Persistent Increase in Serum Ferritin Levels despite Converting to Permanent Vascular Access in Pediatric Hemodialysis Patients: Pediatric Nephrology Research Consortium Study
Abstract
1. Introduction
2. Methods
Statistical Methods
3. Results
3.1. Subjects
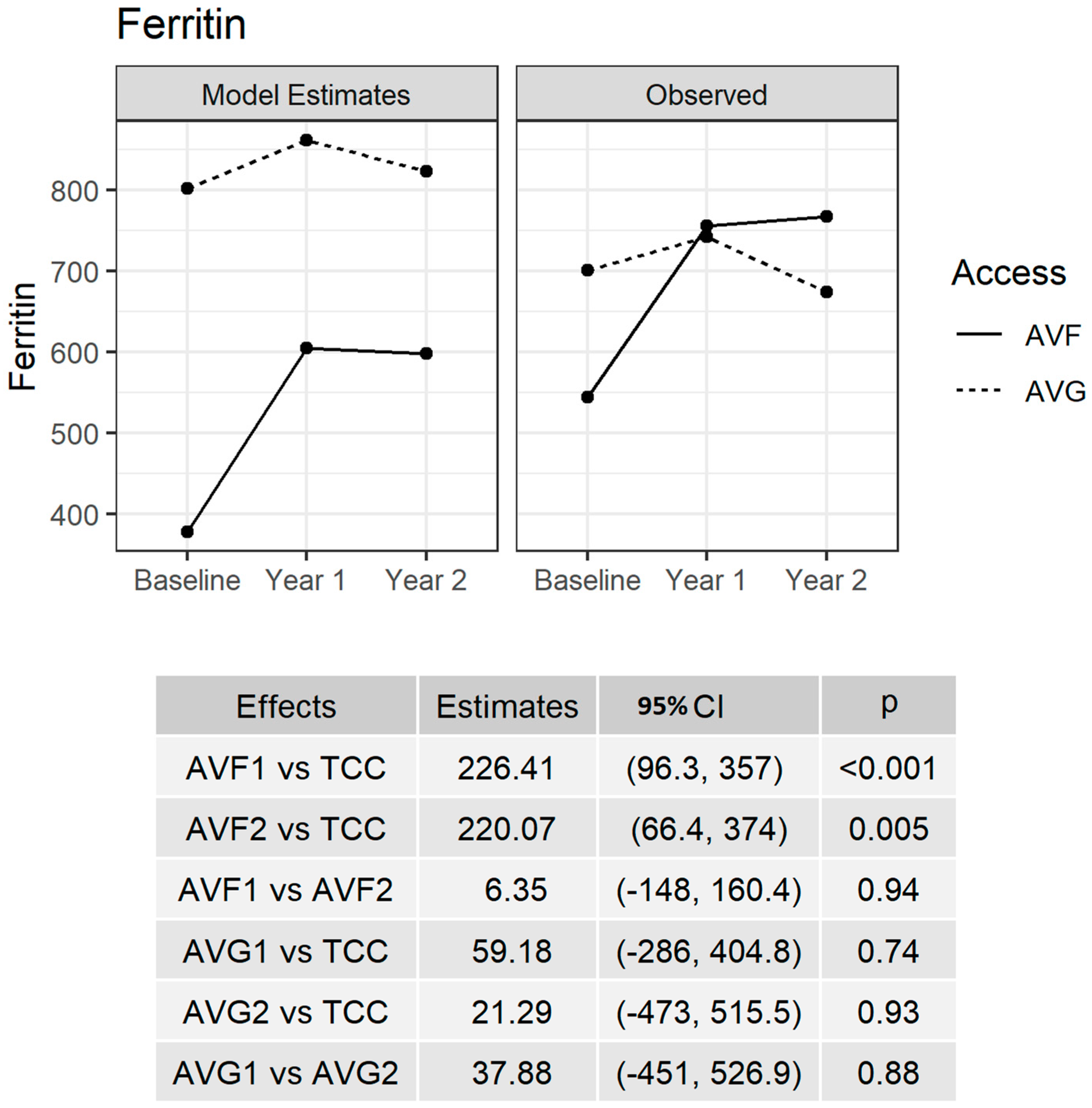
3.2. Serum Ferritin Levels
3.3. Ferritin Trajectory over Time
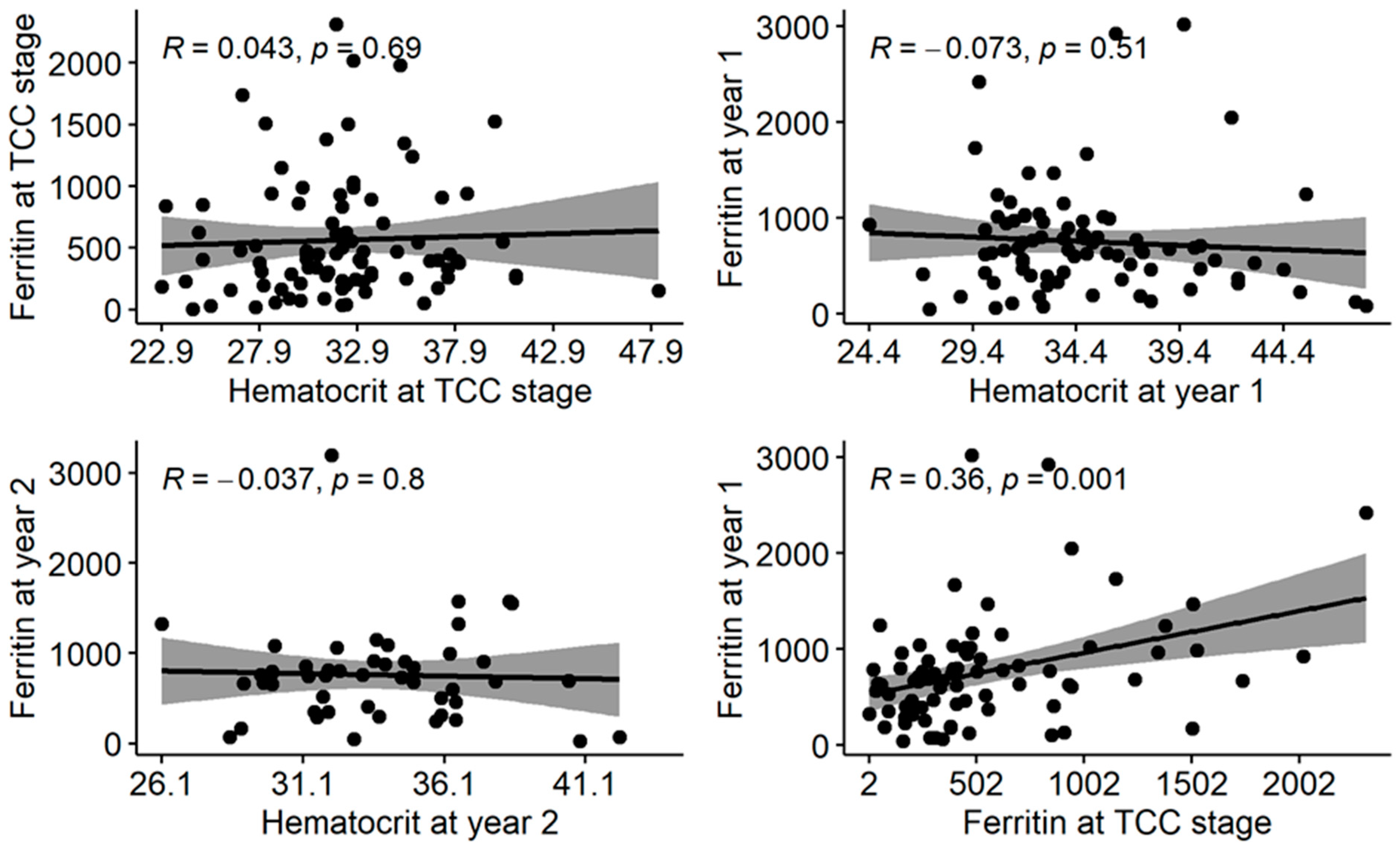
3.4. Association of Serum Ferritin Levels to Serum Hct Levels at Three Data Points
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TCC.Ferritin | Serum ferritin level at the time of PVA creation. |
| PVA1.Ferritin | Serum ferritin level at first year of PVA. |
| PVA2.Ferritin | Serum ferritin level at second year of PVA. |
| AVF1.Ferritin | Serum ferritin level at first year of AVF. |
| AVF2.Ferritin | Serum ferritin level at second year of AVF. |
| AVG1.Ferritin | Serum ferritin level at first year of AVG. |
| AVG2.Ferritin | Serum ferritin level at second year of AVG. |
References
- Warady, B.A.; Ho, M. Morbidity and mortality in children with anemia at initiation of dialysis. Pediatr. Nephrol. 2003, 18, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Furth, S.L. Anemia in children with chronic kidney disease. Nat. Rev. Nephrol. 2011, 7, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Slickers, J.; Duquette, P.; Hooper, S.; Gipson, D. Clinical predictors of neurocognitive deficits in children with chronic kidney disease. Pediatr. Nephrol. 2007, 22, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Warady, B.A.; Silverstein, D.M. Management of anemia with erythropoietic-stimulating agents in children with chronic kidney disease. Pediatr. Nephrol. 2014, 29, 1493–1505. [Google Scholar] [CrossRef]
- Crary, S.E.; Hall, K.; Buchanan, G.R. Intravenous iron sucrose for children with iron deficiency failing to respond to oral iron therapy. Pediatr. Blood Cancer 2011, 56, 615–619. [Google Scholar] [CrossRef]
- Kidney Disease Outcomes Quality Initiative. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am. J. Kidney Dis. 2006, 47 (Suppl. S3), S11–S145. [Google Scholar]
- Balasubramaniam, G.S.; Morris, M.; Gupta, A.; Mesa, I.R.; Thuraisingham, R.; Ashman, N. Allosensitization rate of male patients awaiting first kidney grafts after leuko-depleted blood transfusion. Transplantation 2012, 93, 418–422. [Google Scholar] [CrossRef]
- Rostoker, G.; Vaziri, N.D.; Fishbane, S. Iatrogenic iron overload in dialysis patients at the beginning of the 21st century. Drugs 2016, 76, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, G.; Olynyk, J.K.; Ferrari, P. Diagnosing and preventing iron overload. Hemodial. Int. 2017, S1, S58–S67. [Google Scholar] [CrossRef]
- Coyne, D.W. Iron overload in dialysis patients: Rust or bust? Kidney Int. Rep. 2017, 2, 995–997. [Google Scholar] [CrossRef]
- Mirahmadi, K.S.; Paul, W.L.; Winer, R.L.; Dabir-Vaziri, N.; Byer, B.; Gorman, J.T.; Rosen, S.M. Serum ferritin level; Determinant of iron requirement in hemodialysis patients. JAMA 1977, 238, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.A.; Barreto, D.V.; Barreto, F.C.; Dias, C.B.; Moyses, R.; Silva, M.R.R.; Moura, L.A.R.; Draibe, S.A.; Jorgetti, V.; Carvalho, A.B.; et al. Serum ferritin level remains a reliable marker of bone marrow iron stores evaluated by histomorphometry in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 105–109. [Google Scholar] [CrossRef]
- Fishbane, S.; Kalantar-Zadeh, K.; Nissenson, A.R. Serum ferritin in chronic kidney disease: Reconsidering the upper limit for iron treatment. Semin. Dial. 2004, 17, 336–341. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kalantar-Zadeh, K.; Lee, G.H. The fascinating but deceptive ferritin: To measure it or not to measure it in chronic kidney disease? Clin. J. Am. Soc. Nephrol. 2006, 1, S9–S18. [Google Scholar] [CrossRef] [PubMed]
- Knovich, M.A.; Storey, J.A.; Coffman, L.G.; Torti, S.V.; Torti, F.M. Ferritin for the clinician. Blood Rev. 2009, 23, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Hoffken, B.; Wunsch, H.; Fink, H.; Kliener, M.; Luft, F.C. Diagnosis of iron deficiency anemia in renal failure patients during the post-erythropoietin era. Am. J. Kidney Dis. 1995, 26, 292–299. [Google Scholar] [CrossRef]
- Rogers, J.T.; Bridges, K.R.; Durmowicz, G.P.; Glass, J.; Auron, P.E.; Munro, H.N. Translational control during the acute phase response. Ferritin synthesis in response to interleukin-1. J. Biol. Chem. 1990, 265, 14572–14578. [Google Scholar] [CrossRef]
- Rogers, J.T. Ferritin translation by interleukin-1 and interleukin-6: The role of sequences upstream of the start codons of the heavy and light subunit genes. Blood 1996, 87, 2525–2537. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar]
- Goldstein, S.L.; Ikizler, T.A.; Zappitelli, M.; Silverstein, D.M.; Ayus, J.C. Non-infected hemodialysis catheters are associated with increased inflammation compared to arteriovenous fistulas. Kidney Int. 2009, 76, 1063–1069. [Google Scholar] [CrossRef]
- Goldstein, S.L.; Leung, J.C.; Silverstein, D.M. Pro- and anti-inflammatory cytokines in chronic pediatric dialysis patients: Effect of Aspirin. Clin. J. Am. Soc. Nephrol. 2006, 1, 979–986. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Rodriguez, R.A.; Humphreys, M.H. Association between serum ferritin and measures of inflammation, nutrition and iron in hemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 141–149. [Google Scholar] [CrossRef]
- Rambod, M.; Kovesdy, C.P.; Kalantar-Zadeh, K. Combined high serum ferritin and low iron saturation in hemodialysis patients: The role of inflammation. Clin. J. Am. Soc. Nephrol. 2008, 3, 1691–1701. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Don, B.R.; Rodriguez, R.A.; Humphreys, M.H. Serum ferritin is a marker of morbidity and mortality in hemodialysis patients. Am. J. Kidney Dis. 2001, 37, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ryu, G.W.; Jhee, J.H.; Kim, H.W.; Park, S.; Lee, S.A.; Kwon, Y.E.; Kim, Y.L.; Ryu, H.J.; Lee, M.J.; et al. Serum ferritin predicts mortality regardless of inflammatory and nutritional status in patients starting dialysis: A prospective cohort study. Blood Purif. 2015, 40, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Streja, E.; Soohoo, M.; Rhee, C.M.; Eriguchi, R.; Kim, T.W.; Chang, T.I.; Obi, Y.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum ferritin variations and mortality in incident hemodialysis patients. Am. J. Nephrol. 2017, 46, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Rhee, C.M.; Streja, E.; Obi, Y.; Brunelli, S.M.; Kovesdy, C.P.; Kalantar-Zadeh, K. Longitudinal trends in serum ferritin levels and associated factors in a national incident hemodialysis cohort. Nephrol. Dial Transpl. 2017, 32, 370–377. [Google Scholar] [CrossRef]
- Onder, A.M.; Flynn, J.T.; Billings, A.A.; Deng, F.; DeFreitas, M.; Katsoufis, C.; Grinsell, M.M.; Patterson, L.T.; Jetton, J.; Fathallah-Shaykh, S.; et al. Predictors of patency for arteriovenous fistulae and grafts in pediatric hemodialysis patients. Pediatr. Nephrol. 2019, 34, 329–339. [Google Scholar] [CrossRef]
- Hasuike, Y.; Nonoguchi, H.; Tokuyama, M.; Ohue, M.; Nagai, T.; Yahiro, M.; Nanami, M.; Otaki, Y.; Nakanishi, T. Serum ferritin predicts prognosis in hemodialysis patients: The Nishinomiya study. Clin. Exp. Nephrol. 2010, 14, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Onder, A.M.; Flynn, J.T.; Billings, A.A.; Deng, F.; DeFreitas, M.; Katsoufis, C.; Grinsell, M.M.; Patterson, L.T.; Jetton, J.; Fathallah-Shaykh, S.; et al. Predictors of time to first cannulation for arterivenous fistulae in pediatric hemodialysis patients. Pediatr. Nephrol. 2020, 35, 287–296. [Google Scholar] [CrossRef]
- Bailie, G.R.; Larkina, M.; Goodkin, D.A.; Li, Y.; Pisoni, R.L.; Bieber, B.; Mason, N.; Tong, L.; Locatelli, F.; Marshall, M.R.; et al. Variations in intravenous iron use internationally and over time; the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transpl. 2013, 28, 2570–2579. [Google Scholar] [CrossRef]
- Miskulin, D.C.; Zhou, J.; Tangri, N.; Bandeen-Roche, K.; Cook, C.; Ephraim, P.L.; Crews, D.C.; Scialla, J.J.; Sozio, S.M.; Shafi, T.; et al. Trends in anemia management in US hemodialysis patients 2004–2010. BMC Nephrol. 2013, 14, 264. [Google Scholar] [CrossRef]
- Coyne, D.W.; Kapoian, T.; Suki, W.; Singh, A.K.; Moran, J.E.; Dahl, N.V.; Rizkala, A.R.; DRIVE Study Group. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: Results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J. Am. Soc. Nephrol. 2007, 18, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Brookhart, M.A.; Schneeweiss, S.; Avorn, J.; Bradbury, B.D.; Liu, J.; Winkelamyer, W.C. Comparative mortality risk of anemia management practices in incident hemodialysis patients. JAMA 2010, 303, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Thamer, M.; Stefanik, K.; Kaufman, J.; Cotter, D.J. Epoetin requirements predict mortality in hemodialysis patients. Am. J. Kidney Dis. 2004, 44, 866–876. [Google Scholar] [CrossRef] [PubMed]
| Baseline Demographic Information | All Subjects n = 98 | AVG Subjects n = 11 | AVF Subjects n = 87 | p-Value |
|---|---|---|---|---|
| Average number of TCC used, mean ± sd | 2.9 ± 1.1 | 3.2 ± 1.4 | 1.60 ± 1.37 | 0.037 |
| Average duration of TCC vintage (months) mean ± sd | 10.4 ± 17.3 | 28.8 ± 29.5 | 8.0 ± 13.6 | 0.043 |
| CAKUT as primary etiology of ESRD | 33 (33.7%) | 3 (27.3%) | 30 (34.5%) | 0.746 |
| Male (%) | 58 (59.2%) | 5 (45.5%) | 53 (60.9%) | 0.348 |
| African American (%) | 48 (49%) | 8 (72.7%) | 40 (46.0%) | 0.176 |
| Subjects < 10 years of age | 9 (9.2%) | 1 (9.1%) | 8 (9.2%) | 0.99 |
| Age at AVF/AVG creation (years), median (IQR) | 15.3 (13.2; 17.11) | 15.2 (13.2; 15.5) | 15.2 (13.2; 17.2) | 0.468 |
| Weight at AVF/AVG creation (kg), median (IQR) | 48.7 (39.1; 64.8) | 47.4 (43.0; 60.3) | 48.9 (38.9; 64.5) | 0.879 |
| Height at AVF/AVG creation (m), median (IQR) | 1.55 (1.48; 1.65) | 1.52 (1.47; 1.61) | 1.55 (1.48; 1.65) | 0.57 |
| Body mass index at AVF/AVG creation (BMI, kg/m2), median (IQR) | 20.0 (17.2; 25.6) | 19.1 (17.2; 26.6) | 20.1 (17.3; 24.1) | 0.973 |
| HD Biomarkers | TCC (n = 98) | PVA.1 | p Value | PVA.2 | p Value * | p Value ** |
|---|---|---|---|---|---|---|
| Ferritin (ng/mL) mean ± sd | 562.64 ± 492.34 | 753.84 ± 561.54 | <0.001 | 759.60 ± 528.11 | 0.004 | 0.77 |
| Hematocrit (%), mean ± sd | 32.04 ± 4.43 | 34.92 ± 4.66 | <0.0001 | 34.04 ± 3.64 | 0.003 | 0.24 |
| Albumin (gram/dL), mean ± sd | 3.59 ± 0.76 | 3.91 ± 0.47 | <0.0001 | 3.88 ± 0.53 | 0.001 | 0.91 |
| Single pool Kt/V, mean ± sd | 1.49 ± 0.45 | 1.67 ± 0.41 | 0.02 | 1.63 ± 0.26 | 0.01 | 0.45 |
| Predictors | All Subjects (n = 79) | Improved Ferritin at PVA1 (n = 26) | Worsened Ferritin at PVA1 (n = 53) | p-Value Overall |
|---|---|---|---|---|
| TCC.Ferritin, median [IQR] | 398 [230; 700] | 856 [403; 1320] | 305 [173; 458] | <0.001 |
| PVA1.Ferritin, median [IQR] | 675 [398; 964] | 467 [144; 750] | 767 [535; 1013] | 0.002 |
| PVA2.Ferritin, median [IQR] | 746 [380; 910] | 678 [198; 832] | 762 [506; 1061] | 0.120 |
| Age at AVF/AVG creation (years), median [IQR] | 15.3 [12.9; 17.1] | 14.4 [11.2; 16.7] | 15.5 [14.0; 17.2] | 0.317 |
| Weight at AVF/AVG creation (kg), median [IQR] | 48.3 [36.4; 64.6] | 43.2 [32.1; 54.6] | 50.1 [39.5; 67.0] | 0.11 |
| Height at AVF/AVG creation (m), median [IQR] | 1.55 [1.48; 1.65] | 1.52 [1.42; 1.62] | 1.57 [1.50; 1.65] | 0.269 |
| BMI at AVF/AVG creation (kg/m2), median [IQR] | 19.7 [17.1; 26.3] | 18.7 [16.6; 20.8] | 20.4 [17.4; 27.5] | 0.138 |
| Male | 48 (60.8%) | 18 (69.2%) | 30 (56.6%) | 0.404 |
| African American | 38 (48.1%) | 17 (65.4%) | 21 (39.6%) | 0.056 |
| CAKUT as etiology | 29 (36.7%) | 7 (26.9%) | 22 (41.5%) | 0.310 |
| Primary etiology # | 0.113 | |||
| Congenital | 29 (36.7%) | 7 (26.9%) | 22 (41.5%) | |
| Glomerulonephritis | 21 (26.6%) | 7 (26.9%) | 14 (26.4%) | |
| Other | 16 (20.3%) | 4 (15.4%) | 12 (22.6%) | |
| SRNS | 13 (16.5%) | 8 (30.8%) | 5 (9.43%) | |
| Average duration of TCC vintage prior to PVA (months), median [IQR] | 10.5 ± 17.4 | 11.3 ± 14.7 | 10.27 ± 13.2 | 0.101 |
| PVA1.Hct | 34.0 [31.7; 37.4] | 33.4 [30.8; 36.9] | 34.3 [31.9; 37.5] | 0.425 |
| PVA1.Albumin | 3.90 [3.70; 4.20] | 3.80 [3.60; 4.00] | 4.00 [3.70; 4.30] | 0.021 |
| PVA1.Kt/V | 1.65 [1.39; 1.90] | 1.71 [1.42; 2.03] | 1.65 [1.39; 1.87] | 0.581 |
| Conversion to PVA | 0.722 | |||
| AVG (n = 10) | 10 (12.7%) | 4 (15.4%) | 6 (11.3%) | |
| AVF (n= 69) | 69 (87.3%) | 22 (84.6%) | 47 (88.7%) |
| Predictors | ß (SE) | 95% CI | p-Value |
|---|---|---|---|
| Baseline ferritin (per 50 ng/mL) | 17.96 (7.30) | 3.38–32.53 | 0.017 |
| Age at AVF/AVG creation | 1.49 (2.07) | 5.62–2.65 | 0.475 |
| Dialysis vintage at AVF/AVG creation | 2.82 (5.21) | −7.58–13.23 | 0.590 |
| BMI at AVF/AVG creation | 1.72 (9.65) | −17.54–20.98 | 0.859 |
| Primary etiology | |||
| CAKUT (Reference) | |||
| Chronic glomerulonephritis | −210.4 (165.62) | −541.06–120.27 | 0.208 |
| SRNS | −438.93 (203.59) | −845.41–−32.46 | 0.035 |
| Other | −156.6 (187.61) | −531.19–217.98 | 0.407 |
| Study sites according to the PVA created | |||
| 5–10 AVF/AVG (Reference) | |||
| <5 AVF/AVG | −102.98 (184.07) | −470.38–264.42 | 0.578 |
| >10 AVF/AVG | 319.85 (159.19) | 2.02–637.68 | 0.049 |
| AVF vs. AVG | −134.19 (247.88) | −629–360.72 | 0.590 |
| AVF/AVG site | |||
| Brachial AVF/AVG (Reference) | |||
| Radial AVF/AVG | 8.09 (138.79) | −269.02–285.19 | 0.954 |
| Femoral AVF/AVG | −390 (344.04) | −1076–296.90 | 0.261 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onder, A.M.; Ansari, M.A.Y.; Deng, F.; Grinsell, M.M.; Patterson, L.; Jetton, J.; Fathallah-Shaykh, S.; Ranch, D.; Aviles, D.; Copelovitch, L.; et al. Persistent Increase in Serum Ferritin Levels despite Converting to Permanent Vascular Access in Pediatric Hemodialysis Patients: Pediatric Nephrology Research Consortium Study. J. Clin. Med. 2023, 12, 4251. https://doi.org/10.3390/jcm12134251
Onder AM, Ansari MAY, Deng F, Grinsell MM, Patterson L, Jetton J, Fathallah-Shaykh S, Ranch D, Aviles D, Copelovitch L, et al. Persistent Increase in Serum Ferritin Levels despite Converting to Permanent Vascular Access in Pediatric Hemodialysis Patients: Pediatric Nephrology Research Consortium Study. Journal of Clinical Medicine. 2023; 12(13):4251. https://doi.org/10.3390/jcm12134251
Chicago/Turabian StyleOnder, Ali Mirza, Md Abu Yusuf Ansari, Fang Deng, Matthew M. Grinsell, Larry Patterson, Jennifer Jetton, Sahar Fathallah-Shaykh, Daniel Ranch, Diego Aviles, Lawrence Copelovitch, and et al. 2023. "Persistent Increase in Serum Ferritin Levels despite Converting to Permanent Vascular Access in Pediatric Hemodialysis Patients: Pediatric Nephrology Research Consortium Study" Journal of Clinical Medicine 12, no. 13: 4251. https://doi.org/10.3390/jcm12134251
APA StyleOnder, A. M., Ansari, M. A. Y., Deng, F., Grinsell, M. M., Patterson, L., Jetton, J., Fathallah-Shaykh, S., Ranch, D., Aviles, D., Copelovitch, L., Ellis, E., Chadha, V., Elmaghrabi, A., Lin, J.-J., Butani, L., Haddad, M., Marsenic, O., Brakeman, P., Quigley, R., ... Langman, C. B. (2023). Persistent Increase in Serum Ferritin Levels despite Converting to Permanent Vascular Access in Pediatric Hemodialysis Patients: Pediatric Nephrology Research Consortium Study. Journal of Clinical Medicine, 12(13), 4251. https://doi.org/10.3390/jcm12134251






