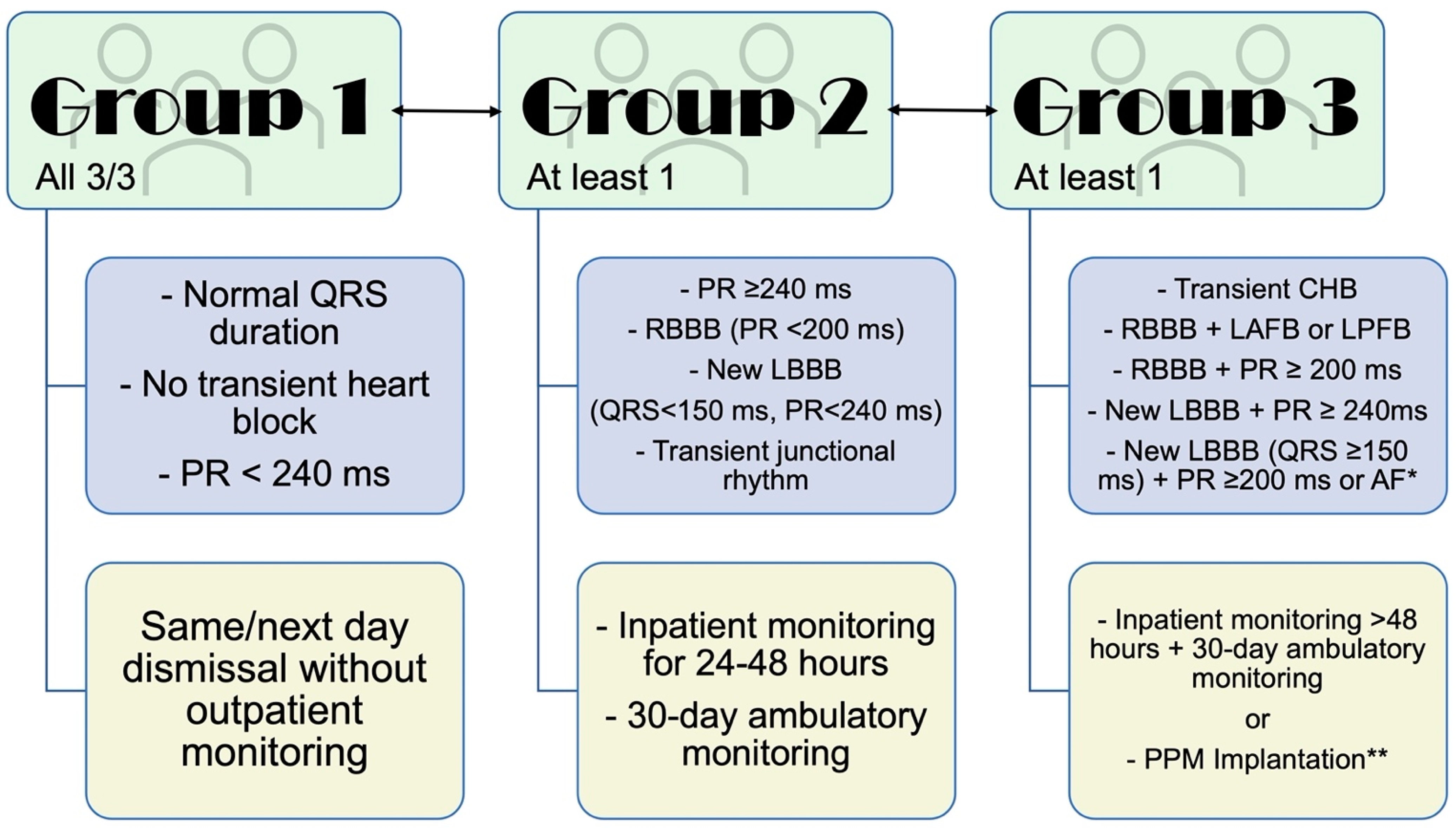
3. Prevention and Detection of Conduction System Disease after TAVR
4. Management of Conduction System Disease after TAVR
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | aortic stenosis |
| AV | atriventricular |
| BEV | balloon-expandable valves |
| CHB | complete heart block |
| HAVB | high-grade AV block |
| HBP | His bundle pacing |
| HDT | high deployment technique |
| HPCSP | His–Purkinje conduction system pacing |
| LBBB/RBBB | left/right bundle branch block |
| LPS | leadless pacemaker systems |
| NCC | non-coronary cusp |
| MEV | mechanically expandable valve |
| MS | membranous septum |
| PPM | permanent pacemaker |
| SEV | self-expanding valve |
| TAVR | transcatheter aortic valve replacement |
References
- Nathaniel, S.; Saligram, S.; Innasimuthu, A.L. Aortic stenosis: An update. World J. Cardiol. 2010, 2, 135. [Google Scholar] [CrossRef] [PubMed]
- Grube, E.; Sinning, J.M. The “Big Five” complications after transcatheter aortic valve replacement: Do we still have to be afraid of them? JACC Cardiovasc. Interv. 2019, 12, 370–372. [Google Scholar] [CrossRef] [PubMed]
- El-Sabawi, B.; Welle, G.A.; Cha, Y.M.; Espinosa, R.E.; Gulati, R.; Sandhu, G.S.; Greason, K.L.; Crestanello, J.A.; Friedman, P.A.; Munger, T.M.; et al. Temporal Incidence and Predictors of High-Grade Atrioventricular Block After Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2021, 10, e020033. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Puri, R.; Urena, M.; Chamandi, C.; Rodriguez-Gabella, T.; Philippon, F.; Rodes-Cabau, J. Conduction Disturbances After Transcatheter Aortic Valve Replacement. Circulation 2017, 136, 1049–1069. [Google Scholar] [CrossRef]
- Nuis, R.-J.; Van Mieghem, N.M.; Schultz, C.J.; Tzikas, A.; Van Der Boon, R.M.; Maugenest, A.-M.; Cheng, J.; Piazza, N.; Van Domburg, R.T.; Serruys, P.W.; et al. Timing and potential mechanisms of new conduction abnormalities during the implantation of the Medtronic CoreValve System in patients with aortic stenosis. Eur. Heart J. 2011, 32, 2067–2074. [Google Scholar] [CrossRef]
- Sammour, Y.; Krishnaswamy, A.; Kumar, A.; Puri, R.; Tarakji, K.G.; Bazarbashi, N.; Harb, S.; Griffin, B.; Svensson, L.; Wazni, O.; et al. Incidence, Predictors, and Implications of Permanent Pacemaker Requirement After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 115–134. [Google Scholar] [CrossRef]
- Xi, Z.; Liu, T.; Liang, J.; Zhou, Y.-J.; Liu, W. Impact of postprocedural permanent pacemaker implantation on clinical outcomes after transcatheter aortic valve replacement: A systematic review and meta-analysis. J. Thorac. Dis. 2019, 11, 5130–5139. [Google Scholar] [CrossRef]
- Faroux, L.; Chen, S.; Muntané-Carol, G.; Regueiro, A.; Philippon, F.; Sondergaard, L.; Jørgensen, T.H.; Lopez-Aguilera, J.; Kodali, S.; Leon, M.; et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: A systematic review and meta-analysis. Eur. Heart J. 2020, 41, 2771–2781. [Google Scholar] [CrossRef]
- Fujita, B.; Schmidt, T.; Bleiziffer, S.; Bauer, T.; Beckmann, A.; Bekeredjian, R.; Möllmann, H.; Walther, T.; Landwehr, S.; Hamm, C.; et al. Impact of new pacemaker implantation following surgical and transcatheter aortic valve replacement on 1-year outcome. Eur. J. Cardio-Thorac. Surg. 2019, 57, 151–159. [Google Scholar] [CrossRef]
- Lilly, S.M.; Deshmukh, A.J.; Epstein, A.E.; Ricciardi, M.J.; Shreenivas, S.; Velagapudi, P.; Wyman, J.F. 2020 ACC expert consensus decision pathway on management of conduction disturbances in patients undergoing transcatheter aortic valve replacement: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 2391–2411. [Google Scholar] [CrossRef]
- VARC-3 Writing Committee Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Friedman, H.S.; Zaman, Q.; Haft, J.I.; Melendez, S. Assessment of atrioventricular conduction in aortic valve disease. Heart 1978, 40, 911–917. [Google Scholar] [CrossRef]
- Marchandise, B.; Piette, F.; Chalant, C.H.; Kremer, R. Conduction disorders in aortic valve diseases. Acta Cardiol. 1975, 30, 111–128. [Google Scholar]
- Prihadi, E.A.; Leung, M.; Vollema, E.M.; Ng, A.; Marsan, N.A.; Bax, J.J.; Delgado, V. Prevalence and Prognostic Relevance of Ventricular Conduction Disturbances in Patients With Aortic Stenosis. Am. J. Cardiol. 2017, 120, 2226–2232. [Google Scholar] [CrossRef]
- Lee, M.Y.; Yeshwant, S.C.; Chava, S.; Lustgarten, D.L. Mechanisms of Heart Block after Transcatheter Aortic Valve Replacement –Cardiac Anatomy, Clinical Predictors and Mechanical Factors that Contribute to Permanent Pacemaker Implantation. Arrhythmia Electrophysiol. Rev. 2015, 4, 81–85. [Google Scholar] [CrossRef]
- Kawashima, T.; Sato, F. Visualizing anatomical evidences on atrioventricular conduction system for TAVI. Int. J. Cardiol. 2014, 174, 1–6. [Google Scholar] [CrossRef]
- Karyofillis, P.; Kostopoulou, A.; Thomopoulou, S.; Habibi, M.; Livanis, E.; Karavolias, G.; Voudris, V. Conduction abnormalities after transcatheter aortic valve implantation. J. Geriatr. Cardiol. 2018, 15, 105–112. [Google Scholar] [CrossRef]
- Tretter, J.T.; Mori, S.; Anderson, R.H.; Taylor, M.D.; Ollberding, N.; Truong, V.; Choo, J.; Kereiakes, D.; Mazur, W. Anatomical predictors of conduction damage after transcatheter implantation of the aortic valve. Open Heart 2019, 6, e000972. [Google Scholar] [CrossRef]
- Lin, S.I.; Miura, M.; Tagliari, A.P.; Lee, Y.H.; Shirai, S.; Puri, R.; Maisano, F.; Taramasso, M. Intraventricular conduction disturbances after transcatheter aortic valve implantation. Interv. Cardiol. Rev. 2020, 15, e11. [Google Scholar] [CrossRef]
- Dhakal, B.P.; Skinner, K.A.; Kumar, K.; Lotun, K.; Shetty, R.; Kazui, T.; Lee, K.; Indik, J.H. Arrhythmias in Relation to Mortality After Transcatheter Aortic Valve Replacement. Am. J. Med. 2020, 133, 1336–1342.e1. [Google Scholar] [CrossRef]
- Guo, R.; Xie, M.; Yim, W.Y.; Wu, W.; Jiang, W.; Wang, Y.; Hu, X. Dose approach matter? A meta-analysis of outcomes following transfemoral versus transapical transcatheter aortic valve replacement. BMC Cardiovasc. Disord. 2021, 21, 358. [Google Scholar] [CrossRef] [PubMed]
- Sohal, S.; Mehta, H.; Kurpad, K.; Mathai, S.V.; Tayal, R.; Visveswaran, G.K.; Wasty, N.; Waxman, S.; Cohen, M. Declining Trend of Transapical Access for Transcatheter Aortic Valve Replacement in Patients with Aortic Stenosis. J. Interv. Cardiol. 2022, 19, 5688026. [Google Scholar] [CrossRef] [PubMed]
- Murarka, S.; Lazkani, M.; Neihaus, M.; Boggess, M.; Morris, M.; Gellert, G.; Fang, H.K.; Pershad, A. Comparison of 30-Day Outcomes of Transfemoral Versus Transapical Approach for Transcatheter Aortic Valve Replacement: A Single-Center US Experience. Ann. Thorac. Surg. 2015, 99, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; McCauley, B.; Ghosalkar, D.S.; Atalay, M.; Collins, S.; Parulkar, A.; Sheikh, W.; Ahmed, M.B.; Chu, A. Aortic Valve Calcification as a Predictor of Post-Transcatheter Aortic Valve Replacement Pacemaker Dependence. Cardiol. Res. 2020, 11, 155–167. [Google Scholar] [CrossRef]
- Hokken, T.W.; van Wiechen, M.P.; Ooms, J.F.; El Azzouzi, I.; de Ronde, M.; Kardys, I.; Budde, R.; Daemen, J.; de Jaegere, P.P.; Van Mieghem, N.M. Impact of Interventricular membranous septum length on pacemaker need with different Transcatheter aortic valve implantation systems. Int. J. Cardiol. 2021, 333, 152–158. [Google Scholar] [CrossRef]
- Sá, M.P.; Eynde, J.V.D.; Jacquemyn, X.; Erten, O.; Rodriguez, R.; Goldman, S.; Coady, P.M.; Gnall, E.; Gray, W.A.; Jarrett, H.; et al. Computed tomography-derived membranous septum length as predictor of conduction abnormalities and permanent pacemaker implantation after TAVI: A meta-analysis of observational studies. Catheter. Cardiovasc. Interv. 2023, 101, 1203–1213. [Google Scholar] [CrossRef]
- Siontis, G.C.; Jüni, P.; Pilgrim, T.; Stortecky, S.; Büllesfeld, L.; Meier, B.; Wenaweser, P.; Windecker, S. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: A meta-analysis. J. Am. Coll. Cardiol. 2014, 64, 129–140. [Google Scholar] [CrossRef]
- Erkapic, D.; De Rosa, S.; Kelava, A.; Lehmann, R.; Fichtlscherer, S.; Hohnloser, S.H. Risk for Permanent Pacemaker After Transcatheter Aortic Valve Implantation: A Comprehensive Analysis of the Literature. J. Cardiovasc. Electrophysiol. 2012, 23, 391–397. [Google Scholar] [CrossRef]
- Murray, L.E.; Smith, A.H.; Flack, E.C.; Crum, K.; Owen, J.; Kannankeril, P.J. Genotypic and phenotypic predictors of complete heart block and recovery of conduction after surgical repair of congenital heart disease. Heart Rhythm. 2017, 14, 402–409. [Google Scholar] [CrossRef]
- Bleiziffer, S.; Ruge, H.; Hörer, J.; Hutter, A.; Geisbüsch, S.; Brockmann, G.; Mazzitelli, D.; Bauernschmitt, R.; Lange, R. Predictors for New-Onset Complete Heart Block After Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2010, 3, 524–530. [Google Scholar] [CrossRef]
- Nazif, T.M.; Dizon, J.M.; Hahn, R.T.; Xu, K.E.; Babaliaros, V.; Douglas, P.S.; El-Chami, M.F.; Herrmann, H.C.; Mack, M.; Makkar, R.R.; et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: The PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc. Interv. 2015, 8, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Sammour, Y.; Banerjee, K.; Kumar, A.; Lak, H.; Chawla, S.; Incognito, C.; Patel, J.; Kaur, M.; Abdelfattah, O.; Svensson, L.G.; et al. Systematic Approach to High Implantation of SAPIEN-3 Valve Achieves a Lower Rate of Conduction Abnormalities Including Pacemaker Implantation. Circ. Cardiovasc. Interv. 2021, 14, e009407. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.; Lambert, T.; Kellermair, J.; Blessberger, H.; Fellner, A.; Nahler, A.; Grund, M.; Steinwender, C. Delayed Total Atrioventricular Block After Transcatheter Aortic Valve Replacement Assessed by Implantable Loop Recorders. JACC Cardiovasc. Interv. 2021, 14, 2723–2732. [Google Scholar] [CrossRef]
- Muntané-Carol, G.; Okoh, A.K.; Chen, C.; Nault, I.; Kassotis, J.; Mohammadi, S.; Coromilas, J.; Lee, L.Y.; Alperi, A.; Philippon, F.; et al. Ambulatory Electrocardiographic Monitoring Following Minimalist Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2021, 14, 2711–2722. [Google Scholar] [CrossRef] [PubMed]
- Agasthi, P.; Ashraf, H.; Pujari, S.H.; Girardo, M.; Tseng, A.; Mookadam, F.; Venepally, N.; Buras, M.R.; Abraham, B.; Khetarpal, B.K.; et al. Prediction of permanent pacemaker implantation after transcatheter aortic valve replacement: The role of machine learning. World J. Cardiol. 2023, 15, 95–105. [Google Scholar] [CrossRef]
- Nadeem, F.; Tsushima, T.; Ladas, T.P.; Thomas, R.B.; Patel, S.M.; Saric, P.; Patel, T.; Lipinski, J.; Li, J.; Costa, M.A.; et al. Impact of Right Ventricular Pacing in Patients Who Underwent Implantation of Permanent Pacemaker After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018, 122, 1712–1717. [Google Scholar] [CrossRef]
- Tsushima, T.; Al-Kindi, S.; Dallan, L.A.P.; Fares, A.; Yoon, S.-H.; Wheat, H.L.; Attizzani, G.F.; Baeza, C.R.; Pelletier, M.P.; Arruda, M.S.; et al. Clinical impact of right ventricular pacing burden in patients with post-transcatheter aortic valve replacement permanent pacemaker implantation. Europace 2023, 25, 1441–1450. [Google Scholar] [CrossRef]
- Chang, S.; Liu, X.; Lu, Z.-N.; Yao, J.; Yin, C.; Wu, W.; Yuan, F.; Luo, T.; Liu, R.; Yan, Y.; et al. Feasibility study of temporary permanent pacemaker in patients with conduction block after TAVR. Front. Cardiovasc. Med. 2023, 10, 978394. [Google Scholar] [CrossRef]
- Krishnaswamy, A.; Sammour, Y.; Mangieri, A.; Kadri, A.; Karrthik, A.; Banerjee, K.; Kaur, M.; Giannini, F.; Pagliaro, B.; Ancona, M.; et al. The Utility of Rapid Atrial Pacing Immediately Post-TAVR to Predict the Need for Pacemaker Implantation. JACC Cardiovasc. Interv. 2020, 13, 1046–1054. [Google Scholar] [CrossRef]
- Larkin, H.D. Dissolving “Smart” Pacemaker Design for Temporary Heart Pacing. JAMA 2022, 328, 122. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Cano, Ó.; Koruth, J.S.; Subzposh, F.A.; Nanda, S.; Pugliese, J.; Ravi, V.; Naperkowski, A.; Sharma, P.S. His-Purkinje conduction system pacing following transcatheter aortic valve replacement: Feasibility and safety. Clin. Electrophysiol. 2020, 6, 649–657. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Subzposh, F.A.; Beer, D.; Durr, B.; Naperkowski, A.; Sun, H.; Oren, J.W.; Dandamudi, G.; Vijayaraman, P. Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing. J. Am. Coll. Cardiol. 2018, 71, 2319–2330. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Naperkowski, A.; Subzposh, F.A.; Abdelrahman, M.; Sharma, P.S.; Oren, J.W.; Dandamudi, G.; Ellenbogen, K.A. Permanent His-bundle pacing: Long-term lead performance and clinical outcomes. Heart Rhythm. 2018, 15, 696–702. [Google Scholar] [CrossRef]
- Sharma, P.S.; Subzposh, F.A.; Ellenbogen, K.A.; Vijayaraman, P. Permanent His-bundle pacing in patients with prosthetic cardiac valves. Heart Rhythm. 2017, 14, 59–64. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Al-Samadi, F.; Clementy, N.; Garweg, C.; Martinez-Sande, J.L.; Piccini, J.P.; Iacopino, S.; Lloyd, M.; Prat, X.V.; Jacobsen, M.D.; et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: A comparison to the investigational study and a transvenous historical control. Heart Rhythm. 2018, 15, 1800–1807. [Google Scholar] [CrossRef]
- Ngo, L.; Nour, D.; Denman, R.A.; Walters, T.E.; Haqqani, H.M.; Woodman, R.J.; Ranasinghe, I. Safety and Efficacy of Leadless Pacemakers: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e019212. [Google Scholar] [CrossRef]
- Garweg, C.; Vandenberk, B.; Foulon, S.; Poels, P.; Haemers, P.; Ector, J.; Willems, R. Leadless pacemaker for patients following cardiac valve intervention. Arch. Cardiovasc. Dis. 2020, 113, 772–779. [Google Scholar] [CrossRef]
- Mechulan, A.; Prevot, S.; Peret, A.; Nait-Saidi, L.; Miliani, I.; Leong-Feng, L.; Leude-Vaillant, E.; Vaillant, A.; Cornen, A.; Latiere, B.; et al. Micra AV leadless pacemaker implantation after transcatheter aortic valve implantation. Pacing Clin. Electrophysiol. 2022, 45, 1310–1315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabdaljabar, M.S.; Eleid, M.F. Risk Factors, Management, and Avoidance of Conduction System Disease after Transcatheter Aortic Valve Replacement. J. Clin. Med. 2023, 12, 4405. https://doi.org/10.3390/jcm12134405
Alabdaljabar MS, Eleid MF. Risk Factors, Management, and Avoidance of Conduction System Disease after Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine. 2023; 12(13):4405. https://doi.org/10.3390/jcm12134405
Chicago/Turabian StyleAlabdaljabar, Mohamad S., and Mackram F. Eleid. 2023. "Risk Factors, Management, and Avoidance of Conduction System Disease after Transcatheter Aortic Valve Replacement" Journal of Clinical Medicine 12, no. 13: 4405. https://doi.org/10.3390/jcm12134405
APA StyleAlabdaljabar, M. S., & Eleid, M. F. (2023). Risk Factors, Management, and Avoidance of Conduction System Disease after Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine, 12(13), 4405. https://doi.org/10.3390/jcm12134405




