Comparative Investigation of Anti-Inflammatory Effect of Platelet-Rich Fibrin after Mandibular Wisdom Tooth Surgery: A Randomized Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Operations
2.2. PRF Preparation
2.3. Obtaining Edema, Pain Trismus Levels
2.4. Obtaining Serum Marker Data
2.5. Statistical Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rizqiawan, A.; Lesmaya, Y.D.; Rasyida, A.Z.; Amir, M.S.; Ono, S.; Kamadjaja, D.B. Postoperative Complications of Impacted Mandibular Third Molar Extraction Related to Patient’s Age and Surgical Difficulty Level: A Cross-Sectional Retrospective Study. Int. J. Dent. 2022, 7239339. [Google Scholar] [CrossRef] [PubMed]
- Bouloux, G.F.; Steed, M.B.; Perciaccante, V.J. Complications of third molar surgery. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Qiu, Y.; Yang, C.; Yang, J.; Chen, M.; Zhang, Z. Piezoelectric Versus Conventional Rotary Techniques for Impacted Third Molar Extraction: A Meta-analysis of Randomized Controlled Trials. Medicine 2015, 94, e1685. [Google Scholar] [CrossRef] [PubMed]
- Mojsa, I.M.; Pokrowiecki, R.; Lipczynski, K.; Czerwonka, D.; Szczeklik, K.; Zaleska, M. Effect of submucosal dexamethasone injection on postoperative pain, edema, and trismus following mandibular third molar surgery: A prospective, randomized, double-blind clinical trial. Int. J. Oral Maxillofac. Surg. 2017, 46, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Landucci, A.; Wosny, A.C.; Uetanabaro, L.C.; Moro, A.; Araujo, M.R. Efficacy of a single dose of low-level laser therapy in reducing pain, swelling, and trismus following third molar extraction surgery. Int. J. Oral Maxillofac. Surg. 2016, 45, 392–398. [Google Scholar] [CrossRef]
- Esen, E.; Taşar, F.; Akhan, O. Determination of the anti-inflammatory effects of methylprednisolone on the sequelae of third molar surgery. J. Oral Maxillofac. Surg. 1999, 57, 1201–1206. [Google Scholar] [CrossRef]
- Blondeau, F.; Daniel, N.G. Extraction of impacted mandibular third molars: Postoperative complications and their risk factors. J. Can. Dent. Assoc. 2007, 73, 325. [Google Scholar]
- Stevkovska-Veleska, D. Cytokines (IL-1, TNF-α, IL-6) and Oral Surgery Interventions. Balk. J. Stomatol. 2010, 14, 124–132. [Google Scholar]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Reinhart, W.H. Erythrocyte sedimentation rate—More than an old fashion? Ther. Umsch. Rev. Ther. 2006, 63, 108–112. [Google Scholar] [CrossRef]
- Baumann, H.; Gauldie, J. The acute phase response. Immunol. Today 1994, 15, 74–80. [Google Scholar] [CrossRef]
- Eckersall, P.; Bell, R. Acute-phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet. J. 2010, 185, 23–27. [Google Scholar] [CrossRef]
- Del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: Periodontal and dentoalveolar surgery. Curr. Pharm. Biotechnol. 2012, 13, 1207–1230. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Singh, S.; Singh, R. Application of PRF in surgical management of periapical lesions. Natl. J. Maxillofac. Surg. 2013, 4, 94–99. [Google Scholar] [CrossRef]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. The opportunity in perio-implantology: The PRF. Implantodontie 2001, 42, e62. [Google Scholar]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e51–e55. [Google Scholar] [CrossRef]
- Dar, M.M.; Shah, A.A.; Najar, A.L.; Younis, M.; Kapoor, M.; Dar, J.I. Healing Potential of Platelet Rich Fibrin in Impacted Mandibular Third Molar Extraction Sockets. Ann. Maxillofac. Surg. 2018, 8, 206–213. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jiménez, P.; Corso, M.D.; Kang, B.S.; Nally, M.; Lanata, N.; Wang, H.L.; Quirynen, M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growthfactors, and fibrin architecture of a leukocyte-and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef]
- Castro, A.B.; Cortellini, S.; Temmerman, A.; Li, X.; Pinto, N.; Teughels, W.; Quirynen, M. Characterization of the Leukocyte- and Platelet-Rich Fibrin Block: Release of Growth Factors, Cellular Content, and Structure. Int. J. Oral Maxillofac. Implant. 2019, 34, 855–864. [Google Scholar] [CrossRef]
- Lei, L.; Yu, Y.; Han, J.; Shi, D.; Sun, W.; Zhang, D.; Chen, L. Quantification of growth factors in advanced platelet-rich fibrin and concentrated growth factors and their clinical efficacy as adjunctive to the GTR procedure in periodontal intrabony defects. J. Periodontol. 2020, 91, 462–472. [Google Scholar] [CrossRef]
- Lourenço, E.S.; Mourão, C.F.A.B.; Leite, P.E.C.; Granjeiro, J.M.; Calasans-Maia, M.D.; Alves, G.G. The in vitro release of cytokines and growth factors from fibrin membranes produced through horizontal centrifugation. J. Biomed. Mater. Res. A 2018, 106, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vizcaíno, C.; Dohle, E.; Al-Maawi, S.; Booms, P.; Sader, R.; Kirkpatrick, C.J.; Choukroun, J.; Ghanaati, S. Platelet-rich fibrin secretome induces three dimensional angiogenic activation in vitro. Eur. Cells Mater. 2019, 37, 250–264. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Barros Mourão, C.F.; de Mello-Machado, R.C.; Javid, K.; Moraschini, V. The use of leukocyte- and platelet-rich fibrin in the management of soft tissue healing and pain in post-extraction sockets: A randomized clinical trial. J. Cranio-Maxillofac. Surg. 2020, 48, 452–457. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed]
- Alcântara, C.E.; Falci, S.G.; Oliveira-Ferreira, F.; Santos, C.R.; Pinheiro, M.L. Pre-emptive effect of dexamethasone and methylprednisolone on pain, swelling, and trismus after third molar surgery: A split-mouth randomized triple-blind clinical trial. Int. J. Oral Maxillofac. Surg. 2014, 43, 93–98. [Google Scholar] [CrossRef]
- Al-Hamed, F.S.; Tawfik, M.A.M.; Abdelfadil, E.; Al-Saleh, M.A. Efficacy of platelet-rich fibrin after mandibular third molar extraction: A systematic review and meta-analysis. J. Oral Maxillofac. Surg. 2017, 75, 1124–1135. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef]
- Arshad, S.; Tehreem, F.; Rehab Khan, M.; Ahmed, F.; Marya, A.; Karobari, M.I. Platelet-Rich Fibrin Used in Regenerative Endodontics and Dentistry: Current Uses, Limitations, and Future Recommendations for Application. Int. J. Dent. 2021, 2021, 4514598. [Google Scholar] [CrossRef]
- Fan, Y.; Perez, K.; Dym, H. Clinical Uses of Platelet-Rich Fibrin in Oral and Maxillofacial Surgery. Dent. Clin. N. Am. 2020, 64, 291–303. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, D.W.; Ryu, D.M. Effect of platelet-rich fibrin on pain and swelling after surgical extraction of third molars. Tissue Eng. Regen. Med. 2011, 8, 80–86. [Google Scholar]
- Ozgul, O.; Senses, F.; Er, N.; Tekin, U.; Tuz, H.H.; Alkan, A.; Kocyigit, I.D.; Atil, F. Efficacy of platelet-rich fibrin in the reduction of the pain and swelling after impacted third molar surgery: Randomized multicenter split-mouth clinical trial. Head Face Med. 2015, 11, 37. [Google Scholar] [CrossRef]
- Jeyaraj, P.E.; Chakranarayan, A. Soft tissue healing and bony regeneration of impacted mandibular third molar extraction sockets, following postoperative incorporation of platelet-rich fibrin. Ann. Maxillofac. Surg. 2018, 8, 10–18. [Google Scholar] [CrossRef]
- Kumar, N.; Prasad, K.; Ramanujam, L.; Ranganath, K.; Dexith, J.; Chauhan, A. Evaluation of treatment outcome after impacted mandibular third molar surgery with the use of autologous platelet-rich fibrin: A randomized controlled clinical study. J. Oral Maxillofac. Surg. 2015, 73, 1042–1049. [Google Scholar] [CrossRef]
- Canellas, J.V.D.S.; Ritto, F.G.; Medeiros, P.J.D. Evaluation of postoperative complications after mandibular third molar surgery with the use of platelet-rich fibrin: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 1138–1146. [Google Scholar] [CrossRef]
- Graziani, F.; D’Aiuto, F.; Gennai, S.; Petrini, M.; Nisi, M.; Cirigliano, N.; Landini, L.; Bruno, R.M.; Taddei, S.; Ghiadoni, L. Systemic Inflammation after Third Molar Removal: A Case-Control Study. J. Dent. Res. 2017, 96, 1505–1512. [Google Scholar] [CrossRef]
- Freitas, A.C.D.; Pinheiro, A.; Miranda, P.; Thiers, F.A.; Vieira, A. Assessment of the anti-inflammatory effect of 830nm laser light using C-reactive protein levels. Braz. Dent. J. 2001, 12, 187–190. [Google Scholar]
- Ballou, S.; Kushner, I. Chapter 49: Laboratory Evaluation of Inflammation. In Kelly’s Textbook of Rheumatology; Harris: Idaho Falls, ID, USA, 2005; pp. 721–726. [Google Scholar]
- Osei-Bimpong, A.; Meek, J.H.; Lewis, S.M. ESR or CRP? A comparison of their clinical utility. Hematology 2007, 12, 353–357. [Google Scholar] [CrossRef]
- Shetty, L.; Gangwani, K.; Londhe, U.; Bharadwaj, S.; Bakri, M.M.H.; Alamoudi, A.; Reda, R.; Bhandi, S.; Raj, A.T.; Patil, S.; et al. Comparison of the C-Reactive Protein Level and Visual Analog Scale Scores between Piezosurgery and Rotatory Osteotomy in Mandibular Impacted Third Molar Extraction. Life 2022, 12, 923. [Google Scholar] [CrossRef]
- Nibali, L.; Fedele, S.; D’Aiuto, F.; Donos, N. Interleukin-6 in oral diseases: A review. Oral Dis. 2012, 18, 236–243. [Google Scholar] [CrossRef]
- Hall, B.E.; Zhang, L.; Sun, Z.J.; Utreras, E.; Prochazkova, M.; Cho, A.; Terse, A.; Arany, P.; Dolan, J.C.; Schmidt, B.L.; et al. Conditional TNF-alpha Overexpression in the Tooth and Alveolar Bone Results in Painful Pulpitis and Osteitis. J. Dent. Res. 2016, 95, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Sahibzada, H.A.; Khurshid, Z.; Khan, R.S.; Naseem, M.; Siddique, K.M.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6, and TNF-alpha as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, C.; Noyan, T.; Ekin, S.; Babayev, E. Serum IL-6 and CRP levels in patients with trauma involving low-extremity bone fractures. East. J. Med. 2013, 18, 176. [Google Scholar]
- Dağlı, E.; Altunkan, A.A.; Birbiçer, H.; Temel, G.O. Comparison of PCT, CRP, D-Dimer, Lactate, TNF-α, IL-1β, IL-6 and lL-10 in Development of Systemic Inflammatory Response Syndrome and Sepsis on Patients with Isolated Head Trauma and Polytrauma. J. Turk. Soc. Intens. Care 2012, 10, 117–124. [Google Scholar]


| PRF | Control | p | |
|---|---|---|---|
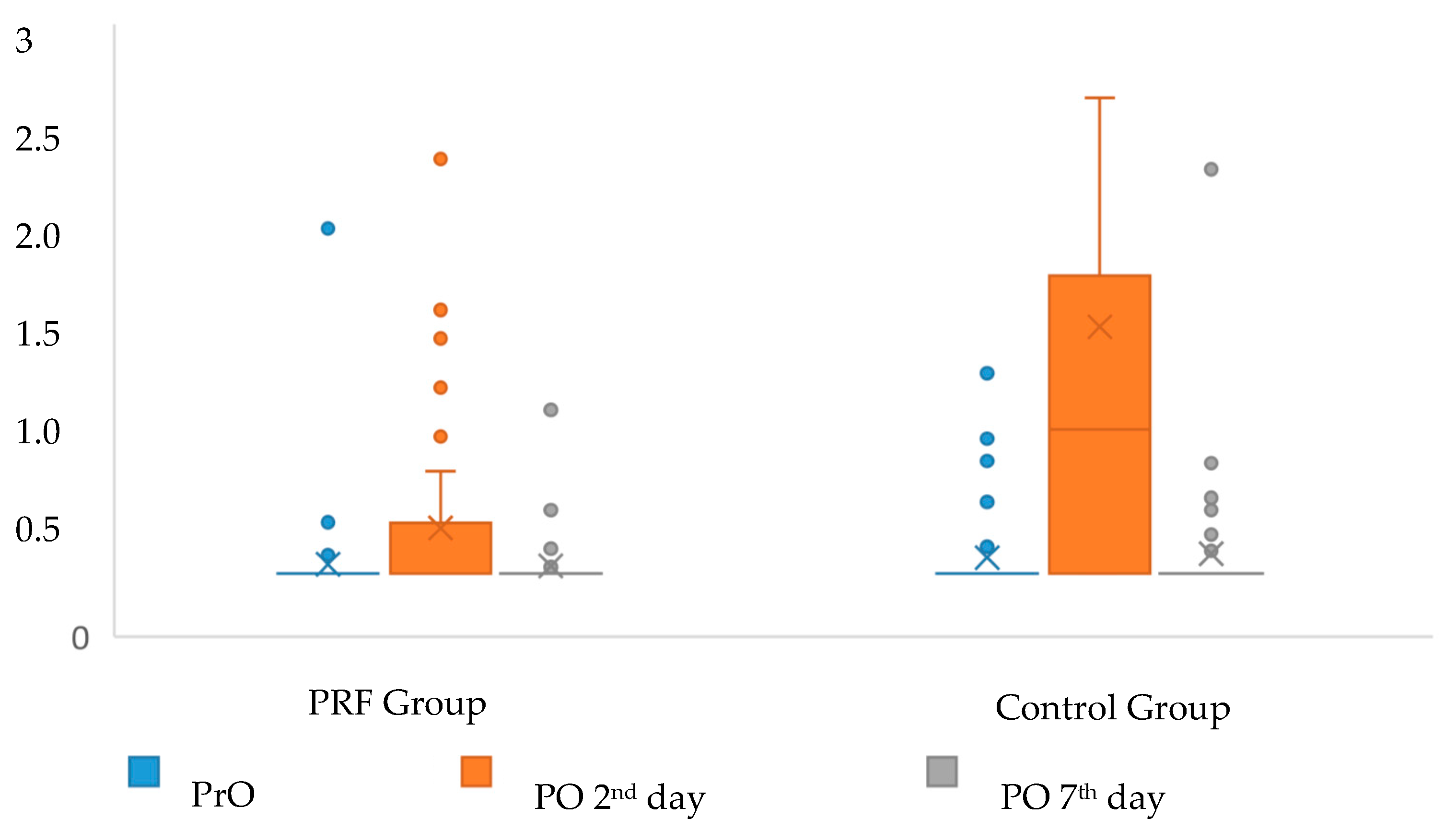
| PrO | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.000 |
| 0.0 (0.0–0.0) a | 0.0 (0.0–0.0) a | ||
| PO 2nd day | 2.88 ± 0.79 | 4.83 ± 1 | <0.001 |
| 3 (1–5) b | 5 (2–7) c | ||
| PO 7th day | 0.31 ± 0.59 | 0.71 ± 0.71 | 0.002 |
| 0 (0–2) a | 1 (0–2) d |
| LC–G | T-MC | |||||
|---|---|---|---|---|---|---|
| PRF | Control | p | PRF | Control | p | |
| PrO | 11.05 ± 1.01 | 11 ± 0.98 | 0.766 | 15.97 ± 1.49 a | 16.03 ± 1.48 a | 0.832 |
| 11 (8.8–3.2) a | 11 (8.8–13) a | 16 (12.4–18.8) | 16.05 (12.4–18.8) | |||
| PO 2nd day | 11.34 ± 1.04 | 12.19 ± 1.03 | <0.001 | 16.25 ± 1.53 b | 16.98 ± 1.5 d | 0.021 |
| 11.4 (9–13.6) b | 12 (10–14.8) c | 16.4 (13–19) | 16.9 (12.6–19.8) | |||
| PO 7th day | 11.12 ± 1.03 | 11.52 ± 0.96 | 0.026 | 16.04 ± 1.51 c | 16.45 ± 1.46 c | 0.179 |
| 11 (9–13.4) a | 11.4 (9–13.8) d | 16 (12.8–18.8) | 16.4 (12.4–19.2) | |||
| PRF | Control | p | |
|---|---|---|---|
| PrO | 4.73 ± 0.62 | 4.76 ± 0.63 | 0.777 |
| 4.65 (3.8–6.2) a | 4.8 (3.8–6.3) a | ||
| PO 2nd day | 4.09 ± 0.64 | 3.61 ± 0.69 | <0.001 |
| 4.2 (2.2–5.8) b | 3.6 (1.8–5) c | ||
| PO 7th day | 4.4 ± 0.58 | 3.86 ± 0.62 | <0.001 |
| 4.4 (3.6–5.8) d | 3.8 (2.2–5) e |
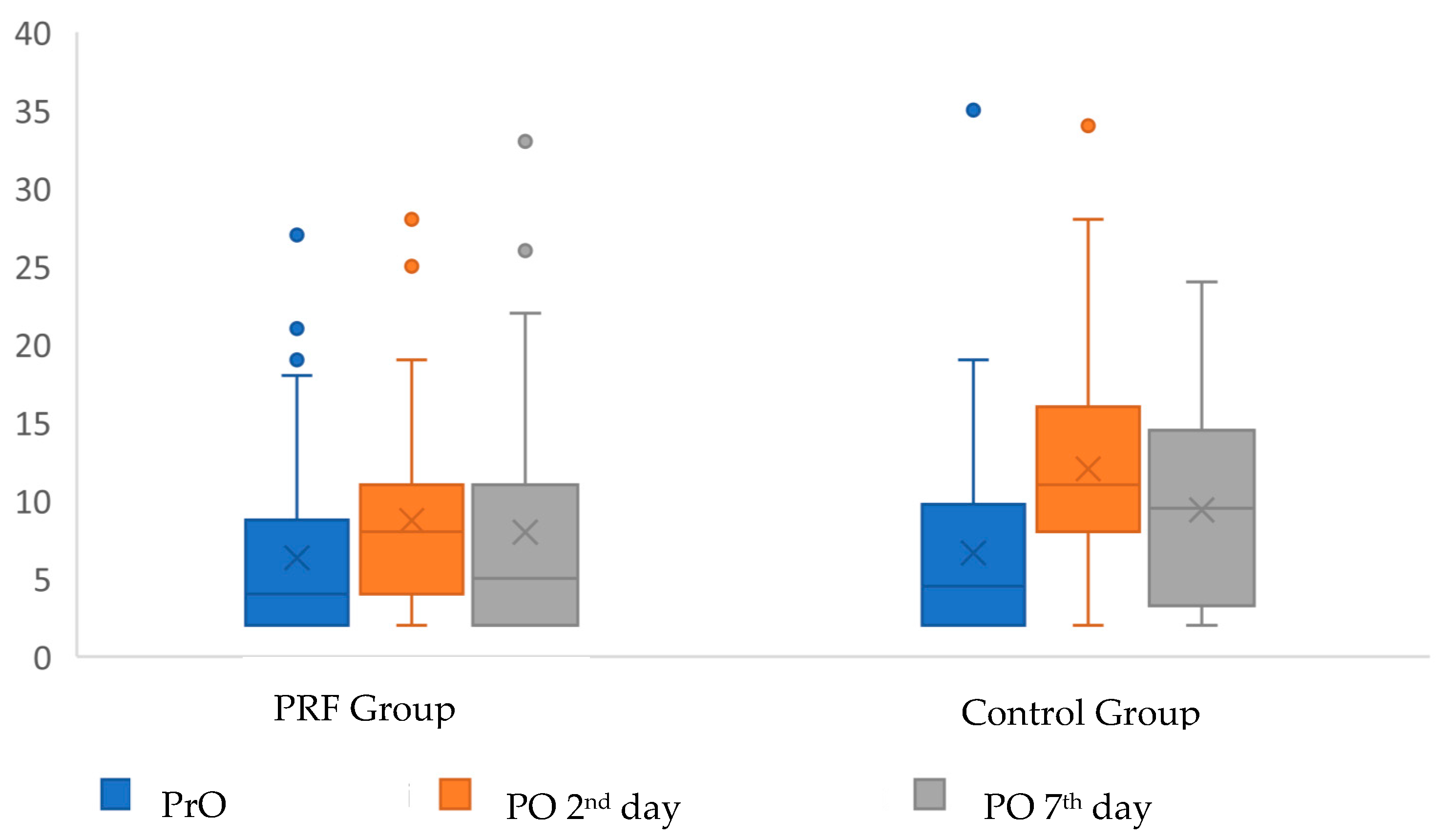
| ESR | CRP | |||||
|---|---|---|---|---|---|---|
| PRF | Control | p | PRF | Control | p | |
| PrO | 6.31 ± 5.87 | 6.63 ± 6.1 | 0.652 | 0.35 ± 0.25 | 0.39 ± 0.21 | 0.301 |
| 4 (2–7) a | 4.5 (2–35) a | 0.31 (0.31–2) a | 0.31 (0.31–1.29) a | |||
| PO 2nd day | 8.71 ± 6.54 | 12..02 ± 7.36 | 0.009 | 0.53 ± 0.45 | 1.52 ± 1.76 | <0.001 |
| 8 (2–28) b | 11 (2–34) c | 0.31 (0.31–2.34) b | 1.02 (0.31–9.44) c | |||
| PO 7th day | 7.96 ± 7.09 | 9.4 ± 6.29 | 0.158 | 0.35 ± 0.13 | 0.41 ± 0.31 | 0.345 |
| 5 (2–33) d | 9.5 (2–24) d | 0.31 (0.31–1.11) d | 0.31 (0.31–2.29) d | |||
| IL-6 | TNF-α | |||||
|---|---|---|---|---|---|---|
| PRF | Control | p | PRF | Control | p | |
| PrO | 10.04 ± 20.09 | 9.7 ± 19.28 | 0.702 | 9.78 ± 6,.4 | 6.57 ± 3.37 | 0.094 |
| 6.71 (0.95–31.08) | 6.47 (0.17–123.81) | 8.9 (3.01–30.23) | 5.46 (3.01–13.31) | |||
| PO 2nd day | 12.23 ± 28.37 | 12.27 ± 20.55 | 0.419 | 6.13 ± 3.09 | 7.73 ± 6.65 | 0.438 |
| 7.11 (0.95–187.7) | 7.43 (1.26–135.97) | 5.22 (3.01–12.33) | 5.71 (3.25–32.19) | |||
| PO 7th day | 1.99 ± 25.45 | 5.52 ± 3.6 | 0.087 | 6.81 ± 2.35 | 8.42 ± 5.1 | 0.574 |
| 6.5 (1.57–163.58) | 4.25 (1.26–15.73) | 6.81 (3.25–10.86) | 7.18 (3.01–23.86) | |||
| Group | Follow-Up | VAS | Trismus | LC–G | T–BC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |||
| PRF group | PrO | ESH | . | . | −0.387 | 0.007 | 0.407 | 0.004 | 0.447 | 0.001 |
| CRP | . | . | 0.018 | 0.903 | −0.049 | 0.739 | −0.002 | 0.990 | ||
| IL-6 | . | . | 0.245 | 0.127 | 0.353 | 0.025 | 0.245 | 0.128 | ||
| TNF-α | . | . | 0.165 | 0.451 | 0.059 | 0.790 | 0.307 | 0.154 | ||
| PO 2nd day | ESH | 0.018 | 0.903 | −0.488 | <0.001 | 0.421 | 0.003 | 0.523 | <0.001 | |
| CRP | 0.054 | 0.713 | −0.240 | 0.100 | −0.237 | 0.105 | −0.261 | 0.073 | ||
| IL-6 | −0.123 | 0.439 | −0.020 | 0.898 | 0.081 | 0.610 | −0.038 | 0.812 | ||
| TNF-α | −0.098 | 0.739 | 0.525 | 0.054 | 0.171 | 0.558 | 0.303 | 0.292 | ||
| PO 7th day | ESH | 0.091 | 0.538 | −0.348 | 0.015 | 0.461 | 0.001 | 0.485 | <0.001 | |
| CRP | −0.073 | 0.624 | 0.061 | 0.682 | −0.131 | 0.374 | −0.131 | 0.376 | ||
| IL-6 | 0.078 | 0.636 | 0.060 | 0.716 | 0.185 | 0.259 | 0.260 | 0.110 | ||
| TNF-α | 0.307 | 0.332 | 0.340 | 0.280 | 0.366 | 0.243 | 0.482 | 0.113 | ||
| Control group | PrO | ESH | . | . | −0.315 | 0.029 | 0.226 | 0.122 | 0.331 | 0.022 |
| CRP | . | . | 0.020 | 0.891 | −0.070 | 0.638 | −0.007 | 0.962 | ||
| IL-6 | . | . | 0.192 | 0.242 | 0.253 | 0.120 | 0.191 | 0.245 | ||
| TNF-α | . | . | 0.006 | 0.982 | −0.022 | 0.939 | −0.025 | 0.929 | ||
| PO 2nd day | ESH | 0.200 | 0.174 | −0.163 | 0.268 | 0.280 | 0.054 | 0.236 | 0.106 | |
| CRP | 0.034 | 0.821 | 0.034 | 0.816 | 0.102 | 0.489 | 0.117 | 0.427 | ||
| IL-6 | −0.212 | 0.173 | −0.157 | 0.316 | −0.183 | 0.241 | −0.189 | 0.225 | ||
| TNF-α | −0.337 | 0.186 | 0.020 | 0.938 | 0.185 | 0.476 | 0.146 | 0.577 | ||
| PO 7th day | ESH | −0.035 | 0.812 | −0.216 | 0.141 | 0.384 | 0.007 | 0.541 | <0.001 | |
| CRP | −0.109 | 0.461 | 0.028 | 0.850 | 0.045 | 0.760 | 0.086 | 0.563 | ||
| IL-6 | −0.061 | 0.703 | 0.055 | 0.728 | 0.238 | 0.128 | 0.084 | 0.598 | ||
| TNF-α | −0.134 | 0.634 | 0.397 | 0.142 | 0.433 | 0.107 | 0.448 | 0.094 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanan Karaca, G.; Duygu, G.; Er, N.; Ozgun, E. Comparative Investigation of Anti-Inflammatory Effect of Platelet-Rich Fibrin after Mandibular Wisdom Tooth Surgery: A Randomized Controlled Study. J. Clin. Med. 2023, 12, 4250. https://doi.org/10.3390/jcm12134250
Tanan Karaca G, Duygu G, Er N, Ozgun E. Comparative Investigation of Anti-Inflammatory Effect of Platelet-Rich Fibrin after Mandibular Wisdom Tooth Surgery: A Randomized Controlled Study. Journal of Clinical Medicine. 2023; 12(13):4250. https://doi.org/10.3390/jcm12134250
Chicago/Turabian StyleTanan Karaca, Gamze, Gonca Duygu, Nilay Er, and Eray Ozgun. 2023. "Comparative Investigation of Anti-Inflammatory Effect of Platelet-Rich Fibrin after Mandibular Wisdom Tooth Surgery: A Randomized Controlled Study" Journal of Clinical Medicine 12, no. 13: 4250. https://doi.org/10.3390/jcm12134250
APA StyleTanan Karaca, G., Duygu, G., Er, N., & Ozgun, E. (2023). Comparative Investigation of Anti-Inflammatory Effect of Platelet-Rich Fibrin after Mandibular Wisdom Tooth Surgery: A Randomized Controlled Study. Journal of Clinical Medicine, 12(13), 4250. https://doi.org/10.3390/jcm12134250






