The Early Effects on Tricuspid Annulus and Right Chambers Dimensions in Successful Tricuspid Valve Bicuspidization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Indications for Intervention
2.2. Image Acquisition
2.2.1. Two-Dimensional and Three-Dimensional Transthoracic Echocardiography
2.2.2. Three-Dimensional Transoesophageal Echocardiography
2.3. Two-Dimensional Echocardiography Measurements
2.4. Three-Dimensional Images Analysis
2.5. Reproducibility Analysis
2.6. Surgical Procedure
2.7. Evaluation of Early Clinical Outcomes
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Patients with Bicuspidization vs. without
3.3. Early Clinical Outcomes
3.4. Impact of Bicuspidization on Tricuspid Valve Dimensions
3.5. Transthoracic vs. Transesophageal 3D Echocardiography
3.6. Comparison between 2D and 3D Transthoracic Echocardiography Measurements
3.7. Interobserver and Intraobserver Variability in Annular Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Topilsky, Y.; Maltais, S.; Medina-Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc. Imaging 2019, 12, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Evans, J.C.; Levy, D.; Larson, M.G.; Freed, L.A.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am. J. Cardiol. 1999, 83, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Nath, J.; Foster, E.; Heidenreich, P.A. Impact of tricuspid regurgitation on long-term survival. J. Am. Coll. Cardiol. 2004, 43, 405–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topilsky, Y.; Nkomo, V.T.; Vatury, O.; Michelena, H.I.; Letourneau, T.; Suri, R.M.; Pislaru, S.; Park, S.; Mahoney, D.W.; Biner, S.; et al. Clinical Outcome of Isolated Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2014, 7, 1185–1194. [Google Scholar] [CrossRef] [Green Version]
- Koelling, T.M.; Aaronson, K.D.; Cody, R.J.; Bach, D.S.; Armstrong, W.F. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am. Heart J. 2002, 144, 524–529. [Google Scholar] [CrossRef]
- Muraru, D.; Parati, G.; Badano, L. The Importance and the Challenges of Predicting the Progression of Functional Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2020, 13, 1652–1654. [Google Scholar] [CrossRef]
- Dreyfus, J.; Ghalem, N.; Garbarz, E.; Cimadevilla, C.; Nataf, P.; Vahanian, A.; Caranhac, G.; Messika-Zeitoun, D. Timing of Referral of Patients With Severe Isolated Tricuspid Valve Regurgitation to Surgeons (from a French Nationwide Database). Am. J. Cardiol. 2018, 122, 323–326. [Google Scholar] [CrossRef]
- Benedetto, U.; Melina, G.; Angeloni, E.; Refice, S.; Roscitano, A.; Comito, C.; Sinatra, R. Prophylactic tricuspid annuloplasty in patients with dilated tricuspid annulus undergoing mitral valve surgery. J. Thorac. Cardiovasc. Surg. 2012, 143, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Cleveland, D.C.; Kirklin, J.K.; Naftel, D.C.; Kirklin, J.W.; Blackstone, E.H.; Pacifico, A.; Bargeron, L. Surgical Treatment of Tricuspid Atresia. Ann. Thorac. Surg. 1984, 38, 447–457. [Google Scholar] [CrossRef]
- Muraru, D.; Hahn, R.T.; Soliman, O.I.; Faletra, F.F.; Basso, C.; Badano, L.P. 3-Dimensional Echocardiography in Imaging the Tricuspid Valve. JACC Cardiovasc. Imaging 2019, 12, 500–515. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Antunes, M.J.; Rodríguez-Palomares, J.; Prendergast, B.; De Bonis, M.; Rosenhek, R.; Al-Attar, N.; Barili, F.; Casselman, F.; Folliguet, T.; Iung, B.; et al. Management of tricuspid valve regurgitation: Position statement of the European Society of Cardiology Working Groups of Cardiovascular Surgery and Valvular Heart Disease. Eur. J. Cardio-Thoracic Surg. 2017, 52, 1022–1030. [Google Scholar] [CrossRef] [Green Version]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; La Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Multi-modality imaging assessment of native valvular regurgitation: An EACVI and ESC council of valvular heart disease position paper. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef] [Green Version]
- 4D Auto TVQ Tricuspid Valve Quantification, White Paper. Available online: https://gevividultraedition.com/storage/app/media/whitepapers/4D-Auto-TVQ-whitepaper-JB03442XX.pdf (accessed on 1 February 2023).
- Hahn, R.T.; Thomas, J.D.; Khalique, O.K.; Cavalcante, J.L.; Praz, F.; Zoghbi, W.A. Imaging Assessment of Tricuspid Regurgitation Severity. JACC Cardiovasc. Imaging 2019, 12, 469–490. [Google Scholar] [CrossRef]
- Fukuda, S.; Saracino, G.; Matsumura, Y.; Daimon, M.; Tran, H.; Greenberg, N.L.; Hozumi, T.; Yoshikawa, J.; Thomas, J.D.; Shiota, T. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgi-tation: A real-time, 3-dimensional echocardiographic study. Circulation 2006, 114, I-492–I-498. [Google Scholar] [CrossRef] [Green Version]
- Anwar, A.M.; Geleijnse, M.L.; Cate, F.J.T.; Meijboom, F.J. Assessment of tricuspid valve annulus size, shape and function using real-time three-dimensional echocardiography. Interact. Cardiovasc. Thorac. Surg. 2006, 5, 683–687. [Google Scholar] [CrossRef] [Green Version]
- Addetia, K.; Muraru, D.; Veronesi, F.; Jenei, C.; Cavalli, G.; Besser, S.A.; Mor-Avi, V.; Lang, R.M.; Badano, L.P. 3-Dimensional Echocardiographic Analysis of the Tricuspid Annulus Provides New Insights Into Tricuspid Valve Geometry and Dynamics. JACC Cardiovasc. Imaging 2019, 12, 401–412. [Google Scholar] [CrossRef]
- Dreyfus, J.; Durand-Viel, G.; Raffoul, R.; Alkhoder, S.; Hvass, U.; Radu, C.; Al-Attar, N.; Ghodbhane, W.; Attias, D.; Nataf, P.; et al. Comparison of 2-dimensional, 3-dimensional, and surgical measurements of the tricuspid annulus size clinical implications. Circ. Cardiovasc. Imaging 2015, 8, e003241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraru, D.; Addetia, K.; Guta, A.C.; Ochoa-Jimenez, R.C.; Genovese, D.; Veronesi, F.; Basso, C.; Iliceto, S.; Badano, L.P.; Lang, R.M. Right atrial volume is a major determinant of tricuspid annulus area in functional tricuspid regurgitation: A three-dimensional echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2020, 22, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Saha-Chaudhuri, P.; Rankin, J.S.; Conte, J.V. Trends and Outcomes of Tricuspid Valve Surgery in North America: An Analysis of More Than 50,000 Patients From The Society of Thoracic Surgeons Database. Ann. Thorac. Surg. 2013, 96, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.N.; Menon, V.; Sammour, Y.M.; Gajulapalli, R.D.; Meenakshisundaram, C.; Nusairat, L.; Mohananey, D.; Hernandez, A.V.; Navia, J.; Krishnaswamy, A.; et al. Outcomes of patients with severe tricuspid regurgitation and congestive heart failure. Heart 2019, 105, 1813–1817. [Google Scholar] [CrossRef]
- Kara, I.; Koksal, C.; Erkin, A.; Sacli, H.; Demirtas, M.; Percin, B.; Diler, M.S.; Kirali, K. Outcomes of Mild to Moderate Functional Tricuspid Regurgitation in Patients Undergoing Mitral Valve Operations: A Meta-Analysis of 2,488 Patients. Ann. Thorac. Surg. 2015, 100, 2398–2407. [Google Scholar] [CrossRef]
- Piperata, A.; Eynde, J.V.D.; Pernot, M.; Busuttil, O.; Avesani, M.; Bottio, T.; Lafitte, S.; Modine, T.; Labrousse, L. Long-term outcomes of concomitant suture bicuspidization technique to treat mild or moderate tricuspid regurgitation in patients undergoing mitral valve surgery. Eur. J. Cardio-Thoracic Surg. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Ghanta, R.K.; Chen, R.; Narayanasamy, N.; McGurk, S.; Lipsitz, S.; Chen, F.Y.; Cohn, L.H. Suture bicuspidization of the tricuspid valve versus ring annuloplasty for repair of functional tricuspid regurgitation: Midterm results of 237 consecutive patients. J. Thorac. Cardiovasc. Surg. 2007, 133, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Hirji, S.; Yazdchi, F.; Kiehm, S.; Landino, S.; McGurk, S.; Muehlschlegel, J.; Singh, S.; Mallidi, H.; Pelletier, M.; Aranki, S.; et al. Outcomes After Tricuspid Valve Repair With Ring Versus Suture Bicuspidization Annuloplasty. Ann. Thorac. Surg. 2020, 110, 821–828. [Google Scholar] [CrossRef]
- Praz, F.; Muraru, D.; Kreidel, F.; Lurz, P.; Hahn, R.T.; Delgado, V.; Senni, M.; von Bardeleben, R.S.; Nickenig, G.; Hausleiter, J.; et al. Transcatheter treatment for tricuspid valve disease. Eurointervention 2021, 17, 791–808. [Google Scholar] [CrossRef]
- Sulejmani, F.; Pataky, J.; Sun, W. Mechanical and Structural Evaluation of Tricuspid Bicuspidization in a Porcine Model. Cardiovasc. Eng. Technol. 2020, 11, 522–531. [Google Scholar] [CrossRef]
- Mathur, M.; Meador, W.D.; Jazwiec, T.; Malinowski, M.; Timek, T.A.; Rausch, M.K. The Effect of Downsizing on the Normal Tricuspid Annulus. Ann. Biomed. Eng. 2019, 48, 655–668. [Google Scholar] [CrossRef]
- Hahn, R.T.; Meduri, C.U.; Davidson, C.J.; Lim, S.; Nazif, T.M.; Ricciardi, M.J.; Rajagopal, V.; Ailawadi, G.; Vannan, M.A.; Thomas, J.D.; et al. Early Feasibility Study of a Transcatheter Tricuspid Valve Annuloplasty: SCOUT Trial 30-Day Results. J. Am. Coll. Cardiol. 2017, 69, 1795–1806. [Google Scholar] [CrossRef]
- Nickenig, G.; Friedrichs, K.P.; Baldus, S.; Arnold, M.; Seidler, T.; Hakmi, S.; Linke, A.; Schäfer, U.; Dreger, H.; Reinthaler, M.; et al. Thirty-day outcomes of the Cardioband tricuspid system for patients with symptomatic functional tricuspid regurgitation: The TriBAND study. Eurointervention 2021, 17, 809–817. [Google Scholar] [CrossRef]
- Fukuda, S.; Song, J.-M.; Gillinov, A.M.; McCarthy, P.M.; Daimon, M.; Kongsaerepong, V.; Thomas, J.D.; Shiota, T. Tricuspid Valve Tethering Predicts Residual Tricuspid Regurgitation After Tricuspid Annuloplasty. Circulation 2005, 111, 975–979. [Google Scholar] [CrossRef] [Green Version]
- Okada, D.R.; Rahmouni, H.W.; Herrmann, H.C.; Bavaria, J.E.; Forfia, P.R.; Han, Y. Assessment of Right Ventricular Function by Transthoracic Echocardiography Following Aortic Valve Replacement. Echocardiography 2013, 31, 552–557. [Google Scholar] [CrossRef]
- Raina, A.; Vaidya, A.; Gertz, Z.; Chambers, S.; Forfia, P.R. Marked changes in right ventricular contractile pattern after cardiothoracic surgery: Implications for post-surgical assessment of right ventricular function. J. Heart Lung Transplant. 2013, 32, 777–783. [Google Scholar] [CrossRef]
- Keyl, C.; Schneider, J.; Beyersdorf, F.; Ruile, P.; Siepe, M.; Pioch, K.; Schneider, R.; Jander, N. Right ventricular function after aortic valve replacement: A pilot study comparing surgical and transcatheter procedures using 3D echocardiography. Eur. J. Cardio-Thoracic Surg. 2015, 49, 966–971. [Google Scholar] [CrossRef] [Green Version]
- Korshin, A.; Grønlykke, L.; Nilsson, J.C.; Møller-Sørensen, H.; Ihlemann, N.; Kjøller, S.M.; Damgaard, S.; Lehnert, P.; Hassager, C.; Kjaergaard, J.; et al. Tricuspid annular plane systolic excursion is significantly reduced during uncomplicated coronary artery bypass surgery: A prospective observational study. J. Thorac. Cardiovasc. Surg. 2019, 158, 480–489. [Google Scholar] [CrossRef]
- Donauer, M.; Schneider, J.; Jander, N.; Beyersdorf, F.; Keyl, C. Perioperative Changes of Right Ventricular Function in Cardiac Surgical Patients Assessed by Myocardial Deformation Analysis and 3-Dimensional Echocardiography. J. Cardiothorac. Vasc. Anesthesia 2020, 34, 708–718. [Google Scholar] [CrossRef] [Green Version]
- Jazwiec, T.; Malinowski, M.; Proudfoot, A.G.; Eberhart, L.; Langholz, D.; Schubert, H.; Wodarek, J.; Timek, T.A. Tricuspid valvular dynamics and 3-dimensional geometry in awake and anesthetized sheep. J. Thorac. Cardiovasc. Surg. 2018, 156, 1503–1511. [Google Scholar] [CrossRef]
- Bach, D.S.; Deeb, G.M.; Bolling, S.F. Accuracy of intraoperative transesophageal echocardiography for estimating the severity of functional mitral regurgitation. Am. J. Cardiol. 1995, 76, 508–512. [Google Scholar] [CrossRef]
- Chin, J.-H.; Lee, E.-H.; Choi, D.-K.; Choi, I.-C. The Effect of Depth of Anesthesia on the Severity of Mitral Regurgitation as Measured by Transesophageal Echocardiography. J. Cardiothorac. Vasc. Anesthesia 2012, 26, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Grewal, K.S.; Malkowski, M.J.; Piracha, A.R.; Astbury, J.C.; Kramer, C.M.; Dianzumba, S.; Reichek, N. Effect of general anesthesia on the severity of mitral regurgitation by transesophageal echocardiography. Am. J. Cardiol. 2000, 85, 199–203. [Google Scholar] [CrossRef] [PubMed]



| Overall, n = 40 | With Bicuspidization, n = 21 | Without Bicuspidization, n = 19 | p-Value | |
|---|---|---|---|---|
| Age, yrs | 60.9 ± 9.5 | 62.7 ± 9.4 | 59.0 ± 9.5 | 0.221 |
| Female/male | 14 (35%)/26 (65%) | 7 (33%)/14 (67%) | 7 (37%)/12 (63%) | 0.816 |
| Weight, kg | 78.6 ± 18.9 | 77.7 ± 18.5 | 79.5 ± 19.8 | 0.818 |
| Height, cm | 171.3 ± 9.6 | 171.2 ± 9.8 | 171.4 ± 9.6 | 0.914 |
| BSA, m2 | 1.92 ± 0.27 | 1.91 ± 0.27 | 1.93 ± 0.28 | 0.819 |
| Hemoglobin, g/L | 140.6 ± 14.7 | 140.7 ± 14.6 | 140.5 ± 15.3 | 0.972 |
| GFR, mL/min | 75.8 ± 18.8 | 67.3 ± 17.9 | 85.1 ± 15.3 | 0.005 |
| BNP, ng/L | 225.2 (76.1–526.2) | 411.7 (210.6–855.0) | 82.0 (49.1–225.2) | 0.003 |
| Atrial fibrillation | 23 (58%) | 14 (67%) | 9 (47%) | 0.218 |
| Coronary artery disease | 14 (35%) | 8 (38%) | 6 (31%) | 0.666 |
| COPD | 2 (5%) | 2 (9%) | 0 (0%) | 0.168 |
| NYHA class: | 0.004 | |||
| I | 2 (5%) | 0 (0%) | 2 (11%) | |
| II | 9 (23%) | 1 (5%) | 8 (42%) | |
| III | 29 (73%) | 20 (95%) | 9 (47%) | |
| Preoperative LVEF, % | 54.2 ± 6.6 | 54.5 ± 5.0 | 53.8 ± 8.2 | 0.510 |
| Preoperative PASP, mmHg | 44.0 ± 16.3 | 44.1 ± 16.1 | 43.8 ± 17.3 | 0.877 |
| Preoperative TR grade: | 0.074 | |||
| No TR | 9 (23%) | 3 (14%) | 6 (32%) | |
| Mild | 19 (48%) | 8 (38%) | 11 (58%) | |
| Moderate | 9 (23%) | 7 (33%) | 2 (10%) | |
| Severe | 3 (8%) | 3 (14%) | 0 (0%) | |
| Preoperative MR grade: | 0.793 | |||
| Mild | 3 (8%) | 1 (5%) | 2 (10%) | |
| Moderate | 1 (3%) | 1 (5%) | 0 (0%) | |
| Severe | 36 (90%) | 19 (90%) | 17 (90%) | |
| Left heart disease: | 0.551 | |||
| Functional MR | 5 (12%) | 2 (10%) | 3 (16%) | |
| MV flail | 18 (45%) | 9 (42%) | 9 (47%) | |
| MV prolapse | 6 (15%) | 2 (10%) | 4 (21%) | |
| Rheumatic MS, MR | 10 (25%) | 7 (33%) | 3 (16%) | |
| Infective endocarditis | 1 (3%) | 1 (5%) | 0 (0%) | |
| MV surgery type: | 0.796 | |||
| MV prosthesis | 16 (40%) | 8 (38%) | 8 (42%) | |
| MV repair | 24 (60%) | 13 (62%) | 11 (58%) | |
| Concomitant surgery: | 0.178 | |||
| CABG | 5 (13%) | 3 (14%) | 2 (11%) | |
| Aortic valve prosthesis | 6 (15%) | 1 (5%) | 5 (26%) |
| With Bicuspidization, n = 21 | Without Bicuspidization, n = 19 | p-Value | |
|---|---|---|---|
| 3DE parameters | |||
| TA area, cm2 | 11.8 ± 3.0 | 8.6 ± 2,5 | 0.001 |
| TA perimeter, cm | 12.5 ± 1.6 | 10,6 ± 1,6 | 0.001 |
| TA major axis, cm | 4.2 ± 0.6 | 3.7 ± 0.5 | 0.002 |
| TA minor axis, cm | 3.4 ± 0.5 | 2.8 ± 0.5 | 0.001 |
| 4Ch diameter, cm | 3.5 ± 0.6 | 3.3 ± 0.6 | 0.257 |
| 2Ch diameter, cm | 3.5 ± 0.4 | 3.1 ± 0.5 | 0.040 |
| Max tenting height, cm | 0.58 ± 0.17 | 0.53 ± 0.19 | 0.439 |
| Coaptation height, cm | 0.52 ± 0.33 | 0.41 ± 0.21 | 0.272 |
| Tenting volume, mL | 1.4 (1.0–2.1) | 1.1 (0.8–1.2) | 0.095 |
| Sphericity index, % | 82.9 ± 9.5 | 77. 7 ± 7.4 | 0.109 |
| 2DE parameters | |||
| Systolic apical 4Ch diameter, cm | 3.4 ± 0.6 | 3.0 ± 0.5 | 0.013 |
| Systolic parasternal long axis diameter, cm | 3.4 ± 0.7 | 3.3 ± 0.6 | 0.447 |
| Systolic parasternal short axis diameter, cm | 3.3 ± 0.6 | 3.1 ± 0.6 | 0.271 |
| 2D TA end-diastolic | 3.9 ± 0.5 | 3.3 ± 0.5 | 0.002 |
| diameter, cm | |||
| RV basal diameter, cm | 4.0 ± 0.5 | 3.5 ± 0.6 | 0.004 |
| RV mid diameter, cm | 3.2 ± 0.5 | 2.8 ± 0.6 | 0.006 |
| RV length, cm | 7.1 ± 0.9 | 7.1 ± 0.8 | 0.878 |
| FAC, % | 36.2 ± 11.2 | 42.6 ± 13.4 | 0.113 |
| RA area, cm2 | 21.5 ± 3.5 | 17.0 ± 4.2 | 0.001 |
| EROA, cm2 | 0.25 (0.07–0.34) | 0.13 (0.12–0.36) | 0.982 |
| Regurgitant volume, mL | 21.0 (7.0–26.0) | 11.5 (8.3–20.3) | 0.387 |
| TR grade: | 0.074 | ||
| No TR | 3 (14%) | 6 (32%) | |
| Mild | 8 (38%) | 11 (58%) | |
| Moderate | 7 (33%) | 2 (10%) | |
| Severe | 3 (14%) | 0 (0%) | |
| MR grade: | 0.793 | ||
| No MR | 0 (0%) | 0 (0%) | |
| Mild | 1 (5%) | 2 (10%) | |
| Moderate | 1 (5%) | 0 (0%) | |
| Severe | 19 (90%) | 17 (90%) | |
| With Bicuspidization, n = 21 | Without Bicuspidization, n = 19 | p-Value | |
|---|---|---|---|
| CPB time | 156 ± 39 | 144 ± 49 | 0.383 |
| Early mortality (≤30 days) | 0 (0%) | 0 (0%) | - |
| ICU stay (days) | 4 (3; 6) | 3 (2; 4) | 0.488 |
| Hospital stay (days) | 17 (13; 28) | 16 (12; 21) | 0.491 |
| Postoperative complications: | |||
| New-onset AF | 2 (9.5%) | 7 (36.8%) | 0.039 |
| IABP or ECMO | 1 (4.8%) | 0 (0%) | 0.335 |
| Resternotomy for bleeding | 1 (4.8%) | 2 (10.5%) | 0.489 |
| AKI requiring dialysis | 1 (4.8%) | 1 (5.3%) | 0.942 |
| Stroke | 1 (4.8%) | 2 (10.5%) | 0.489 |
| Respiratory complication | 4 (19.0%) | 2 (10.5%) | 0.451 |
| Atrioventricular block | 3 (14.3%) | 3 (15.8%) | 0.894 |
| Permanent pacemaker insertion | 2 (9.5%) | 3 (15.8%) | 0.550 |
| Mediastinitis | 2 (9.5%) | 0 (0%) | 0.168 |
| Tracheostomy | 1 (4.8%) | 0 (0%) | 0.335 |
| KERRYPNX | With Bicuspidization, n = 21 | Without Bicuspidization, n = 19 | ||||
|---|---|---|---|---|---|---|
| Before | After | p-Value | Before | After | p-Value | |
| 3DE parameters | ||||||
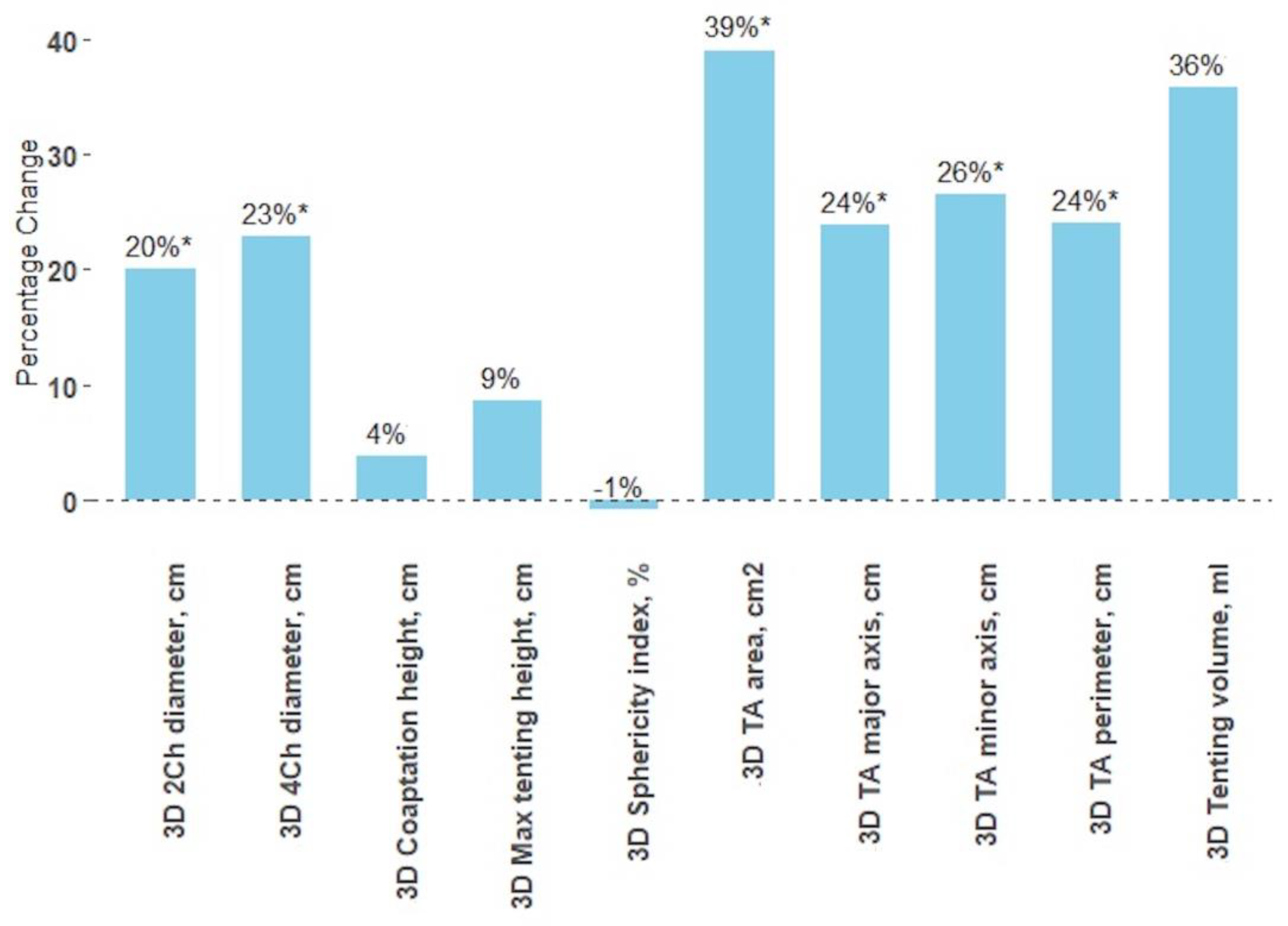
| TA area, cm2 | 11.8 ± 3.0 | 7.2 ± 3.3 | <0.001 | 8.6 ± 2,5 | 9.1 ± 2.2 | 0.346 |
| TA perimeter, cm | 12.5 ± 1.6 | 9.5 ± 2.1 | <0.001 | 10.6 ± 1,6 | 10.9 ± 1.4 | 0.449 |
| TA major axis, cm | 4.2 ± 0.6 | 3.2 ± 0.7 | <0.001 | 3.7 ± 0.5 | 3.7 ± 0.4 | 0.544 |
| TA minor axis, cm | 3.4 ± 0.5 | 2.5 ± 0.6 | <0.001 | 2.8 ± 0.5 | 3.0 ± 0.5 | 0.172 |
| 4Ch diameter, cm | 3.5 ± 0.6 | 2.7 ± 0.6 | 0.010 | 3.3 ± 0.6 | 3.3 ± 0.5 | 0.780 |
| 2Ch diameter, cm | 3.5 ± 0.4 | 2.8 ± 0.5 | 0.049 | 3.1 ± 0.5 | 3.1 ± 0.6 | 0.796 |
| Max tenting height, cm | 0.60 (0.40–0.70) | 0.50 (0.40–0.65) | 0.751 | 0.53 ± 0.19 | 0.48 ± 0.15 | 0.763 |
| Coaptation height, cm | 0.40 (0.33–0.60) | 0.50 (0.40–0.60) | 0.487 | 0.41 ± 0.21 | 0.31 ± 0.20 | 0.262 |
| Tenting volume, mL | 1.4 (1.0–2.1) | 0.9 (0.5–1.3) | 0.068 | 1.1 (0.8–1.2) | 0.8 (0.4–1.7) | 0.962 |
| Sphericity index, % | 82.9 ± 9.5 | 83.6 ± 5.7 | 0.632 | 77.7 ± 7.4 | 78.8 ± 8.0 | 0.207 |
| 2DE parameters | ||||||
| 2D TA end-diastolic diameter, cm | 3.9 ± 0.5 | 3.1 ± 0.6 | <0.001 | 3.3 ± 0.5 | 3.7 ± 0.5 | <0.001 |
| RV basal diameter, cm | 4.0 ± 0.5 | 3.6 ± 0.6 | 0.020 | 3.5 ± 0.6 | 3.9 ± 0.6 | 0.001 |
| RV mid diameter, cm | 3.2 ± 0.5 | 3.0 ± 0.6 | 0.108 | 2.8 ± 0.6 | 3.1 ± 0.6 | 0.052 |
| RV length, cm | 7.1 ± 0.9 | 7.3 ± 0.7 | 0.228 | 7.1 ± 0.8 | 7.1 ± 0.9 | 0.956 |
| FAC, % | 36.2 ± 11.2 | 37 ± 9.2 | 0.863 | 42.6 ± 13.4 | 45.8 ± 11.3 | 0.116 |
| RA area, cm2 | 21.5 ± 3.5 | 15.5 ± 5.1 | <0.001 | 17.0 ± 4.2 | 14.7 ± 4.5 | 0.001 |
| EROA, cm2 | 0.25 (0.07–0.34) | 0.08 (0.08–0.09) | 0.043 | 0.13 (0.12–0.36) | 0.15 (0.10–0.19) | 1.000 |
| Regurgitant volume, mL | 21.0 (7.0–26.0) | 5.0 (4.0–5.0) | 0.041 | 11.5 (8.3–20.3) | 11.0 (6.3–13.3) | 0.364 |
| TR grade: | <0.001 | 0.21 | ||||
| No TR | 3 (14%) | 13 (62%) | 6 (32%) | 10 (53%) | ||
| Mild | 8 (38%) | 8 (38%) | 11 (58%) | 9 (47%) | ||
| Moderate | 7 (33%) | 0 (0%) | 2 (10%) | 0 (0%) | ||
| Severe | 3 (14%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| MR grade: | <0.001 | <0.001 | ||||
| No MR | 0 (0%) | 12 (57%) | 0 (0%) | 16 (84%) | ||
| Mild | 1 (5%) | 6 (29%) | 2 (10%) | 2 (11%) | ||
| Moderate | 1 (5%) | 3 (14%) | 0 (5%) | 1 (5%) | ||
| Severe | 19 (90%) | 0 (0%) | 17 (90%) | 0 (0%) | ||
| Transthoracic 3DE | Transesophageal 3DE | p-Value | |
|---|---|---|---|
| TA area, cm2 | 9.9 ± 3.3 | 11.4 ± 2,5 | 0.002 |
| TA perimeter, cm | 11.4 ± 1.9 | 12.4 ± 2.0 | 0.002 |
| TA major axis, cm | 3.9 ± 0.6 | 4.1 ± 0.5 | 0.016 |
| TA minor axis, cm | 3.1 ± 0.6 | 3.4 ± 0.5 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bieliauskienė, G.; Kažukauskienė, I.; Janušauskas, V.; Zorinas, A.; Ručinskas, K.; Mainelis, A.; Zakarkaitė, D. The Early Effects on Tricuspid Annulus and Right Chambers Dimensions in Successful Tricuspid Valve Bicuspidization. J. Clin. Med. 2023, 12, 4093. https://doi.org/10.3390/jcm12124093
Bieliauskienė G, Kažukauskienė I, Janušauskas V, Zorinas A, Ručinskas K, Mainelis A, Zakarkaitė D. The Early Effects on Tricuspid Annulus and Right Chambers Dimensions in Successful Tricuspid Valve Bicuspidization. Journal of Clinical Medicine. 2023; 12(12):4093. https://doi.org/10.3390/jcm12124093
Chicago/Turabian StyleBieliauskienė, Gintarė, Ieva Kažukauskienė, Vilius Janušauskas, Aleksejus Zorinas, Kęstutis Ručinskas, Antanas Mainelis, and Diana Zakarkaitė. 2023. "The Early Effects on Tricuspid Annulus and Right Chambers Dimensions in Successful Tricuspid Valve Bicuspidization" Journal of Clinical Medicine 12, no. 12: 4093. https://doi.org/10.3390/jcm12124093
APA StyleBieliauskienė, G., Kažukauskienė, I., Janušauskas, V., Zorinas, A., Ručinskas, K., Mainelis, A., & Zakarkaitė, D. (2023). The Early Effects on Tricuspid Annulus and Right Chambers Dimensions in Successful Tricuspid Valve Bicuspidization. Journal of Clinical Medicine, 12(12), 4093. https://doi.org/10.3390/jcm12124093





