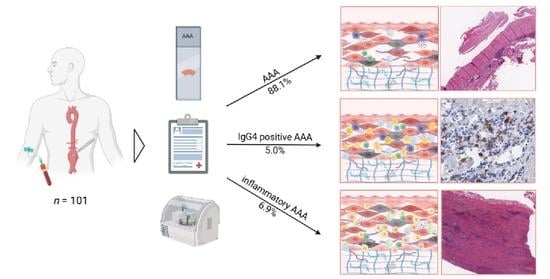
The Incidence of IgG4-Related and Inflammatory Abdominal Aortic Aneurysm Is Rare in a 101 Patient Cohort
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Identification, Inclusion Criteria and Ethical Approval
2.2. Basic Patient and Clinical Data, Procedure Details and Outcomes
2.3. AAA Characteristics
2.4. Sample Acquisition, Preparation and Digitalization
2.5. Immunohistochemistry
2.6. Pathologic Analysis and Definitions
2.7. Serum and Blood Analysis and Definitions
2.8. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakalihasan, N.; Michel, J.B.; Katsargyris, A.; Kuivaniemi, H.; Defraigne, J.O.; Nchimi, A.; Powell, J.T.; Yoshimura, K.; Hultgren, R. Abdominal aortic aneurysms. Nat. Rev. Dis. Prim. 2018, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef]
- Busch, A.; Hartmann, E.; Grimm, C.; Ergun, S.; Kickuth, R.; Otto, C.; Kellersmann, R.; Lorenz, U. Heterogeneous histomorphology, yet homogeneous vascular smooth muscle cell dedifferentiation, characterize human aneurysm disease. J. Vasc. Surg. 2016, 66, 1553–1564.e6. [Google Scholar] [CrossRef] [PubMed]
- Curci, J.A.; Thompson, R.W. Adaptive cellular immunity in aortic aneurysms: Cause, consequence, or context? J. Clin. Investig. 2004, 114, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.J. Intraluminal thrombus: Innocent bystander or factor in abdominal aortic aneurysm pathogenesis? JVS Vasc. Sci. 2021, 2, 159–169. [Google Scholar] [CrossRef]
- Bobryshev, Y.V.; Lord, R.S. Vascular-associated lymphoid tissue (VALT) involvement in aortic aneurysm. Atherosclerosis 2001, 154, 15–21. [Google Scholar] [CrossRef]
- Gabel, G.; Northoff, B.H.; Balboa, A.; Becirovic-Agic, M.; Petri, M.; Busch, A.; Maegdefessel, L.; Mahlmann, A.; Ludwig, S.; Teupser, D.; et al. Parallel Murine and Human Aortic Wall Genomics Reveals Metabolic Reprogramming as Key Driver of Abdominal Aortic Aneurysm Progression. J. Am. Heart Assoc. 2021, 10, e020231. [Google Scholar] [CrossRef]
- Marquez-Sanchez, A.C.; Koltsova, E.K. Immune and inflammatory mechanisms of abdominal aortic aneurysm. Front. Immunol. 2022, 13, 989933. [Google Scholar] [CrossRef]
- Haug, E.S.; Skomsvoll, J.F.; Jacobsen, G.; Halvorsen, T.B.; Saether, O.D.; Myhre, H.O. Inflammatory aortic aneurysm is associated with increased incidence of autoimmune disease. J. Vasc. Surg. 2003, 38, 492–497. [Google Scholar] [CrossRef]
- Caradu, C.; Ammollo, R.P.; Dari, L.; Wanhainen, A.; Van Herzeele, I.; Bellmunt-Montoya, S.; Ducasse, E.; Berard, X. Management of Inflammatory Aortic Aneurysms—A Scoping Review. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 493–502. [Google Scholar] [CrossRef]
- Kim, I.Y.; Eun, Y.H.; Jeong, H.; Park, T.K.; Kim, H.; Lee, J.; Jang, S.Y.; Kim, J.S.; Koh, E.M.; Kim, D.K.; et al. Clinical characteristics and outcomes of 61 patients with chronic periaortitis including IgG4-related and non-IgG4-related cases. Int. J. Rheum. Dis. 2017, 20, 1751–1762. [Google Scholar] [CrossRef]
- Prucha, M.; Sedivy, P.; Stadler, P.; Zdrahal, P.; Prokopova, P.; Voska, L.; Sedlackova, L. Abdominal aortic aneurysm as an IgG4-related disease. Clin. Exp. Immunol. 2019, 197, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Raparia, K.; Molina, C.P.; Quiroga-Garza, G.; Weilbaecher, D.; Ayala, A.G.; Ro, J.Y. Inflammatory aortic aneurysm: Possible manifestation of IgG4-related sclerosing disease. Int. J. Clin. Exp. Pathol. 2013, 6, 469–475. [Google Scholar]
- Kasashima, S.; Kasashima, F.; Kawashima, A.; Endo, M.; Matsumoto, Y.; Kawakami, K. Clinical Outcomes After Endovascular Repair and Open Surgery to Treat Immunoglobulin G4-Related and Nonrelated Inflammatory Abdominal Aortic Aneurysms. J. Endovasc. Ther. 2017, 24, 833–845. [Google Scholar] [CrossRef]
- Sakata, N.; Tashiro, T.; Uesugi, N.; Kawara, T.; Furuya, K.; Hirata, Y.; Iwasaki, H.; Kojima, M. IgG4-positive plasma cells in inflammatory abdominal aortic aneurysm: The possibility of an aortic manifestation of IgG4-related sclerosing disease. Am. J. Surg. Pathol. 2008, 32, 553–559. [Google Scholar] [CrossRef]
- Capoccia, L.; Riambau, V. Endovascular repair versus open repair for inflammatory abdominal aortic aneurysms. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Pelisek, J.; Hegenloh, R.; Bauer, S.; Metschl, S.; Pauli, J.; Glukha, N.; Busch, A.; Reutersberg, B.; Kallmayer, M.; Trenner, M.; et al. Biobanking: Objectives, Requirements, and Future Challenges-Experiences from the Munich Vascular Biobank. J. Clin. Med. 2019, 8, 251. [Google Scholar] [CrossRef]
- Trenner, M.; Radu, O.; Zschapitz, D.; Bohmann, B.; Biro, G.; Eckstein, H.H.; Busch, A. Can We Still Teach Open Repair of Abdominal Aortic Aneurysm in The Endovascular Era? Single-Center Analysis on The Evolution of Procedural Characteristics Over 15 Years. J. Surg. Educ. 2022, 79, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Velu, R.; Quigley, F.; Jenkins, J.; Singh, T.P. Editor’s Choice—Cohort Study Examining the Association Between Abdominal Aortic Size and Major Adverse Cardiovascular Events in Patients with Aortic and Peripheral Occlusive and Aneurysmal Disease. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311; discussion 312–313. [Google Scholar] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, V.; Zen, Y.; Chan, J.K.; Yi, E.E.; Sato, Y.; Yoshino, T.; Kloppel, G.; Heathcote, J.G.; Khosroshahi, A.; Ferry, J.A.; et al. Consensus statement on the pathology of IgG4-related disease. Mod. Pathol. 2012, 25, 1181–1192. [Google Scholar] [CrossRef]
- Hamano, H.; Kawa, S.; Horiuchi, A.; Unno, H.; Furuya, N.; Akamatsu, T.; Fukushima, M.; Nikaido, T.; Nakayama, K.; Usuda, N.; et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N. Engl. J. Med. 2001, 344, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Wallace, Z.S.; Naden, R.P.; Chari, S.; Choi, H.; Della-Torre, E.; Dicaire, J.F.; Hart, P.A.; Inoue, D.; Kawano, M.; Khosroshahi, A.; et al. The 2019 American College of Rheumatology/European League Against Rheumatism Classification Criteria for IgG4-Related Disease. Arthritis Rheumatol. 2020, 72, 7–19. [Google Scholar] [CrossRef]
- Kasashima, S.; Zen, Y.; Kawashima, A.; Konishi, K.; Sasaki, H.; Endo, M.; Matsumoto, Y.; Kawakami, K.; Kasashima, F.; Moriya, M.; et al. Inflammatory abdominal aortic aneurysm: Close relationship to IgG4-related periaortitis. Am. J. Surg. Pathol. 2008, 32, 197–204. [Google Scholar] [CrossRef]
- Kasashima, S.; Zen, Y.; Kawashima, A.; Endo, M.; Matsumoto, Y.; Kasashima, F. A new clinicopathological entity of IgG4-related inflammatory abdominal aortic aneurysm. J. Vasc. Surg. 2009, 49, 1264–1271; discussion 1271. [Google Scholar] [CrossRef]
- Koo, B.S.; Koh, Y.W.; Hong, S.; Kim, Y.J.; Kim, Y.G.; Lee, C.K.; Yoo, B. Frequency of immunoglobulin G4-related aortitis in cases with aortic resection and their clinical characteristics compared to other aortitises. Int. J. Rheum. Dis. 2014, 17, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Sakata, N.; Nabeshima, K.; Iwasaki, H.; Tashiro, T.; Uesugi, N.; Nakashima, O.; Ito, H.; Kawanami, T.; Furuya, K.; Kojima, M. Possible involvement of myofibroblast in the development of inflammatory aortic aneurysm. Pathol. Res. Pract. 2007, 203, 21–29. [Google Scholar] [CrossRef]
- Husmann, L.; Huellner, M.W.; Gruenig, H.; Ledergerber, B.; Messerli, M.; Mestres, C.A.; Rancic, Z.; Hasse, B. Imaging characteristics and diagnostic accuracy of FDG-PET/CT, contrast enhanced CT and combined imaging in patients with suspected mycotic or inflammatory abdominal aortic aneurysms. PLoS ONE 2022, 17, e0272772. [Google Scholar] [CrossRef]
- Kasashima, S.; Zen, Y.; Kawashima, A.; Endo, M.; Matsumoto, Y.; Kasashima, F.; Ohtake, H.; Nakanuma, Y. A clinicopathologic study of immunoglobulin G4-related sclerosing disease of the thoracic aorta. J. Vasc. Surg. 2010, 52, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Hotta, M.; Kushima, R.; Asai, T.; Okabe, H. IgG4-related inflammatory aneurysm of the aortic arch. Pathol. Int. 2009, 59, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Nagai, R.; Saito, K.; Imai, Y.; Takahashi, M.; Hosoya, Y.; Takeda, N.; Hirano, K.; Koike, K.; Enomoto, Y.; et al. Idiopathic retroperitoneal fibrosis, inflammatory aortic aneurysm, and inflammatory pericarditis--retrospective analysis of 11 case histories. J. Cardiol. 2012, 59, 139–146. [Google Scholar] [CrossRef]
- Agaimy, A.; Weyand, M.; Strecker, T. Inflammatory thoracic aortic aneurysm (lymphoplasmacytic thoracic aortitis): A 13-year-experience at a German Heart Center with emphasis on possible role of IgG4. Int. J. Clin. Exp. Pathol. 2013, 6, 1713–1722. [Google Scholar]
- Tang, J.; Cai, S.; Ye, C.; Dong, L. Biomarkers in IgG4-related disease: A systematic review. Semin. Arthritis Rheum. 2020, 50, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Castelein, T.; Coudyzer, W.; Blockmans, D. IgG4-related periaortitis vs idiopathic periaortitis: Is there a role for atherosclerotic plaque in the pathogenesis of IgG4-related periaortitis? Rheumatology 2015, 54, 1250–1256. [Google Scholar] [CrossRef]
- Perugino, C.A.; Wallace, Z.S.; Meyersohn, N.; Oliveira, G.; Stone, J.R.; Stone, J.H. Large vessel involvement by IgG4-related disease. Medicine 2016, 95, e3344. [Google Scholar] [CrossRef]
- Kan-o, M.; Kado, Y.; Sadanaga, A.; Tamiya, S.; Toyoshima, S.; Sakamoto, M. Immunoglobulin G4-related multiple cardiovascular lesions successfully treated with a combination of open surgery and corticosteroid therapy. J. Vasc. Surg. 2015, 61, 1599–1603. [Google Scholar] [CrossRef]
- Suehiro, Y.; Seo, H.; Suehiro, S.; Hirai, H. Surgical strategy of IgG4-related inflammatory abdominal aortic aneurysm with preoperative steroid therapy: A case report. Ann. Vasc. Surg. 2021, 77, 351.E1–351.E6. [Google Scholar] [CrossRef]



| Patient Cohort | IgG4-Positive AAA | Inflammatory AAA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 101 | n = 5 | n = 7 | |||||||||||||
| Patient Characteristics, Co-morbidities and Frailty | |||||||||||||||
| sex (male) | 88 (87.1) | m | m | m | f | m | m | f | m | f | f | m | m | ||
| metrics | age (y; mean ± SD) | 67 ± 8.2 | 70 | 63 | 71 | 70 | 62 | 69 | 76 | 65 | 64 | 74 | 71 | 55 | |
| height (cm) | 176.3 ± 7.5 | 176 | 187 | - | - | - | 178 | 175 | 173 | 165 | 168 | 189 | - | ||
| weight (kg) | 83.4 ± 14.1 | 90 | 95 | - | - | - | 101 | 74 | 70 | 70 | 41 | 85 | - | ||
| BMI | 26.7 ± 3.7 | 29 | 27 | - | - | - | 32 | 24 | 23 | 26 | 15 | 24 | - | ||
| body surface (m2) | 1.99 ± 0.19 | 2.05 | 2.20 | - | - | - | 2.2 | 1.9 | 1.8 | 1.8 | 1.4 | 2.1 | - | ||
| aortic size index (cm/m2) | 2.99 ± 0.68 | 2.97 | 2.32 | - | - | - | 2.6 | 2.4 | 3.4 | 3.0 | 3.7 | 2.4 | - | ||
| psoas volume (cm3) | 188.2 ± 57.5 | 222 | 256 | - | - | - | 210 | 130 | 190 | 91 | 32 | 174 | - | ||
| psoas area (cm2) | 18.77 ± 5.8 | 23.3 | 28.6 | - | - | - | 26.8 | 11.6 | 13.2 | 5.9 | 3.6 | 20.9 | - | ||
| co-morbidities | hypertension | 83 (82.2) | x | x | x | x | x | x | x | x | x | x | |||
| diabetes | 13 (12.9) | ||||||||||||||
| hyperlipidemia | 59 (58.4) | x | x | x | x | x | x | x | x | x | |||||
| CAD | 42 (41.6) | x | x | x | x | x | |||||||||
| COPD | 21 (20.8) | x | x | x | |||||||||||
| PAOD | 26 (25.7) | x | x | x | |||||||||||
| renal insufficiency | 27 (26.7) | x | x | ||||||||||||
| dialysis | 2 (2) | ||||||||||||||
| smoking (current/ex) | 81 (80.2) | x | x | x | x | x | x | x | x | x | x | ||||
| medication | platelet inhibitor | 64 (63.4) | x | x | x | x | x | x | x | x | x | x | |||
| ACE inhibitor | 32 (31.7) | x | x | x | x | ||||||||||
| statin | 51 (50.5) | x | x | x | x | x | x | x | |||||||
| metformin | 3 (3) | ||||||||||||||
| insulin | 2 (2) | ||||||||||||||
| AAA Characteristics | |||||||||||||||
| diameter (mm) | 58 ± 11.1 | 61 | 51 | 55 | 64 | 73 | 56 | 46 | 62 | 53 | 53 | 50 | 56 | ||
| volume (cm3) | 186 ± 121 | 273 | 132 | 215 | - | 316 | 202 | 74 | 257 | 156 | 191 | 117 | 99 | ||
| ratio lumen/total volume | 0.54 ± 0.19 | 0.61 | 0.64 | 0.63 | - | 0.49 | 0.63 | 0.73 | 0.23 | 0.44 | 0.27 | 0.48 | 0.58 | ||
| extent | infrarenal | 50 (49.5) | x | x | x | x | x | ||||||||
| juxtarenal | 32 (31.7) | x | x | x | x | x | x | x | |||||||
| suprarenal | 19 (18.8) | ||||||||||||||
| + iliac aneurysm | uni | 10 (9.9) | x | ||||||||||||
| bi | 12 (11.9) | x | x | ||||||||||||
| state | symptomatic | 8 (7.9) | x | ||||||||||||
| asymptomatic | 84 (83.2) | x | x | x | x | x | x | x | x | x | x | x | |||
| ruptured | 9 (8.9) | ||||||||||||||
| Endosize® | α angulation (°) | 20 ± 20.2 | 12 | 14 | 24 | 11 | 50 | 45 | 13 | 23 | 22 | 13 | 13 | 43 | |
| β angulation (°) | 32.6 ± 14.8 | 36 | 12 | 47 | 39 | 54 | 52 | 53 | 31 | 46 | 17 | 17 | 63 | ||
| aortic tortuosity index | 1.1 ± 0.06 | 1.2 | 1.0 | 1.1 | 1.2 | 1.2 | 1.1 | 1.2 | 1.0 | 1.1 | 1.1 | 1.0 | 1.3 | ||
| iliac tortuosity index | 1.35 ± 0.22 | 1.2 | 1.3 | 1.3 | 1.4 | 1.7 | 1.5 | 1.4 | 1.5 | 1.1 | 2.4 | 1.3 | 1.3 | ||
| iliac calcification (%) | 8.6 ± 11.8 | 7 | 12 | 10 | 2 | 1 | - | 3 | 0 | 6 | 7 | 5 | 2 | ||
| Patient Cohort | IgG4-Positive AAA | Inflammatory AAA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 101 | n = 5 | n = 7 | ||||||||||||||
| Histopathologic Features | ||||||||||||||||
| adventitia | degree inflammation (0–3) | 1.3 ± 0.69 | 1 | 2 | 2 | 2 | 2 | 2 | 3 | 1 | 2 | 3 | 1 | 3 | ||
| no | type of inflammation | 9 (8.9) | ||||||||||||||
| monocyte | 49 (48.5) | x | x | x | x | x | x | x | x | x | ||||||
| granulocyte | 1 (1) | |||||||||||||||
| plasma cell | 30 (29.7) | x | ||||||||||||||
| mix | 12 (11.9) | x | x | |||||||||||||
| media | degree inflammation (0–3) | 0.3 ± 0.5 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | ||
| no | type of inflammation | 68 (67.3) | x | x | x | x | x | x | x | x | ||||||
| monocyte | 24 (23.8) | x | x | x | ||||||||||||
| granulocyte | 0 | |||||||||||||||
| plasma cell | 5 (5) | x | ||||||||||||||
| mix | 4 (4) | |||||||||||||||
| intima | 5 | AHA classification | 11 (10.9) | x | x | |||||||||||
| 6 | 76 (75.2) | x | x | x | x | x | x | x | x | x | ||||||
| 7 | 0 | |||||||||||||||
| 8 | 12 (11.9) | x | ||||||||||||||
| Serologic Analysis | ||||||||||||||||
| leucocyte count (×103/µL) | 7.9 ± 2.5 | 7.8 | 8.5 | 5.1 | 6.1 | 8.0 | 8.2 | 6.8 | 10.8 | 11.6 | 8.8 | 9.6 | 7.4 | |||
| thrombocyte count (×103/µL) | 215.2 ± 55.9 | 284 | 206 | 202 | 229 | 215 | 261 | 336 | 323 | 200 | 279 | 223 | 250 | |||
| C-reactive protein (mg/dL) | 1.5 ± 4.5 | 1.2 | 0.9 | 0.9 | 0.7 | 0.8 | 1 | 0.8 | 1 | 0.9 | 0.5 | 1.5 | 0.9 | |||
| sC3 (mg/dL) | 128.4 ± 26.8 | 127 | 150 | 74 | 220 | 179 | 111 | 145 | 127 | 135 | 114 | 86 | 101 | |||
| sC4 (mg/dL) | 25.8 ± 8.3 | 21 | 26 | 13 | 47 | 27 | 20 | 36 | 33 | 36 | 30 | 25 | 17 | |||
| IgG (mg/dL) | 1000 ± 278.6 | 923 | 1160 | 476 | 500 | 560 | 1840 | 770 | 874 | 785 | 1210 | 755 | 657 | |||
| IgG2 (mg/dL) | 319.3 ± 143.3 | 275 | 405 | 135 | 127 | - | 642 | 192 | 239 | 177 | 188 | 129 | 250 | |||
| IgG4 (mg/dL) | 92.5 ± 102 | 27 | 61 | 51 | 24 | 29 | 59 | 6.3 | 79 | 64 | 34 | 17 | 38 | |||
| IgE (IU/mL) | 330.1 ± 1004 | 10 | 90 | 4 | 192 | - | 21 | 4 | 237 | 17 | 81 | 157 | 41 | |||
| Patient Cohort | IgG4-Positive AAA | Inflammatory AAA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 101 | n = 5 | n = 7 | |||||||||||
| Procedural Details | |||||||||||||
| tube graft (vs. Y graft) | 53 (52.5) | x | x | x | x | x | x | x | x | ||||
| retroperitoneal access | 47 (46.5) | x | x | x | x | ||||||||
| additional anastomosis (any) | 16 (15.8) | x | |||||||||||
| procedure time (min) | 242.1 ± 94.9 | 227 | 176 | 233 | 165 | 145 | 257 | 132 | 320 | 178 | 149 | 181 | 218 |
| Clinical Outcome | |||||||||||||
| days in-hospital | 15 ± 11 | 11 | 7 | 7 | 12 | 9 | 8 | 7 | 13 | 9 | 12 | 8 | 7 |
| days on ICU | 4 ± 5 | 1 | 3 | 3 | 3 | 8 | 7 | 1 | 1 | 2 | 1 | 1 | |
| surgical complication | 20 (19.8) | x | x | x | |||||||||
| medical complication | 40 (39.6) | x | x | x | |||||||||
| in-hospital mortality | 3 (3.0) | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nackenhorst, M.C.; Kapalla, M.; Weidle, S.; Kirchhoff, F.; Zschäpitz, D.; Sieber, S.; Reeps, C.; Eckstein, H.-H.; Schneider, H.; Thaler, M.; et al. The Incidence of IgG4-Related and Inflammatory Abdominal Aortic Aneurysm Is Rare in a 101 Patient Cohort. J. Clin. Med. 2023, 12, 4029. https://doi.org/10.3390/jcm12124029
Nackenhorst MC, Kapalla M, Weidle S, Kirchhoff F, Zschäpitz D, Sieber S, Reeps C, Eckstein H-H, Schneider H, Thaler M, et al. The Incidence of IgG4-Related and Inflammatory Abdominal Aortic Aneurysm Is Rare in a 101 Patient Cohort. Journal of Clinical Medicine. 2023; 12(12):4029. https://doi.org/10.3390/jcm12124029
Chicago/Turabian StyleNackenhorst, Maja Carina, Marvin Kapalla, Simon Weidle, Felix Kirchhoff, David Zschäpitz, Sabine Sieber, Christian Reeps, Hans-Henning Eckstein, Heike Schneider, Markus Thaler, and et al. 2023. "The Incidence of IgG4-Related and Inflammatory Abdominal Aortic Aneurysm Is Rare in a 101 Patient Cohort" Journal of Clinical Medicine 12, no. 12: 4029. https://doi.org/10.3390/jcm12124029
APA StyleNackenhorst, M. C., Kapalla, M., Weidle, S., Kirchhoff, F., Zschäpitz, D., Sieber, S., Reeps, C., Eckstein, H.-H., Schneider, H., Thaler, M., Moog, P., Busch, A., & Sachs, N. (2023). The Incidence of IgG4-Related and Inflammatory Abdominal Aortic Aneurysm Is Rare in a 101 Patient Cohort. Journal of Clinical Medicine, 12(12), 4029. https://doi.org/10.3390/jcm12124029