Comparison of Periprocedural and Intermediate-Term Outcomes of TAVI in Patients with Ejection Fraction ≤ 20% vs. Patients with 20% < EF ≤ 40%
Abstract
1. Introduction
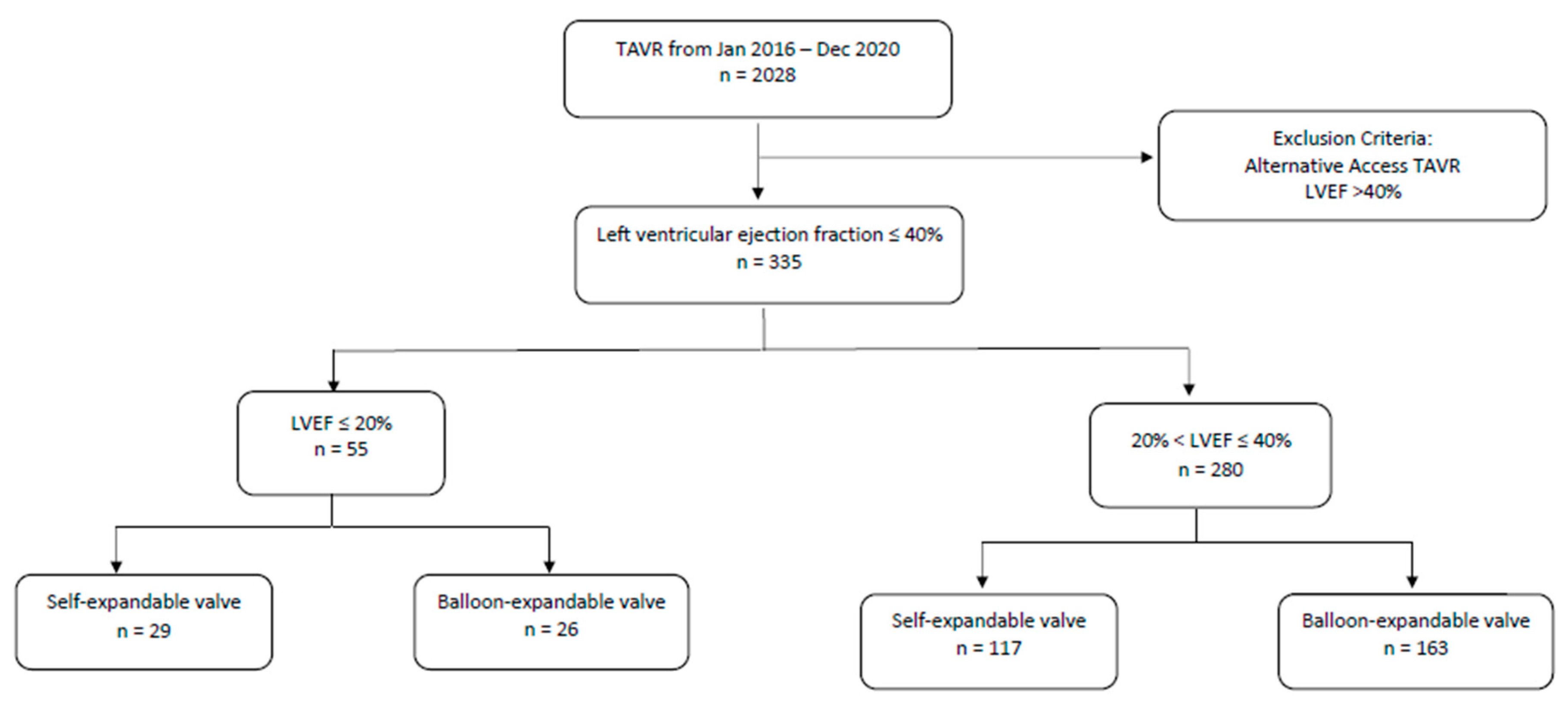
2. Methods
2.1. TAVR Procedure
2.2. Baseline Characteristics
2.3. Endpoints
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Peri-Procedural Outcomes
3.3. Clinical Outcomes at One Month and One Year
3.4. Subgroup Analysis within VLEF Group (Table 4)
4. Discussion
4.1. TAVR in Low EF vs. Very Low EF
4.2. Choice of THV among Patients with Very Low EF
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ribeiro, H.B.; Lerakis, S.; Gilard, M.; Cavalcante, J.L.; Makkar, R.; Herrmann, H.C.; Windecker, S.; Enriquez-Sarano, M.; Cheema, A.N.; Nombela-Franco, L. Outcomes From Transcatheter Aortic Valve Replacement in Patients with Low-Flow, Low-Gradient Aortic Stenosis and Left Ventricular Ejection Fraction Less Than 30%: A Substudy From the TOPAS-TAVI Registry. JAMA Cardiol. 2019, 4, 64–70. [Google Scholar]
- Baron, S.J.; Arnold, S.V.; Herrmann, H.C.; Holmes, D.R.; Szeto, W.Y.; Allen, K.B.; Chhatriwalla, A.K.; Vemulapali, S.; O’Brien, S.; Dai, D.; et al. Impact of Ejection Fraction and Aortic Valve Gradient on Outcomes of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, E.; Van Mieghem, N.M.; Pibarot, P.; Hahn, R.T.; Kodali, S.; Maurer, M.S.; Nazif, T.M.; Rodés-Cabau, J.; Paradis, J.-M.; Nazif, T.M.; et al. Rationale and design of the Transcatheter Aortic Valve Replacement to UNload the Left ventricle in patients with ADvanced heart failure (TAVR UNLOAD) trial. Am. Heart J. 2016, 182, 80–88. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032, Erratum in Circulation 2022, 145, e1033; Erratum in Circulation 2022, 146, e185. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Rasokat, U.; Renker, M.; Liebetrau, C.; van Linden, A.; Arsalan, M.; Weferling, M.; Rolf, A.; Doss, M.; Möllmann, H.; Walther, T.; et al. PARTNER Investigators. 1-Year Survival After TAVR of Patients with Low-Flow, Low-Gradient and High-Gradient Aortic Valve Stenosis in Matched Study Populations. JACC Cardiovasc. Interv. 2019, 12, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Kliger, C.; Pirelli, L.; Kodra, A.; Wang, D.; Singh, P.; Arnone, P.; Patel, A.; Liu, S.; Mihelis, E.; et al. Transcatheter heart valve selection in patients with low ejection fraction and aortic stenosis. J. Card. Surg. 2022, 37, 4937–4943. [Google Scholar] [CrossRef] [PubMed]
- Elmariah, S.; Palacios, I.F.; McAndrew, T.; Hueter, I.; Inglessis, I.; Baker, J.N.; Kodali, S.; Leon, M.B.; Svensson, L.; Pibarot, P.; et al. Outcomes of transcatheter and surgical aortic valve replacement in high-risk patients with aortic stenosis and left ventricular dysfunction: Results from the Placement of Aortic Transcatheter Valves (PARTNER) trial (cohort A). Circ. Cardiovasc. Interv. 2013, 6, 604–614. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; Writing Committee Members; et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef] [PubMed]
- Dauerman, H.L.; Reardon, M.J.; Popma, J.J.; Little, S.H.; Cavalcante, J.L.; Adams, D.H.; Kleiman, N.S.; Oh, J.K. Early recovery of left ventricular systolic function after CoreValve transcatheter aortic valve replacement. Circ. Cardiovasc. Interv. 2016, 9, e003425. [Google Scholar] [CrossRef] [PubMed]
- Deste, W.; Gulino, S.; Zappulla, P.; Iacono, F.; Sicuso, R.; Indelicato, A.; Monte, P.I.; Rapisarda, G.; Trovato, D.; Cirasa, A.; et al. Early recovery of left ventricular systolic function after transcatheter aortic valve implantation. J. Cardiovasc. Echogr. 2018, 28, 166–170. [Google Scholar] [PubMed]
- Kolte, D.; Bhardwaj, B.; Lu, M.; Alu, M.C.; Passeri, J.J.; Inglessis, I.; Vlahakes, G.J.; Garcia, S.; Cohen., D.J.; Lindman, B.R.; et al. Association between Early Left Ventricular Ejection Fraction Improvement After Transcatheter Aortic Valve Replacement and 5-Year Clinical Outcomes. JAMA Cardiol. 2022, 7, 934–944. [Google Scholar] [CrossRef]
- Merdler, I.; Loewenstein, I.; Hochstadt, A.; Morgan, S.; Schwarzbard, S.; Sadeh, B.; Peri, Y.; Shacham, Y.; Finkelstein, A.; Steinvil, A. Effectiveness and Safety of Transcatheter Aortic Valve Implantation in Patients with Aortic Stenosis and Variable Ejection Fractions (<40%, 40%–49%, and >50%). Am. J. Cardiol. 2020, 125, 583–588. [Google Scholar]
- Sharobeem, S.; Boulmier, D.; Leurent, G.; Bedossa, M.; Leclercq, C.; Mabo, P.; Martins, R.P.; Tomasi, J.; Verhoye, J.-P.; Donal, E.; et al. Prognostic impact of permanent pacemaker implantation after transcatheter aortic valve replacement. Heart Rhythm. 2022, 19, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | EF ≤ 20% | 20% < EF ≤ 40% | p-Value |
|---|---|---|---|
| Age | 79.8 ± 9.6 | 81.1 ± 8.5 | 0.33 |
| Female (%) | 22/55 (40) | 85/280 (30%) | 0.21 |
| Prior CIED/PPM (%) | 22/55 (40) | 80/280 (29%) | 0.11 |
| Prior PCI/CABG (%) | 23/55 (42) | 161/280 (57%) | 0.04 |
| Other cardiac surgery (%) | 9/55 (16) | 56/280 (20%) | 0.71 |
| DM (%) | 21/55 (38) | 112/280 (40%) | 0.88 |
| PVD (%) | 18/55 (33) | 71/280 (25%) | 0.32 |
| Baseline Cr | 1.3 ± 0.7 | 1.6 ± 1.4 | 0.1 |
| Baseline Mg | 33 ± 13 | 35 ± 14 | 0.29 |
| Baseline KCCQ | 39 ± 24 | 47 ± 24 | 0.02 |
| STS | 11.0 ± 7.9 | 9.3 ± 7.3 | 0.12 |
| Smoking (%) | 3/55 (5) | 12/280 (4%) | 0.72 |
| Baseline hemoglobin | 11.8 ± 1.7 | 11.8 ± 1.8 | 0.79 |
| Prior stroke (%) | 1/55 (2) | 28/280 (10%) | 0.06 |
| Corevalve (%) | 29/55 (53) | 117/280 (42%) | 0.14 |
| Baseline NYHA III/IV (%) | 53/55 (96) | 236/280 (84%) | 0.02 |
| Baseline NYHA I/II) (%) | 2/55 (3.6%) | 44/280 (16%) | 0.02 |
| Baseline peak gradient (mmHg) | 54 ± 20 | 59 ± 22 | 0.18 |
| Baseline AVA (cm2) | 0.63 ± 0.17 | 0.69 ± 0.25 | 0.06 |
| Mean AV gradient (mmHg) | 31.621 ± 12.588 | 35.214 ± 14.225 | 0.67 |
| EF ≤ 20 | 20 < EF ≤ 40 | p-Value | |
|---|---|---|---|
| Primary composite endpoint | 14/55 (24%) | 55/280 (20%) | 0.47 |
| Discharge deceased (%) | 1/55 (2%) | 5/280 (2%) | 0.99 |
| ≥Moderate PVL (%) | 1/55 (2%) | 14/280 (5%) | 0.48 |
| Stroke (%) | 1/55 (2%) | 4/280 (1%) | 0.59 |
| Conversion to surgery | 1/55 (2%) | 0/280 (0%) | 0.16 |
| Aortic re-intervention | 1/55 (2%) | 1/280 (<1%) | 0.30 |
| Need for PPM (%) | 10/55 (18%) | 48/280 (17%) | 0.85 |
| IABP use (%) | 5/55 (9%) | 2/280 (1%) | <0.01 |
| ECMO | 1/55 (2%) | 0/280 (0%) | 0.16 |
| Transfusion (%) | 21/55 (38%) | 59/280 (21%) | <0.01 |
| Use of 2+ pressors intraop. | 22/55 (40%) | 60/280 (21%) | <0.01 |
| Total LOS | 6 ± 3 Days | 3 ± 2 Days | <0.01 |
| EF ≤ 20 | 20 < EF ≤ 40 | p-Value | |
|---|---|---|---|
| Mortality at 1 month | 5/55 (9%) | 13/280 (5%) | 0.19 |
| Mean AVG at 1 month | 11 ± 10, n = 45 | 9 ± 5, n = 224 | 0.07 |
| ≥Moderate PVL at 1 month | 2/49 (4%) | 14/220 (6%) | 0.74 |
| 1M LVEF (%) | 30% ± 13, n =49 | 40.2% ± 14, n = 220 | <0.01 |
| 1M average change in EF (%) | 10% | 9% | 0.89 |
| KCCQ score at 1 month | 67 ± 24, n = 34 | 69 ± 26, n = 168 | 0.74 |
| NYHA III/IV at 1 month | 3/52 (6%) | 34/257 (13%) | 0.54 |
| Rehospitalization at 1M | 13/52 (25%) | 51/257 (20%) | 0.74 |
| Mortality at 1 year | 7/55 (13%) | 30/280 (11%) | 0.64 |
| Mean AVG at 1 year | 12 ± 9, n = 22 | 11 ± 8, n = 122 | 0.48 |
| ≥Moderate PVL at 1 year | 3/24 (12%) | 3/116 (5%) | 0.18 |
| KCCQ score at 1 year | 77.54 ± 20.43 (n = 18) | 74.97 ± 23.58 (n= 127) | 0.56 |
| NYHA III/IV classification at 1 year | 4/18 (22%) | 9/127 (7%) | 0.06 |
| Characteristic | Self-Expandable Valve (SEV) | Balloon-Expandable Valve (BEV) | p-Value |
|---|---|---|---|
| Type of valve placed during TAVR | 29/55 (53%) | 26/55 (47%) | |
| Corevalve: 4 | Sapien XT: 3 | ||
| Evolut R: 18 | Sapien 3: 23 | ||
| Evolut Pro: 6 | |||
| Evolut Pro+: 1 | |||
| Intra-aortic balloon pump (IABP) | 3/29 (10%) | 0/26 (0%) | 0.24 |
| Permanent pacemaker (PPM) after TAVR | 5/29 (17%) | 5/26 (19%) | 0.99 |
| Extracorporeal membrane oxygenation (ECMO) | 1/29 (3%) | 0/26 (0%) | 0.99 |
| Pre-valve balloon aortic valvuloplasty (BAV) | 9/29 (31%) | 10/26 (38%) | 0.58 |
| Post-valve balloon aortic valvuloplasty (BAV) | 8/29 (28%) | 3/26 (12%) | 0.18 |
| Hypotension during TAVR | 6/29 (21%) | 3/26 (12%) | 0.47 |
| Vasopressor support during TAVR | 8/29 (28%) | 5/26 (19%) | 0.54 |
| Stroke | 1/29 (3%) | 1/26 (4%) | 0.99 |
| ≥Moderate paravalvular leak (PVL) | 2/29 (7%) | 1/29 (4%) | 0.99 |
| Length of stay (LOS), days | 9 ± 3 | 5.5 ± 2 | 0.03 |
| Alive at time of discharge | 29/29 (100%) | 25/26 (96%) | 0.47 |
| Alive at 1 month post discharge | 26/29 (93%) | 23/26 (88%) | 0.99 |
| Average LVEF at 1 month post discharge (%) | 29 ± 9 (n = 22) | 31 ± 17 (n = 21) | 0.62 |
| Change in LVEF at 1 month (%) | 11 (5, 20) | 7.5 (0, 22) | 0.53 |
| Rehospitalization within 30 days of discharge | 6/25 (46%) | 7/27 (54%) | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodra, A.; Cinelli, M.; Alexander, R.; Hamfreth, R.; Wang, D.; Thampi, S.; Basman, C.; Kliger, C.; Scheinerman, J.; Pirelli, L. Comparison of Periprocedural and Intermediate-Term Outcomes of TAVI in Patients with Ejection Fraction ≤ 20% vs. Patients with 20% < EF ≤ 40%. J. Clin. Med. 2023, 12, 2390. https://doi.org/10.3390/jcm12062390
Kodra A, Cinelli M, Alexander R, Hamfreth R, Wang D, Thampi S, Basman C, Kliger C, Scheinerman J, Pirelli L. Comparison of Periprocedural and Intermediate-Term Outcomes of TAVI in Patients with Ejection Fraction ≤ 20% vs. Patients with 20% < EF ≤ 40%. Journal of Clinical Medicine. 2023; 12(6):2390. https://doi.org/10.3390/jcm12062390
Chicago/Turabian StyleKodra, Arber, Michael Cinelli, Renita Alexander, Rahming Hamfreth, Denny Wang, Shankar Thampi, Craig Basman, Chad Kliger, Jacob Scheinerman, and Luigi Pirelli. 2023. "Comparison of Periprocedural and Intermediate-Term Outcomes of TAVI in Patients with Ejection Fraction ≤ 20% vs. Patients with 20% < EF ≤ 40%" Journal of Clinical Medicine 12, no. 6: 2390. https://doi.org/10.3390/jcm12062390
APA StyleKodra, A., Cinelli, M., Alexander, R., Hamfreth, R., Wang, D., Thampi, S., Basman, C., Kliger, C., Scheinerman, J., & Pirelli, L. (2023). Comparison of Periprocedural and Intermediate-Term Outcomes of TAVI in Patients with Ejection Fraction ≤ 20% vs. Patients with 20% < EF ≤ 40%. Journal of Clinical Medicine, 12(6), 2390. https://doi.org/10.3390/jcm12062390




