Treatment of Pediatric Anogenital Warts in the Era of HPV-Vaccine: A Literature Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient-Applied Topical Therapies
3.2. Healthcare Provider Administered Topical Therapies
3.3. Healthcare Provider-Administered Physical Destruction Methods
3.4. Healthcare Provider-Administered Intralesional Therapies
3.5. Healthcare Provider Administered HPV-Vaccination
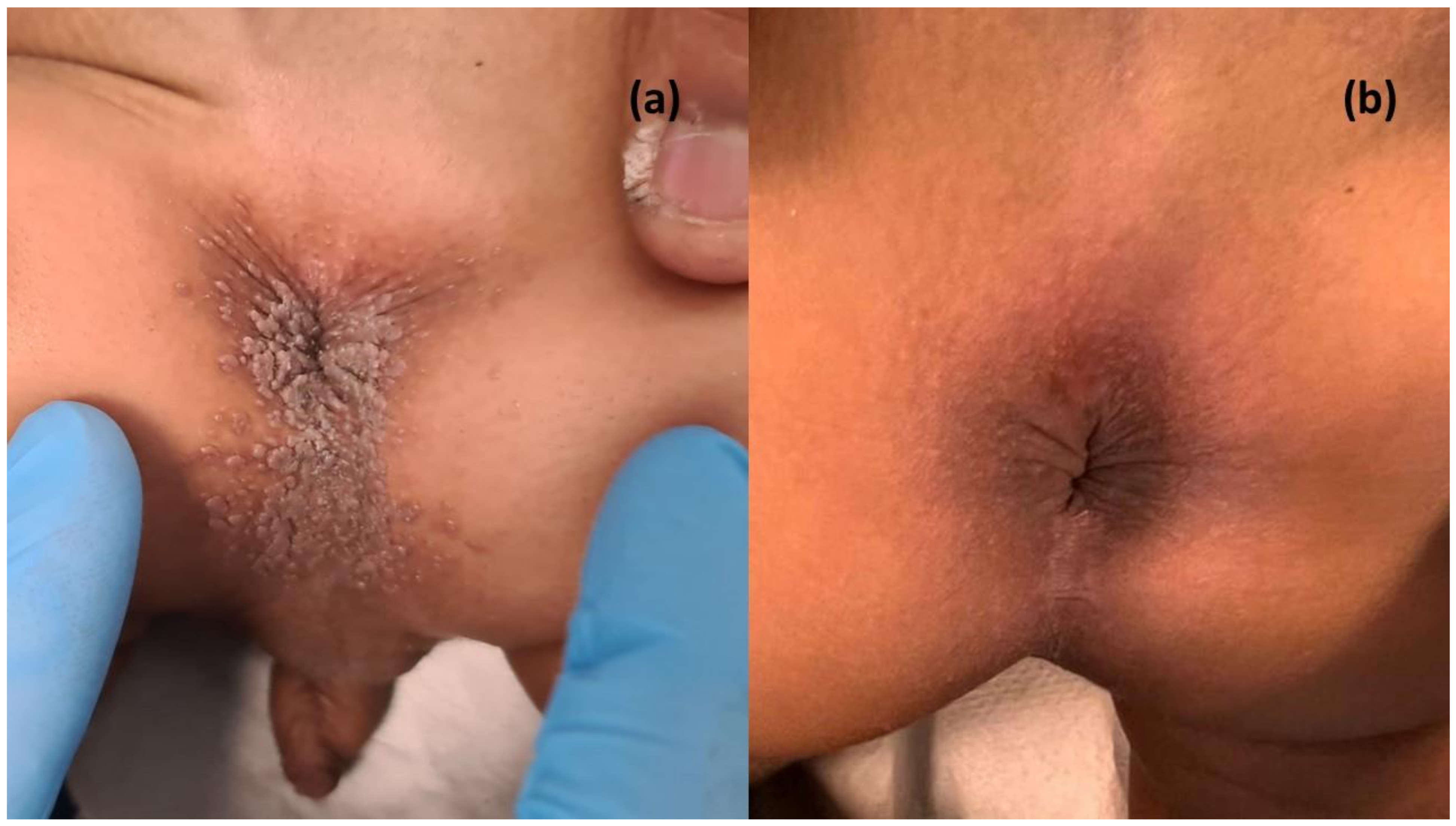
3.6. Case in Point Report
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa-Silva, M.; Fernandes, I.; Rodrigues, A.G.; Lisboa, C. Anogenital warts in pediatric population. An. Bras. Dermatol. 2017, 92, 675–681. [Google Scholar] [CrossRef] [Green Version]
- Thornsberry, L.; English, J.C., 3rd. Evidence-based treatment and prevention of external genital warts in female pediatric and adolescent patients. J. Pediatr. Adolesc. Gynecol. 2012, 25, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.; Barkas, G.; Valari, M.; Bethimoutis, G.; Nicolaidou, E.; Vosynioti, V.; Kontochristopoulos, G.; Papadogeorgaki, H.; Verra, P.; Katsarou, A. Condylomata acuminata in children. Pediatr. Infect. Dis. J. 2012, 31, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Righolt, C.H.; Willows, K.; Kliewer, E.V.; Mahmud, S.M. Incidence of anogenital warts after the introduction of the quadrivalent HPV vaccine program in Manitoba, Canada. PLoS ONE 2022, 17, e0267646. [Google Scholar] [CrossRef]
- Di Spirito, F.; Pantaleo, G.; Di Palo, M.P.; Amato, A.; Raimondo, A.; Amato, M. Oral Human Papillomavirus Benign Lesions and HPV-Related Cancer in Healthy Children: A Systematic Review. Cancers 2023, 15, 1096. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S. Prevalence and genotype distribution of human papillomavirus infection among female outpatients in Northeast China: A population-based survey of 110,927 women. Arch. Gynecol. Obstet. 2023, 308, 35–41. [Google Scholar] [CrossRef]
- Egawa, N. Papillomaviruses and cancer: Commonalities and differences in HPV carcinogenesis at different sites of the body. Int. J. Clin. Oncol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Ciccarese, G.; Drago, F.; Copello, F.; Bodini, G.; Rebora, A.; Parodi, A. Study on the impact of sexually transmitted infections on Quality of Life, mood and sexual function. Ital. J. Dermatol. Venerol. 2021, 156, 686–691. [Google Scholar] [CrossRef]
- Seyoum, A.; Assefa, N.; Gure, T.; Seyoum, B.; Mulu, A.; Mihret, A. Prevalence and Genotype Distribution of High-Risk Human Papillomavirus Infection Among Sub-Saharan African Women: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 890880. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Hao, J.Q.; Mendez, M.J.G.; Mohamed, S.B.; Fu, S.L.; Zhao, F.H.; Qiao, Y.L. The Prevalence of Cervical HPV Infection and Genotype Distribution in 856,535 Chinese Women with Normal and Abnormal Cervical Lesions: A Systemic Review. J. Cytol. 2022, 39, 137–147. [Google Scholar] [CrossRef]
- Chaux, A.; Sanchez, D.F.; Fernández-Nestosa, M.J.; Cañete-Portillo, S.; Rodríguez, I.M.; Giannico, G.A.; Cubilla, A.L. The dual pathogenesis of penile neoplasia: The heterogeneous morphology of human papillomavirus-related tumors. Asian J. Urol. 2022, 9, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Shiraishi, K.; Takashima, A.; Takayanagi, D.; Saiki, Y.; Takano, S.; Tanaka, M.; Fukunaga, M.; Sugimoto, K.; Iwasaki, Y.; et al. Characteristics of anal canal squamous cell carcinoma as an HPV-associated cancer in Japan. Int. J. Clin. Oncol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Broccolo, F.; Fusetti, L.; Rosini, S.; Caraceni, D.; Zappacosta, R.; Ciccocioppo, L.; Matteoli, B.; Halfon, P.; Malnati, M.S.; Ceccherini-Nelli, L. Comparison of oncogenic HPV type-specific viral DNA load and E6/E7 mRNA detection in cervical samples: Results from a multicenter study. J. Med. Virol. 2013, 85, 472–482. [Google Scholar] [CrossRef]
- Ren, Z.; Silverberg, J.I. Association of Atopic Dermatitis With Bacterial, Fungal, Viral, and Sexually Transmitted Skin Infections. Dermatitis 2020, 31, 157–164. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Silverberg, N.B. Childhood atopic dermatitis and warts are associated with increased risk of infection: A US population-based study. J. Allergy Clin. Immunol. 2014, 133, 1041–1047. [Google Scholar] [CrossRef]
- Murao, K.; Nakasuka, A.; Keyama, T.; Hashimoto, I.; Kubo, Y. Case of multiple Bowen diseases associated with human papillomavirus type 16 in a patient with atopic dermatitis. J. Dermatol. 2016, 43, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, K.H.; Rady, P.; Tyring, S.; Stone, M.S. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis. Pediatr. Dermatol. 2014, 31, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Igawa, M.; Nakano, H. Condyloma acuminatum in three-year-old girl. Urology 1992, 39, 484–486. [Google Scholar] [CrossRef]
- Moresi, J.M.; Herbert, C.R.; Cohen, B.A. Treatment of anogenital warts in children with topical 0.05% podofilox gel and 5% imiquimod cream. Pediatr. Dermatol. 2001, 18, 448–450, discussion 452. [Google Scholar] [CrossRef]
- Bussen, S.; Sütterlin, M.; Schmidt, U.; Bussen, D. Anogenital Warts in Childhood—Always a Marker for Sexual Abuse? Geburtshilfe Frauenheilkd. 2012, 72, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Ting, P.T.; Dytoc, M.T. Therapy of external anogenital warts and molluscum contagiosum: A literature review. Dermatol. Ther. 2004, 17, 68–101. [Google Scholar] [CrossRef]
- Majewski, S.; Pniewski, T.; Malejczyk, M.; Jablonska, S. Imiquimod is highly effective for extensive, hyperproloferative condyloma in children. Pediatr. Dermatol. 2003, 20, 440–442. [Google Scholar] [CrossRef]
- Schaen, L.; Mercurio, M.G. Treatment of human papilloma virus in a 6-month-old infant with imiquimod 5% cream. Pediatr. Dermatol. 2001, 18, 450–452. [Google Scholar] [CrossRef]
- Gruber, P.; Wilkinson, J. Successful treatment of perianal warts in a child with 5% imiquimod cream. J. Dermatolog. Treat. 2001, 12, 215–217. [Google Scholar]
- Tatti, S.; Swinehart, J.M.; Thielert, C.; Tawfik, H.; Mescheder, A.; Beutner, K.R. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts. Obstet. Gynecol. 2008, 111, 1371–1379. [Google Scholar]
- Gupta, A.K.; Daigle, D. Sinecatechins 10% ointment: A green tea extract for the treatment of external genital warts. Skin Therapy Lett. 2015, 20, 6–8. [Google Scholar]
- Rob, F.; Jůzlová, K.; Sečníková, Z.; Jiráková, A.; Hercogová, J. Successful Treatment with 10% Sinecatechins Ointment for Recurrent Anogenital Warts in an Eleven-Year-Old Child. Pediatr. Infect. Dis. J. 2017, 36, 235–236. [Google Scholar] [CrossRef]
- Godoy-Gijon, E.; Fraile-Alonso, M.C.; Alonso-Vicente, C.; Rojo-Rello, S. Treatment of pediatric anogenital condyloma acuminata with sinecatechins ointment. Dermatol. Ther. 2017, 30, e12562. [Google Scholar] [CrossRef]
- Fernández-Morano, T.; del Boz, J.; González-Carrascosa, M.; Tortajada, B.; de Troya, M. Topical cidofovir for viral warts in children. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1487–1489. [Google Scholar] [CrossRef]
- Calisto, D.; Arcangeli, F. Topical cidofovir for condyloma acumiata of the genitaliaof a 3-year-old child. J. Am. Acad. Dermatol. 2003, 49, 1192–1193. [Google Scholar] [CrossRef]
- Varma, S.; Lathrop, E.; Haddad, L.B. Pediatric condyloma acuminata. J. Pediatr. Adolesc. Gynecol. 2013, 26, e121–e122. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, G.; Drago, F.; Granger, C.; Parodi, A. Efficacy Assessment of a Topically Applied Nitric-Zinc Complex Solution for the Treatment of External Ano-genital Warts in 100 Patients. Dermatol. Ther. 2019, 9, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cusini, M.; Micali, G.; Lacarrubba, F.; Puviani, M.; Barcella, A.; Milani, M. Efficacy and tolerability of nitric-zinc complex in the treatment of external genital warts and “difficult-to-treat” warts: A “proof of concept”, prospective, multicentre, open study. G Ital. Dermatol. Venereol. 2015, 150, 643–648. [Google Scholar] [PubMed]
- Giacaman, A.; Granger, C.; Aladren, S.; Bauzá, A.; Alomar Torrens, B.; Riutort Mercant, M.; Martin-Santiago, A. Use of Topical Nitric-Zinc Complex Solution to Treat Palmoplantar and Periungual Warts in a Pediatric Population. Dermatol. Ther. 2019, 9, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, K.A.; Woods, C.R.; Sinal, S.H. Venereal warts in children. Pediatr. Rev. 2011, 32, 115–121, quiz 121. [Google Scholar] [CrossRef] [Green Version]
- Tuncel, A.; Görgü, M.; Ayhan, M.; Deren, O.; Erdogan, B. Treatment of anogenital warts by pulsed dye laser. Dermatol. Surg. 2002, 28, 350–352. [Google Scholar] [CrossRef]
- Fields, J.R.; Saikaly, S.K.; Schoch, J.J. Intralesional immunotherapy for pediatric warts: A review. Pediatr. Dermatol. 2020, 37, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Nofal, A.; Soliman, M.; Hamdy, F.; Alakad, R. Intralesional candidaantigen versus intralesional tuberculin in the treatment of recalcitrant genital warts: A comparative study. J. Am. Acad. Dermatol. 2020, 82, 1512–1514. [Google Scholar] [CrossRef]
- Nofal, A.; Nofal, E. Intralesional immunotherapy of common warts: Successful treatment with mumps, measles and rubella vaccine. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1166–1170. [Google Scholar]
- Horn, T.D.; Johnson, S.M.; Helm, R.M.; Roberson, P.K. Intralesional immunotherapy of warts with mumps, Candida and trichophyton skin test antigens: A single-blinded, random-ized and controlled trial. Arch. Dermatol. 2005, 141, 589–594. [Google Scholar]
- Nofal, A.; Alakad, R. Intralesional immunotherapy for the treatment of anogenital warts in pediatric population. J. Dermatolog. Treat. 2022, 33, 1042–1046. [Google Scholar] [CrossRef]
- Illah, O.; Olaitan, A. Updates on HPV Vaccination. Diagnostics 2023, 13, 243. [Google Scholar] [CrossRef]
- Herrin, D.M.; Coates, E.E.; Costner, P.J.; Kemp, T.J.; Nason, M.C.; Saharia, K.K.; Pan, Y.; Sarwar, U.N.; Holman, L.; Yamshchikov, G.; et al. Comparison of adaptive and innate immune responses induced by licensed vaccines for Human Papillomavirus. Hum. Vaccin. Immunother. 2014, 10, 3446–3454. [Google Scholar]
- Nofal, A.; Marei, A.; Al-shimaa, M.I.; Nofal, E.; Nabil, M. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J. Am. Acad. Dermatol. 2020, 82, 94–100. [Google Scholar]
- Kreuter, A.; Wieland, U. Lack of efficacy in treating condyloma acuminata and preventing recurrences with the recombinant quadrivalent human papillomavirus vaccine in a case series of immunocompetent patients. J. Am. Acad. Dermatol. 2013, 68, 179–180. [Google Scholar] [CrossRef]
- Kreuter, A.; Waterboer, T.; Wieland, U. Regression of cutaneous warts in a patient with WILD syndrome following recombinant quadrivalent human papillomavirus vaccination. Arch. Dermatol. 2010, 146, 1196–1197. [Google Scholar]
- Landini, M.; Borgogna, C.; Peretti, A.; Doorbar, J.; Griffin, H.; Mignone, F.; Lai, A.; Urbinati, L.; Matteelli, A.; Gariglio, M.; et al. Identification of the skin virome in a boy with widespread human papillomavirus-2-positive warts that completely regressed after administration of tetravalent human papillomavirus vaccine. Br. J. Dermatol. 2015, 173, 597–600. [Google Scholar]
- Martín, J.M.; Escandell, I.; Ayala, D.; Jordá, E. Spontaneous Remission of Recalcitrant Warts in Girls After Human Papillomavirus Vaccination. Actas Dermosifiliogr. 2016, 107, 533–535, (In English and Spanish). [Google Scholar] [CrossRef]
- Martin, J.M.; Mateo, E.; Ramón, D. Spontaneous Regression of a Recalcitrant Wart after Bivalent Papillomavirus Vaccination. J. Pediatr. 2018, 194, 259. [Google Scholar]
- Abeck, D.; Folster-Holst, R. Quadrivalent human papillomavirus vaccination: A promising treatment for recalcitrant cutaneous warts in children. Acta Derm. Venereol. 2015, 95, 1017–1019. [Google Scholar]
- Daniel, B.S.; Murrell, D.F. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013, 149, 370–372. [Google Scholar] [PubMed]
- Smith, S.P.; Baxendale, H.E.; Sterling, J.C. Clearance of recalcitrant warts in a patient with idiopathic immune deficiency following administration of the quadrivalent human papillomavirus vaccine. Clin. Exp. Dermatol. 2017, 42, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Couselo-Rodríguez, C.; Pérez-Feal, P.; Alarcón-Pérez, C.E.; Bou-Boluda, L.; Baselga, E. Clearance of genital warts in a pediatric patient following administration of the nonavalent human papillomavirus vaccine. Int. J. Dermatol. 2021, 60, e377–e379. [Google Scholar] [CrossRef] [PubMed]
- Dianzani, C.; Neagu, N.; Venuti, A.; Coscarella, G.; DE Franciscis, B.; Montini, F.; Zalaudek, I.; Conforti, C. Efficacy of nonavalent human papillomavirus vaccine for recalcitrant warts. Ital. J. Dermatol. Venerol. 2023, 158, 67–68. [Google Scholar] [CrossRef]
- Choi, H. Can quadrivalent human papillomavirus prophylactic vaccine be an effective alternative for the therapeutic management of genital warts? an exploratory study. Int. Braz. J. Urol. 2019, 45, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Kim, J.K.; Kim, D.H.; Yoon, M.S. Condyloma accuminatum treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, 18). J. Am. Acad. Dermatol. 2011, 64, e130–e132. [Google Scholar] [CrossRef]
- Bossart, S.; Imstepf, V.; Hunger, R.E.; Seyed Jafari, S.M. Nonavalent Human Papillomavirus Vaccination as a Treatment for Skin Warts in Immunosuppressed Adults: A Case Series. Acta Derm. Venereol. 2020, 100, adv00078. [Google Scholar] [CrossRef]
- Moscato, G.; DI Matteo, G.; Ciotti, M.; Di Bonito, P.; Andreoni, M.; Moschese, V. Dual response to human papilloma virus vaccine in an immunodeficiency disorder: Resolution of plantar warts and persistence of condylomas. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1212–1213. [Google Scholar]
- Bellone, S.; El-Sahwi, K.; Cocco, E.; Casagrande, F.; Cargnelutti, M.; Palmieri, M.; Bignotti, E.; Romani, C.; Silasi, D.-A.; Azodi, M.; et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: Implications for L1 dendritic cell-based therapeutic vaccines. J. Virol. 2009, 83, 6779–6789. [Google Scholar]
- Govan, V.A. A novel vaccine for cervical cancer: Quadrivalent human papillomavirus (types 6, 11, 16 and 18) recombinant vaccine (Gardasil). Ther. Clin. Risk Manag. 2008, 4, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- Bossart, S.; Gabutti, M.P.; Seyed Jafari, S.M.; Hunger, R.E. Nonavalent human papillomavirus vaccination as alternative treatment for genital warts. Dermatol. Ther. 2020, 33, e13771. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Joura, E.A.; Garland, S.M.; Huh, W.K.; Iversen, O.E.; Kjaer, S.K.; Ferenczy, A.; Kurman, R.J.; Ronnett, B.M.; Stoler, M.H.; et al. Nine-valent HPV vaccine efficacy against related diseases and definitive therapy: Comparison with historic placebo population. Gynecol. Oncol. 2019, 154, 110–117. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.M.; Fregnani, J.H.T.G.; Villa, L.L. HPV Vaccine: Updates and Highlights. Acta Cytol. 2019, 63, 159–168. [Google Scholar] [CrossRef]
- Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D., Jr.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. Broad Spectrum HPV Vaccine Study. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Schiller, J.T. Human Papillomavirus Vaccines. J. Infect. Dis. 2021, 224 (Suppl. S2), S367–S378. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef]
- Herzum, A.; Ciccarese, G.; Drago, F.; Pastorino, A.; Dezzana, M.; Mavilia, M.G.; Sola, S.; Copello, F.; Parodi, A. Cervical, oral and anal Human papillomavirus infection in women attending the Dermatology Unit of a regional reference hospital in Genoa, Italy: A prevalence study. J. Prev. Med. Hyg. 2022, 63, E415–E419. [Google Scholar] [CrossRef]
- Ciccarese, G.; Herzum, A.; Pastorino, A.; Dezzana, M.; Casazza, S.; Mavilia, M.G.; Copello, F.; Parodi, A.; Drago, F. Prevalence of genital HPV infection in STI and healthy populations and risk factors for viral persistence. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 885–888. [Google Scholar] [CrossRef]
- Broccolo, F.; Ciccarese, G.; Rossi, A.; Anselmi, L.; Drago, F.; Toniolo, A. Human papillomavirus (HPV) and Epstein-Barr virus (EBV) in keratinizing versus non- keratinizing squamous cell carcinoma of the oropharynx. Infect. Agent Cancer 2018, 13, 32. [Google Scholar] [CrossRef]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 (Suppl. S5), F12–F23. [Google Scholar] [PubMed] [Green Version]
- Delmonte, S.; Benardon, S.; Cariti, C.; Ribero, S.; Ramoni, S.; Cusini, M. Anogenital warts treatment options: A practical approach. G. Ital. Dermatol. Venereol. 2020, 155, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Steinau, M.; Hariri, S.; Gillison, M.L.; Broutian, T.R.; Dunne, E.F.; Tong, Z.Y.; Markowitz, L.E.; Unger, E.R. Prevalence of cervical and oral human papillomavirus infections among US women. J. Infect. Dis. 2014, 209, 1739–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.; Wagner, M.; Singhal, P.; Kothari, S. Systematic review of the incidence and prevalence of genital warts. BMC Infect. Dis. 2013, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Salfa, M.C.; Regine, V.; Ferri, M. Le infezioni sessualmente trasmesse:i dati dei due sistemi di sorveglianza sentinella attivi in Italia. Not. Ist. Super. Sanità 2014, 27, 3–39. [Google Scholar]
- Dias, J.V.; Gomes, S.; Afonso, H.; Teles, R. Anogenital condylomata acuminata in young children: Not always result of sexual transmission. BMJ Case Rep. 2022, 15, e250591. [Google Scholar] [CrossRef]
- Jones, V.; Smith, S.J.; Omar, H.A. Nonsexual transmission of anogenital warts in children: A retrospective analysis. Sci. World J. 2007, 7, 1896–1899. [Google Scholar] [CrossRef] [Green Version]
- Ciccarese, G.; Trave, I.; Herzum, A.; Gariazzo, L.; Cozzani, E.; Rebora, A.; Parodi, A.; Drago, F. Dermatological infections in organ transplant recipients: A retrospective study on 222 patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e36–e38. [Google Scholar] [CrossRef] [Green Version]
- Smolen, K.K.; Gelinas, L.; Franzen, L.; Dobson, S.; Dawar, M.; Ogilvie, G.; Krajden, M.; Fortuno, E.S., 3rd; Kollmann, T.R. Age of recipient and number of doses differentially impact human B and T cell immune memory responses to HPV vaccination. Vaccine 2012, 30, 3572–3579. [Google Scholar] [CrossRef]
- Pham, C.T.; Juhasz, M.; Sung, C.T.; Mesinkovska, N.A. The human papillomavirus vaccine as a treatment for human papillomavirus-related dysplastic and neoplastic conditions: A literature review. J. Am. Acad. Dermatol. 2020, 82, 202–212. [Google Scholar] [CrossRef]
- Culton, D.A.; Morrell, D.S.; Burkhart, C.N. The management of condyloma acuminata in the pediatric population. Pediatr. Ann. 2009, 38, 368–372. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herzum, A.; Ciccarese, G.; Occella, C.; Gariazzo, L.; Pastorino, C.; Trave, I.; Viglizzo, G. Treatment of Pediatric Anogenital Warts in the Era of HPV-Vaccine: A Literature Review. J. Clin. Med. 2023, 12, 4230. https://doi.org/10.3390/jcm12134230
Herzum A, Ciccarese G, Occella C, Gariazzo L, Pastorino C, Trave I, Viglizzo G. Treatment of Pediatric Anogenital Warts in the Era of HPV-Vaccine: A Literature Review. Journal of Clinical Medicine. 2023; 12(13):4230. https://doi.org/10.3390/jcm12134230
Chicago/Turabian StyleHerzum, Astrid, Giulia Ciccarese, Corrado Occella, Lodovica Gariazzo, Carlotta Pastorino, Ilaria Trave, and Gianmaria Viglizzo. 2023. "Treatment of Pediatric Anogenital Warts in the Era of HPV-Vaccine: A Literature Review" Journal of Clinical Medicine 12, no. 13: 4230. https://doi.org/10.3390/jcm12134230
APA StyleHerzum, A., Ciccarese, G., Occella, C., Gariazzo, L., Pastorino, C., Trave, I., & Viglizzo, G. (2023). Treatment of Pediatric Anogenital Warts in the Era of HPV-Vaccine: A Literature Review. Journal of Clinical Medicine, 12(13), 4230. https://doi.org/10.3390/jcm12134230





