Uncovering Predictors of Lipid Goal Attainment in Type 2 Diabetes Outpatients Using Logic Learning Machine: Insights from the AMD Annals and AMD Artificial Intelligence Study Group
Abstract
:1. Introduction
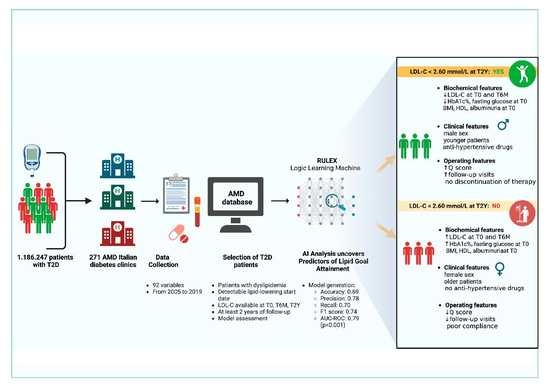
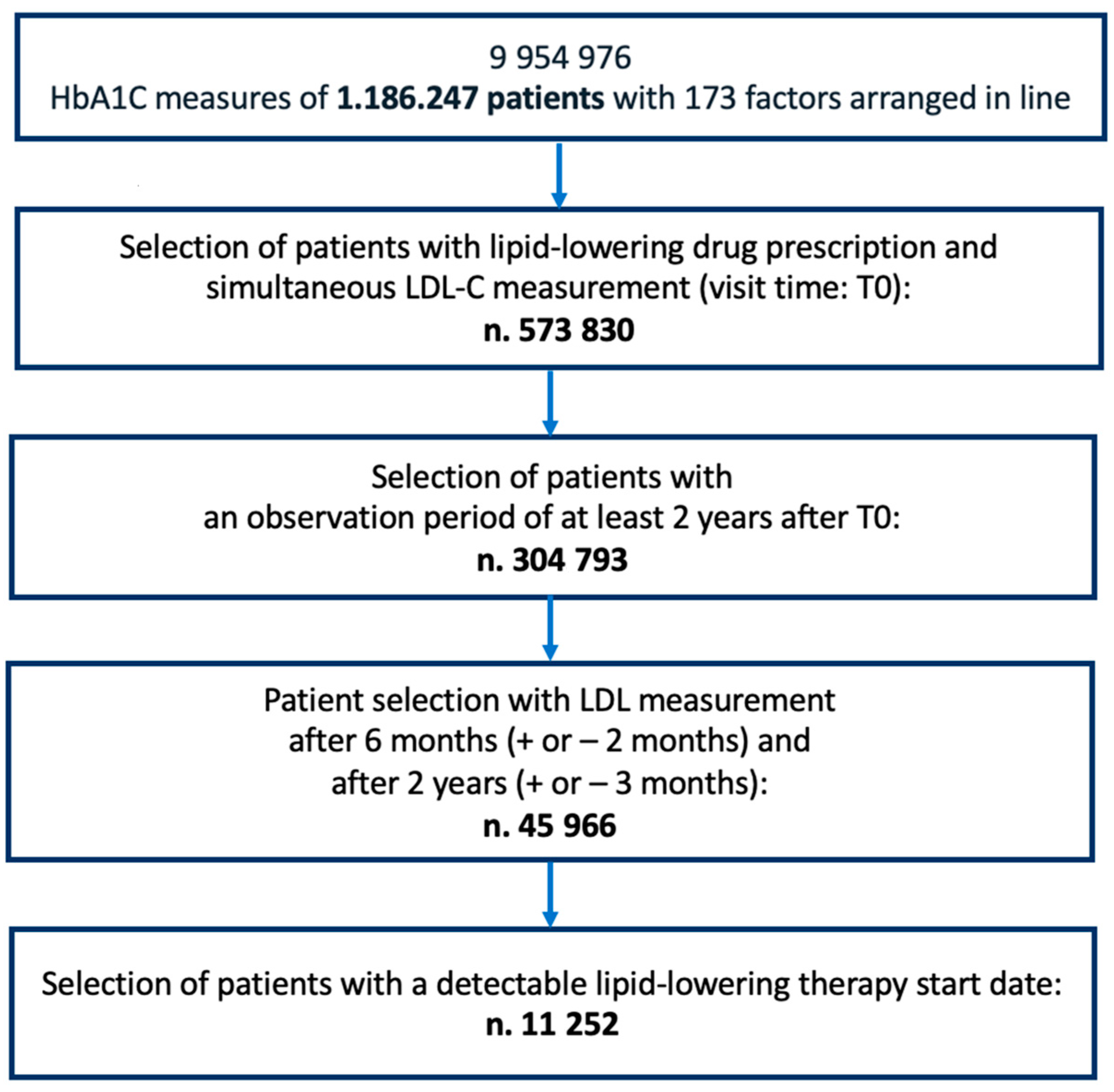
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Database Description
2.3. Lipid Target
2.4. LLM Characteristics and ML Modelling
3. Results
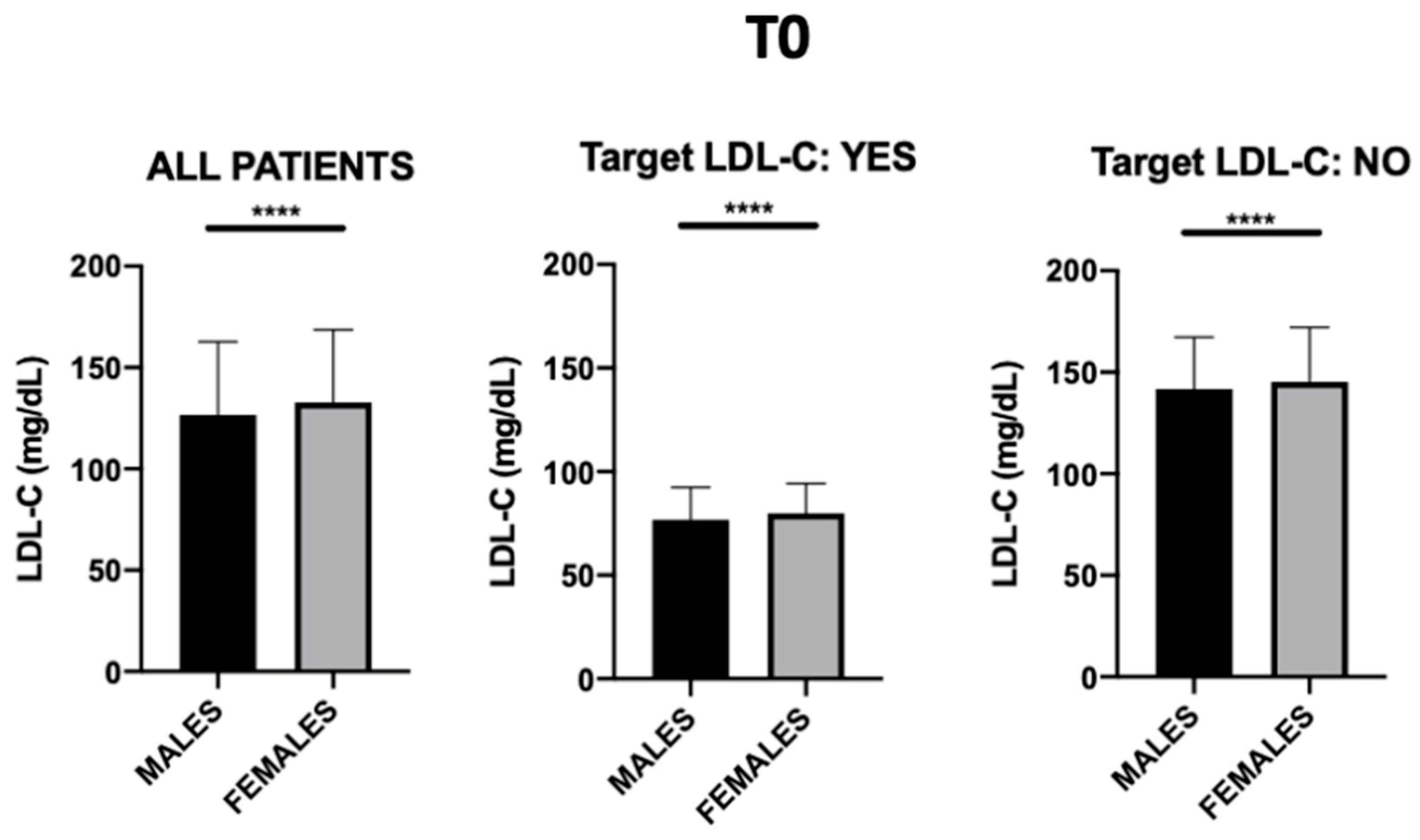
3.1. General Patient Characteristics
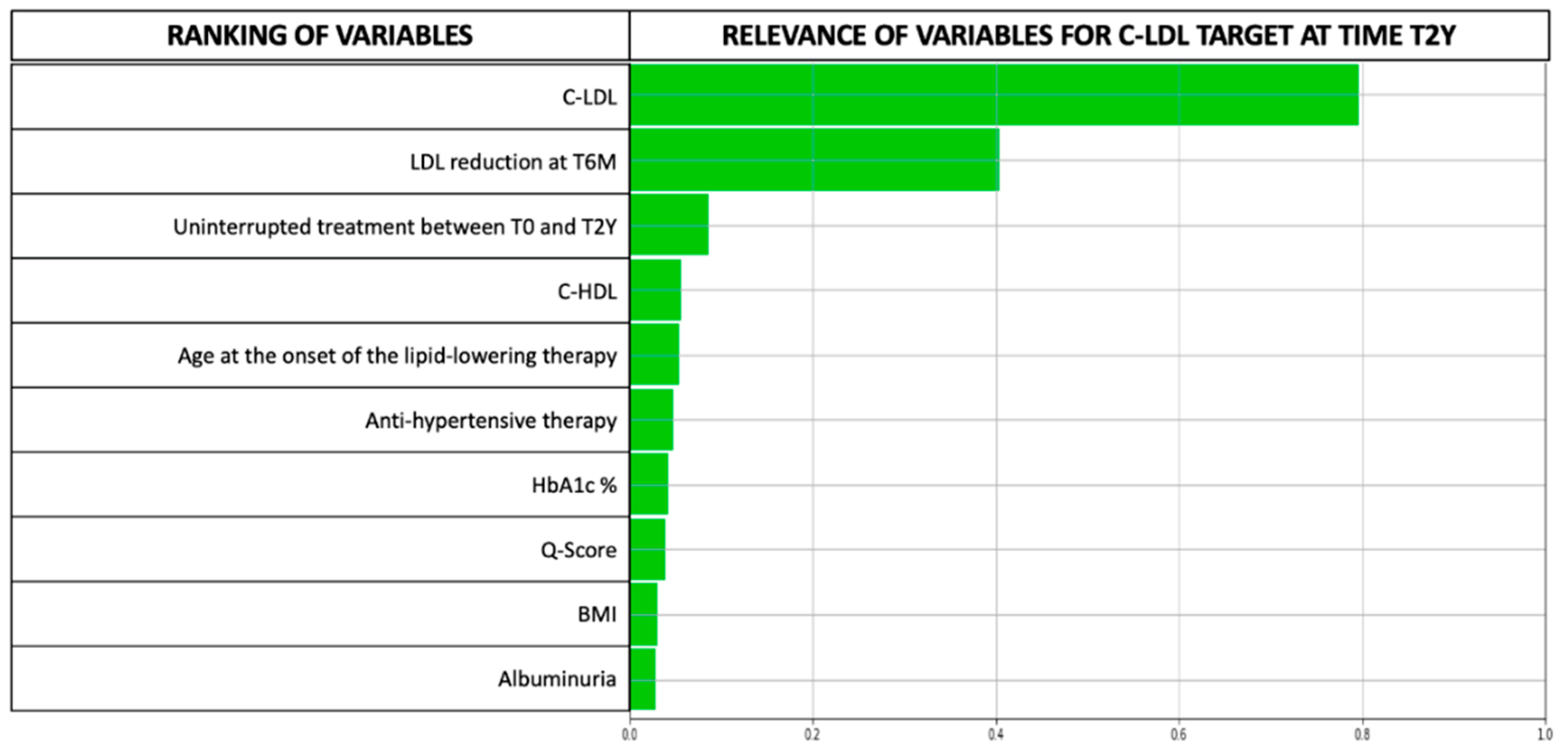
3.2. LLM Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, N.D.; Sattar, N. Cardiovascular Risk in Diabetes Mellitus: Epidemiology, Assessment and Prevention. Nat. Rev. Cardiol. 2023, 1, 1–11. [Google Scholar] [CrossRef]
- Ali, M.K.; Pearson-Stuttard, J.; Selvin, E.; Gregg, E.W. Interpreting Global Trends in Type 2 Diabetes Complications and Mortality. Diabetologia 2022, 65, 3–13. [Google Scholar] [CrossRef]
- Wu, L.; Parhofer, K.G. Diabetic Dyslipidemia. Metabolism 2014, 63, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.M. Trends in Medication Use Among US Adults with Diabetes Mellitus: Glycemic Control at the Expense of Controlling Cardiovascular Risk Factors. Arch. Intern Med. 2009, 169, 1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyrbus, K.; Gasior, M.; Desperak, P.; Nowak, J.; Osadnik, T.; Banach, M. Characteristics of Lipid Profile and Effectiveness of Management of Dyslipidaemia in Patients with Acute Coronary Syndromes–Data from the TERCET Registry with 19,287 Patients. Pharmacol. Res. 2019, 139, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Kearney, P.M.; Perry, I.J. The Cumulative Effect of Core Lifestyle Behaviours on the Prevalence of Hypertension and Dyslipidemia. BMC Public Health 2008, 8, 210. [Google Scholar] [CrossRef] [Green Version]
- Cholesterol Treatment Trialists’ (CTT) Collaboration Efficacy and Safety of More Intensive Lowering of LDL Cholesterol: A Meta-Analysis of Data from 170,000 Participants in 26 Randomised Trials. Lancet 2010, 376, 1670–1681. [CrossRef] [Green Version]
- American Diabetes Association Standards of Medical Care in Diabetes—2010. Diabetes Care 2010, 33, S11–S61. [CrossRef] [Green Version]
- Marathe, P.H.; Gao, H.X.; Close, K.L. American Diabetes Association Standards of Medical Care in Diabetes 2017. J. Diabetes 2017, 9, 320–324. [Google Scholar] [CrossRef] [Green Version]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Das, S.R.; Gibbons, C.H.; et al. Introduction and Methodology: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S1–S4. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Pintaudi, B.; Scatena, A.; Piscitelli, G.; Frison, V.; Corrao, S.; Manicardi, V.; Graziano, G.; Rossi, M.C.; Gallo, M.; Mannino, D.; et al. Clinical Profiles and Quality of Care of Subjects with Type 2 Diabetes According to Their Cardiovascular Risk: An Observational, Retrospective Study. Cardiovasc. Diabetol. 2021, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Postmus, I.; Trompet, S.; Deshmukh, H.A.; Barnes, M.R.; Li, X.; Warren, H.R.; Chasman, D.I.; Zhou, K.; Arsenault, B.J.; Donnelly, L.A.; et al. Pharmacogenetic Meta-Analysis of Genome-Wide Association Studies of LDL Cholesterol Response to Statins. Nat. Commun. 2014, 5, 5068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trompet, S.; Postmus, I.; Slagboom, P.E.; Heijmans, B.T.; Smit, R.A.J.; Maier, A.B.; Buckley, B.M.; Sattar, N.; Stott, D.J.; Ford, I.; et al. Non-Response to (Statin) Therapy: The Importance of Distinguishing Non-Responders from Non-Adherers in Pharmacogenetic Studies. Eur. J. Clin. Pharmacol. 2016, 72, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilmanski, T.; Kornilov, S.A.; Diener, C.; Conomos, M.P.; Lovejoy, J.C.; Sebastiani, P.; Orwoll, E.S.; Hood, L.; Price, N.D.; Rappaport, N.; et al. Heterogeneity in Statin Responses Explained by Variation in the Human Gut Microbiome. Med 2022, 3, 388–405.e6. [Google Scholar] [CrossRef]
- Rai, S.; Bhatia, V.; Bhatnagar, S. Drug Repurposing for Hyperlipidemia Associated Disorders: An Integrative Network Biology and Machine Learning Approach. Comput. Biol. Chem. 2021, 92, 107505. [Google Scholar] [CrossRef]
- Rawshani, A.; Eliasson, B.; Rawshani, A.; Henninger, J.; Mardinoglu, A.; Carlsson, Å.; Sohlin, M.; Ljungberg, M.; Hammarstedt, A.; Rosengren, A.; et al. Adipose Tissue Morphology, Imaging and Metabolomics Predicting Cardiometabolic Risk and Family History of Type 2 Diabetes in Non-Obese Men. Sci. Rep. 2020, 10, 9973. [Google Scholar] [CrossRef]
- Nicolucci, A.; Rossi, M.C.; Arcangeli, A.; Cimino, A.; De Bigontina, G.; Fava, D.; Gentile, S.; Giorda, C.; Meloncelli, I.; Pellegrini, F.; et al. Four-Year Impact of a Continuous Quality Improvement Effort Implemented by a Network of Diabetes Outpatient Clinics: The AMD-Annals Initiative: Quality Initiative in Diabetes Care. Diabet. Med. 2010, 27, 1041–1048. [Google Scholar] [CrossRef]
- Rossi, M.C.; Candido, R.; Ceriello, A.; Cimino, A.; Di Bartolo, P.; Giorda, C.; Esposito, K.; Lucisano, G.; Maggini, M.; Mannucci, E.; et al. Trends over 8 Years in Quality of Diabetes Care: Results of the AMD Annals Continuous Quality Improvement Initiative. Acta Diabetol. 2015, 52, 557–571. [Google Scholar] [CrossRef]
- Masi, D.; Risi, R.; Biagi, F.; Vasquez Barahona, D.; Watanabe, M.; Zilich, R.; Gabrielli, G.; Santin, P.; Mariani, S.; Lubrano, C.; et al. Application of a Machine Learning Technology in the Definition of Metabolically Healthy and Unhealthy Status: A Retrospective Study of 2567 Subjects Suffering from Obesity with or without Metabolic Syndrome. Nutrients 2022, 14, 373. [Google Scholar] [CrossRef]
- Musacchio, N.; Giancaterini, A.; Guaita, G.; Ozzello, A.; Pellegrini, M.A.; Ponzani, P.; Russo, G.T.; Zilich, R.; de Micheli, A. Artificial Intelligence and Big Data in Diabetes Care: A Position Statement of the Italian Association of Medical Diabetologists. J. Med. Internet Res. 2020, 22, e16922. [Google Scholar] [CrossRef]
- De Berardis, G.; Pellegrini, F.; Franciosi, M.; Belfiglio, M.; Di Nardo, B.; Greenfield, S.; Kaplan, S.H.; Rossi, M.C.E.; Sacco, M.; Tognoni, G.; et al. Quality of Diabetes Care Predicts the Development of Cardiovascular Events: Results of the QuED Study. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 57–65. [Google Scholar] [CrossRef]
- Rossi, M.C.E.; Lucisano, G.; Comaschi, M.; Coscelli, C.; Cucinotta, D.; Di Blasi, P.; Bader, G.; Pellegrini, F.; Valentini, U.; Vespasiani, G.; et al. Quality of Diabetes Care Predicts the Development of Cardiovascular Events: Results of the AMD-QUASAR Study. Diabetes Care 2011, 34, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Cleeman, J.I.; Bairey Merz, C.N.; Brewer, H.B.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith, S.C.; Stone, N.J. Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J. Am. Coll. Cardiol. 2004, 44, 720–732. [Google Scholar] [CrossRef] [Green Version]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Giorda, C.B.; Pisani, F.; De Micheli, A.; Ponzani, P.; Russo, G.; Guaita, G.; Zilich, R.; Musacchio, N. Determinants of Good Metabolic Control without Weight Gain in Type 2 Diabetes Management: A Machine Learning Analysis. BMJ Open Diab. Res. Care 2020, 8, e001362. [Google Scholar] [CrossRef]
- Verda, D.; Parodi, S.; Ferrari, E.; Muselli, M. Analyzing Gene Expression Data for Pediatric and Adult Cancer Diagnosis Using Logic Learning Machine and Standard Supervised Methods. BMC Bioinform. 2019, 20, 390. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, N.A.; Al-Baity, H.H. Augmented Analytics Driven by AI: A Digital Transformation beyond Business Intelligence. Sensors 2022, 22, 8071. [Google Scholar] [CrossRef] [PubMed]
- Bazoukis, G.; Hall, J.; Loscalzo, J.; Antman, E.M.; Fuster, V.; Armoundas, A.A. The Inclusion of Augmented Intelligence in Medicine: A Framework for Successful Implementation. Cell Rep. Med. 2022, 3, 100485. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, N.; Zilich, R.; Ponzani, P.; Guaita, G.; Giorda, C.; Heidbreder, R.; Santin, P.; Di Cianni, G. Transparent Machine Learning Suggests a Key Driver in the Decision to Start Insulin Therapy in Individuals with Type 2 Diabetes. J. Diabetes 2023, 15, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.A.; Strümke, I.; Thambawita, V.; Hammou, M.; Riegler, M.A.; Halvorsen, P.; Parasa, S. On Evaluation Metrics for Medical Applications of Artificial Intelligence. Sci. Rep. 2022, 12, 5979. [Google Scholar] [CrossRef]
- Thorsen-Meyer, H.-C.; Nielsen, A.B.; Nielsen, A.P.; Kaas-Hansen, B.S.; Toft, P.; Schierbeck, J.; Strøm, T.; Chmura, P.J.; Heimann, M.; Dybdahl, L.; et al. Dynamic and Explainable Machine Learning Prediction of Mortality in Patients in the Intensive Care Unit: A Retrospective Study of High-Frequency Data in Electronic Patient Records. Lancet Digit. Health 2020, 2, e179–e191. [Google Scholar] [CrossRef] [PubMed]
- Billimek, J.; Malik, S.; Sorkin, D.H.; Schmalbach, P.; Ngo-Metzger, Q.; Greenfield, S.; Kaplan, S.H. Understanding Disparities in Lipid Management Among Patients with Type 2 Diabetes: Gender Differences in Medication Nonadherence after Treatment Intensification. Women’s Health Issues 2015, 25, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Chin, S.H.; Crothers, D.; Giles, P.; Al-allaf, K.; Khan, J.M. Time Trends of Gender-Based Differences in Lipid Goal Attainments During Secondary Prevention of Coronary Artery Disease: Results of a 5-Year Survey. Am. J. Ther. 2013, 20, 613–617. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, M.; Emerson, S.; Catalano, L.; Hossain, E.; Julien, H.M.; Jacoby, D.S. Post–Acute Coronary Syndrome Disparities in Guideline-Directed Lipid Therapy and Insufficient Achievement of Optimal Low-Density Lipoprotein. Am. J. Cardiol. 2023, 190, 8–16. [Google Scholar] [CrossRef]
- Parhofer, K.G. Interaction between Glucose and Lipid Metabolism: More than Diabetic Dyslipidemia. Diabetes Metab. J. 2015, 39, 353. [Google Scholar] [CrossRef] [Green Version]
- Kasiske, B.L. Effects of Antihypertensive Therapy on Serum Lipids. Ann. Intern Med. 1995, 122, 133. [Google Scholar] [CrossRef]
- Grimm, R.H. Long-Term Effects on Plasma Lipids of Diet and Drugs to Treat Hypertension. JAMA 1996, 275, 1549. [Google Scholar] [CrossRef]
- Fleg, J.L.; Forman, D.E.; Berra, K.; Bittner, V.; Blumenthal, J.A.; Chen, M.A.; Cheng, S.; Kitzman, D.W.; Maurer, M.S.; Rich, M.W.; et al. Secondary Prevention of Atherosclerotic Cardiovascular Disease in Older Adults: A Scientific Statement from the American Heart Association. Circulation 2013, 128, 2422–2446. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Deedwania, P.; Acharya, T.; Aguilar, D.; Bhatt, D.L.; Chyun, D.A.; Di Palo, K.E.; Golden, S.H.; Sperling, L.S.; on behalf of the American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Comprehensive Management of Cardiovascular Risk Factors for Adults With Type 2 Diabetes: A Scientific Statement From the American Heart Association. Circulation 2022, 145, e722–e759. [Google Scholar] [CrossRef]
- Yamashita, S.; Tsubakio-Yamamoto, K.; Ohama, T.; Nakagawa-Toyama, Y.; Nishida, M. Molecular Mechanisms of HDL-Cholesterol Elevation by Statins and Its Effects on HDL Functions. JAT 2010, 17, 436–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilaine, B.; Emily, B.; Sonja, S.; Cheryl, W. The Pleiotropic Vasoprotective Functions of High Density Lipoproteins (HDL). J. Biomed. Res. 2018, 32, 164. [Google Scholar] [CrossRef] [PubMed]
- Sharpton, R.A.; Laucka, P.J.; McKeller, R.N.; Dangler, M.A.; Horne, J.S.; Dangler, J.Y. The Impact of Obesity on the Efficacy of Simvastatin for Lowering Low-Density Lipoprotein Cholesterol in a Veteran Population. Fed. Pract. 2017, 34, 41–44. [Google Scholar]
- Shen, X.; Zhang, Z.; Zhang, X.; Zhao, J.; Zhou, X.; Xu, Q.; Shang, H.; Dong, J.; Liao, L. Efficacy of Statins in Patients with Diabetic Nephropathy: A Meta-Analysis of Randomized Controlled Trials. Lipids Health Dis. 2016, 15, 179. [Google Scholar] [CrossRef] [Green Version]
- Gurwitz, D.; McLeod, H.L. Genome-Wide Studies in Pharmacogenomics: Harnessing the Power of Extreme Phenotypes. Pharmacogenomics 2013, 14, 337–339. [Google Scholar] [CrossRef]



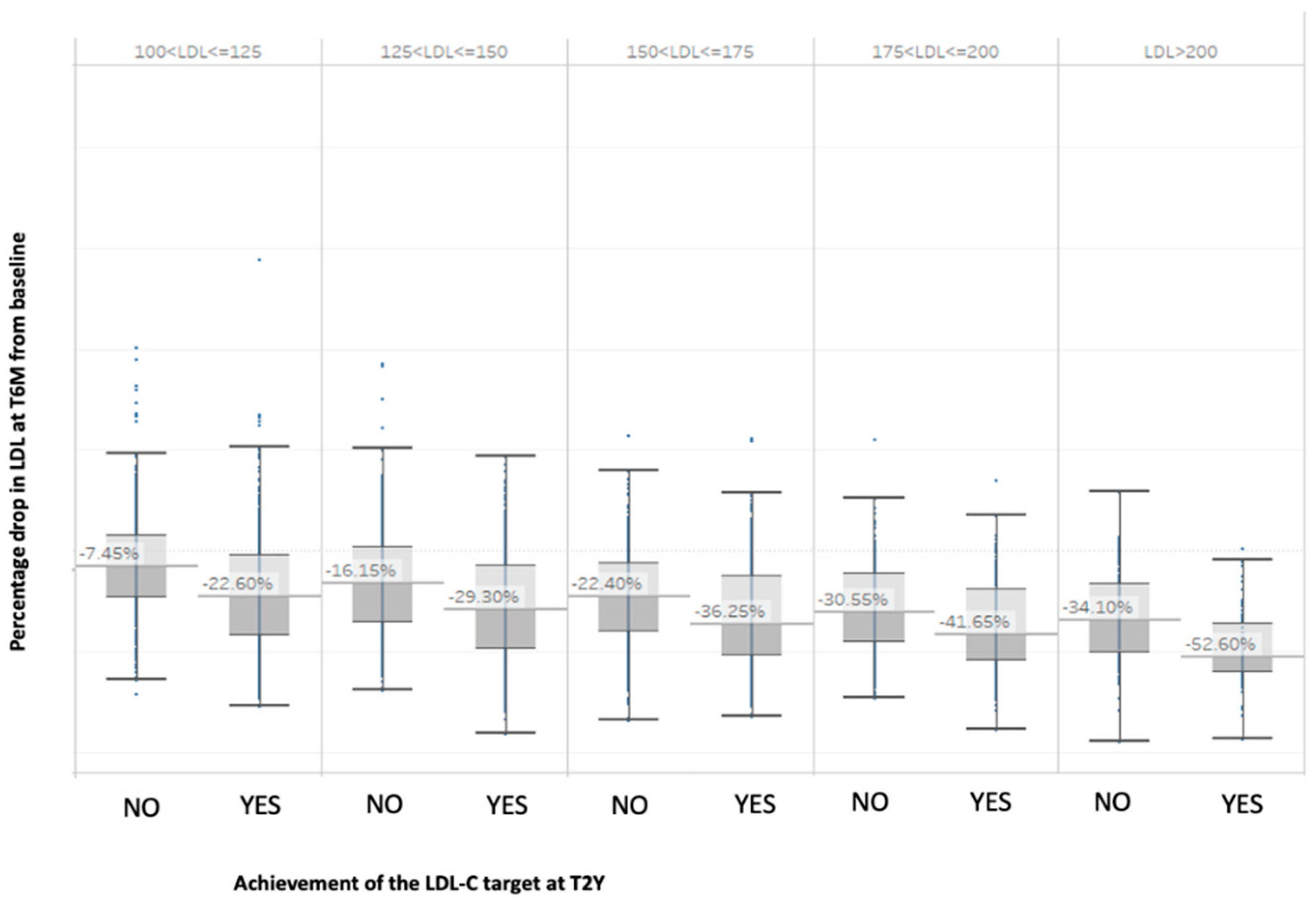
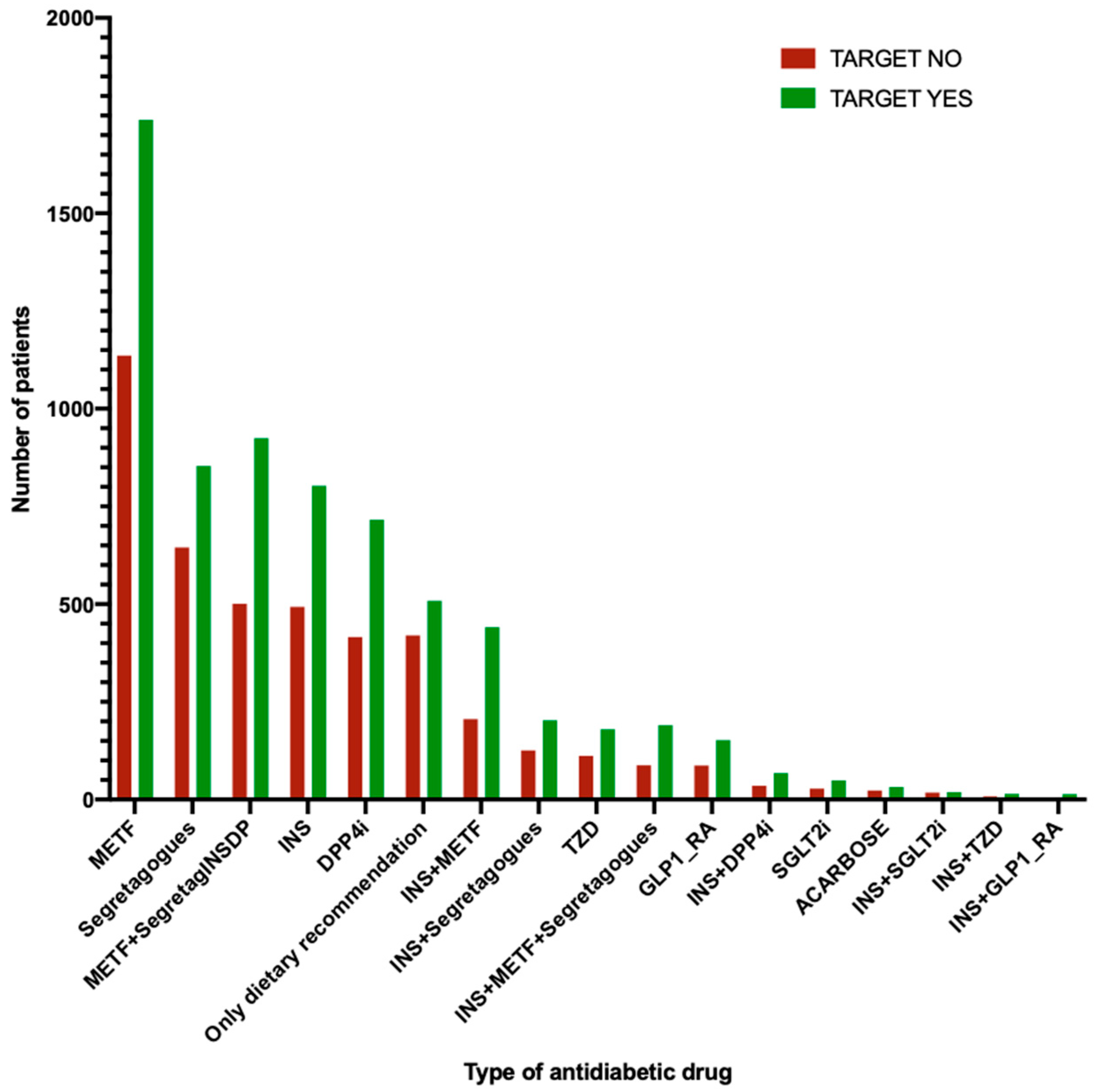
| T0 | T6M | T2Y | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Achieved Target at T2Y | Mean | SD | p-Value | Mean | SD | Mean | SD |
| Age | NO | 65.06 | 10.03 | <0.001 | 65.55 | 10.03 | 67.05 | 10.02 |
| (y) | YES | 65.96 | 9.49 | 66.47 | 9.5 | 67.96 | 9.49 | |
| HbA1c | NO | 7.29 | 1.22 | 0.92 | 7.19 | 1.13 | 7.28 | 1.16 |
| (%) | YES | 7.28 | 1.21 | 7.2 | 1.11 | 7.21 | 1.06 | |
| LDL-C | NO | 142.64 | 32.26 | <0.001 | 117.08 | 32.5 | 127.52 | 23.72 |
| (mg/dL) | YES | 120.86 | 36 | 88.87 | 30.65 | 74.35 | 16.01 | |
| HDL-C | NO | 51.75 | 13.08 | <0.001 | 51.01 | 13.33 | 51.18 | 12.98 |
| (mg/dL) | YES | 50.15 | 13.39 | 49.54 | 13.6 | 49.47 | 14.04 | |
| TGD | NO | 143.6 | 73.43 | 0.32 | 135.74 | 77.61 | 139.33 | 69.97 |
| (mg/dL) | YES | 142.62 | 72.49 | 129.05 | 64.41 | 125.08 | 66.23 | |
| BMI | NO | 29.64 | 5.29 | 0.11 | 29.6 | 5.23 | 29.6 | 5.3 |
| (Kg/m2) | YES | 29.79 | 5.24 | 29.77 | 5.25 | 29.71 | 5.3 | |
| eGFR | NO | 79.09 | 20.3 | <0.001 | 78.36 | 20.36 | 76.66 | 20.9 |
| (mL/min/m2) | YES | 76.92 | 20.47 | 76.34 | 20.69 | 75.34 | 21.67 | |
| Glucose | NO | 147.01 | 44.4 | 0.12 | 143.39 | 43.69 | 144.89 | 43.96 |
| (mg/dL) | YES | 148.07 | 45.28 | 143.05 | 40.05 | 142.05 | 42.95 | |
| DBP | NO | 79.75 | 9.58 | 0.08 | 79.26 | 9.62 | 78.83 | 9.62 |
| (mmHg) | YES | 79.39 | 9.44 | 78.23 | 9.15 | 77.76 | 9.22 | |
| SBP | NO | 137.89 | 18.21 | 0.39 | 137.28 | 17.99 | 137.72 | 18.32 |
| (mmHg) | YES | 138.11 | 18.33 | 136.47 | 17.83 | 136.38 | 17.59 | |
| TC | NO | 223.02 | 36.92 | <0.001 | 195.08 | 37.54 | 206.57 | 29.81 |
| (mg/dL) | YES | 199.79 | 41.35 | 164.16 | 36.57 | 148.75 | 22.72 | |
| Uric A. | NO | 5.51 | 1.96 | 0.05 | 5.47 | 1.46 | 5.53 | 1.55 |
| (mg/dL) | YES | 5.59 | 1.84 | 5.56 | 1.59 | 5.47 | 1.53 | |
| LDL-C ≤ 75 | 75 < LDL-C ≤ 100 | 100 < LDL-C ≤ 125 | 125 < LDL-C ≤ 150 | 150 < LDL-C ≤ 175 | 175 < LDL-C ≤ 200 | LDL > 200 | ||
|---|---|---|---|---|---|---|---|---|
| at T0 | Patients n (%) | 959 (8.52%) | 1509 (13.41%) | 2307 (20.5%) | 3266 (29.03%) | 2207 (19.61%) | 759 (6.75%) | 245 (2.18%) |
| at T6M | Patients n (%) | 2908 (25.84%) | 3390 (30.13%) | 2480 (22.04%) | 1504 (13.37%) | 686 (6.1%) | 221 (1.96%) | 63 (0.56%) |
| at T2Y | Patients n (%) | 3363 (29.89%) | 3607 (32.06%) | 2370 (21.06%) | 1222 (10.86%) | 497 (4.42%) | 141 (1.25%) | 52 (0.46%) |
| Baseline LDL-C Range | Probable LDL-C Target After 2 Years: YES | Probable LDL-C Target After 2 Years: NO |
|---|---|---|
| 100 < LDL-C ≤ 125 | LDL-C reduction 6 months after T0 > 14% | LDL-C reduction 6 months after T0 < 14% |
| 125 < LDL-C ≤ 150 | LDL-C reduction 6 months after T0 > 32% | LDL-C reduction 6 months after T0 < 30% |
| 150 < LDL-C ≤ 175 | LDL-C reduction 6 months after T0 > 33% | LDL-C reduction 6 months after T0 < 33% |
| 175 < LDL-C ≤ 200 | LDL-C reduction 6 months after T0 > 45% | LDL-C reduction 6 months after T0 < 45% |
| 200 < LDL-C ≤ 250 | LDL-C reduction 6 months after T0 > 47% | LDL-C reduction 6 months after T0 < 47% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, D.; Zilich, R.; Candido, R.; Giancaterini, A.; Guaita, G.; Muselli, M.; Ponzani, P.; Santin, P.; Verda, D.; Musacchio, N., on behalf of the Associazione Medici Diabetologi (AMD) Annals Study Group and AMD Artificial Intelligence Study Group. Uncovering Predictors of Lipid Goal Attainment in Type 2 Diabetes Outpatients Using Logic Learning Machine: Insights from the AMD Annals and AMD Artificial Intelligence Study Group. J. Clin. Med. 2023, 12, 4095. https://doi.org/10.3390/jcm12124095
Masi D, Zilich R, Candido R, Giancaterini A, Guaita G, Muselli M, Ponzani P, Santin P, Verda D, Musacchio N on behalf of the Associazione Medici Diabetologi (AMD) Annals Study Group and AMD Artificial Intelligence Study Group. Uncovering Predictors of Lipid Goal Attainment in Type 2 Diabetes Outpatients Using Logic Learning Machine: Insights from the AMD Annals and AMD Artificial Intelligence Study Group. Journal of Clinical Medicine. 2023; 12(12):4095. https://doi.org/10.3390/jcm12124095
Chicago/Turabian StyleMasi, Davide, Rita Zilich, Riccardo Candido, Annalisa Giancaterini, Giacomo Guaita, Marco Muselli, Paola Ponzani, Pierluigi Santin, Damiano Verda, and Nicoletta Musacchio on behalf of the Associazione Medici Diabetologi (AMD) Annals Study Group and AMD Artificial Intelligence Study Group. 2023. "Uncovering Predictors of Lipid Goal Attainment in Type 2 Diabetes Outpatients Using Logic Learning Machine: Insights from the AMD Annals and AMD Artificial Intelligence Study Group" Journal of Clinical Medicine 12, no. 12: 4095. https://doi.org/10.3390/jcm12124095
APA StyleMasi, D., Zilich, R., Candido, R., Giancaterini, A., Guaita, G., Muselli, M., Ponzani, P., Santin, P., Verda, D., & Musacchio, N., on behalf of the Associazione Medici Diabetologi (AMD) Annals Study Group and AMD Artificial Intelligence Study Group. (2023). Uncovering Predictors of Lipid Goal Attainment in Type 2 Diabetes Outpatients Using Logic Learning Machine: Insights from the AMD Annals and AMD Artificial Intelligence Study Group. Journal of Clinical Medicine, 12(12), 4095. https://doi.org/10.3390/jcm12124095