Relationship between Serum Cortisol, Dehydroepiandrosterone Sulfate (DHEAS) Levels, and Natural Killer Cell Activity: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
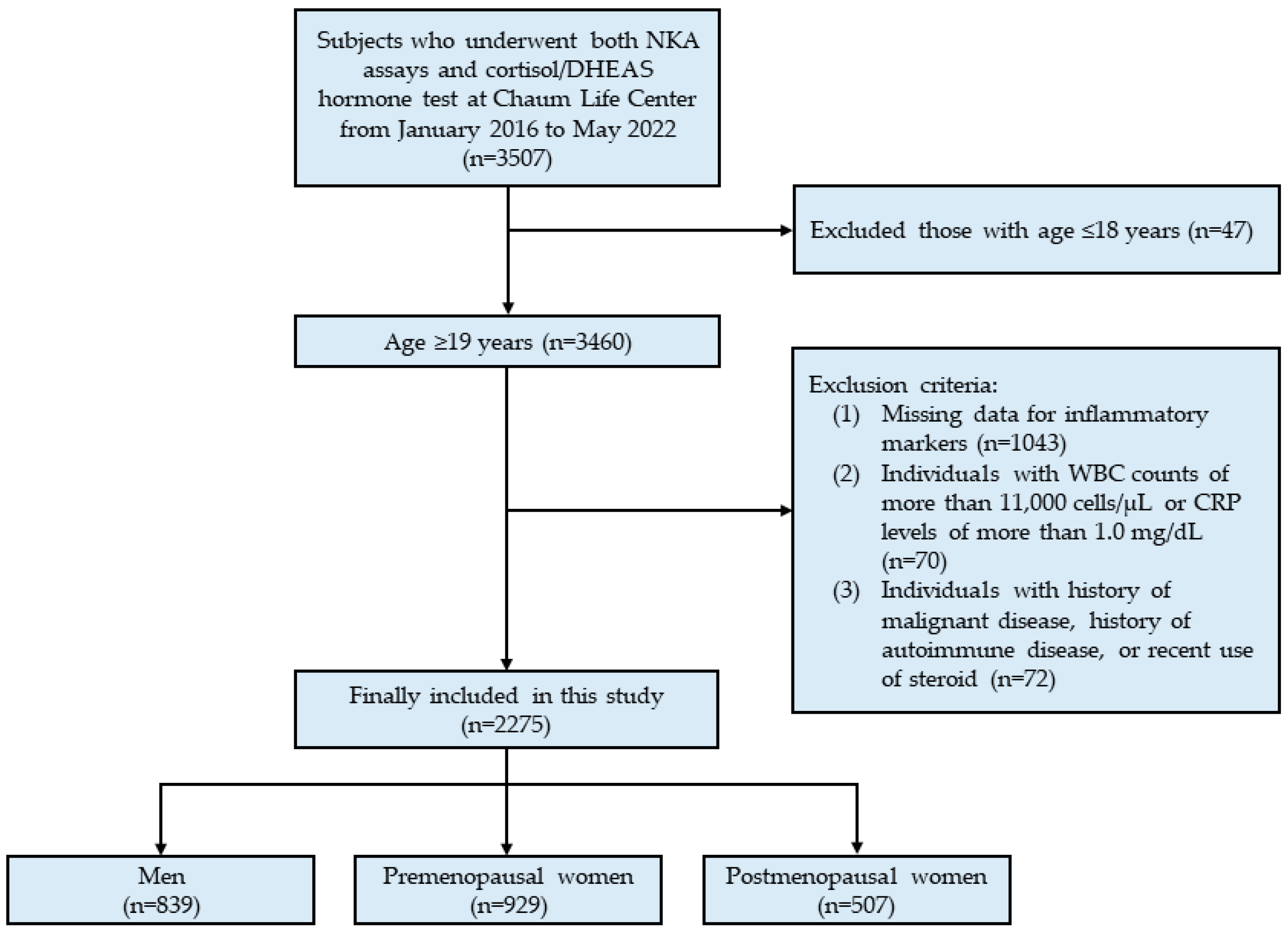
2.1. Study Population
2.2. Data Collection
2.3. IFN-γ Measurement for NKA
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
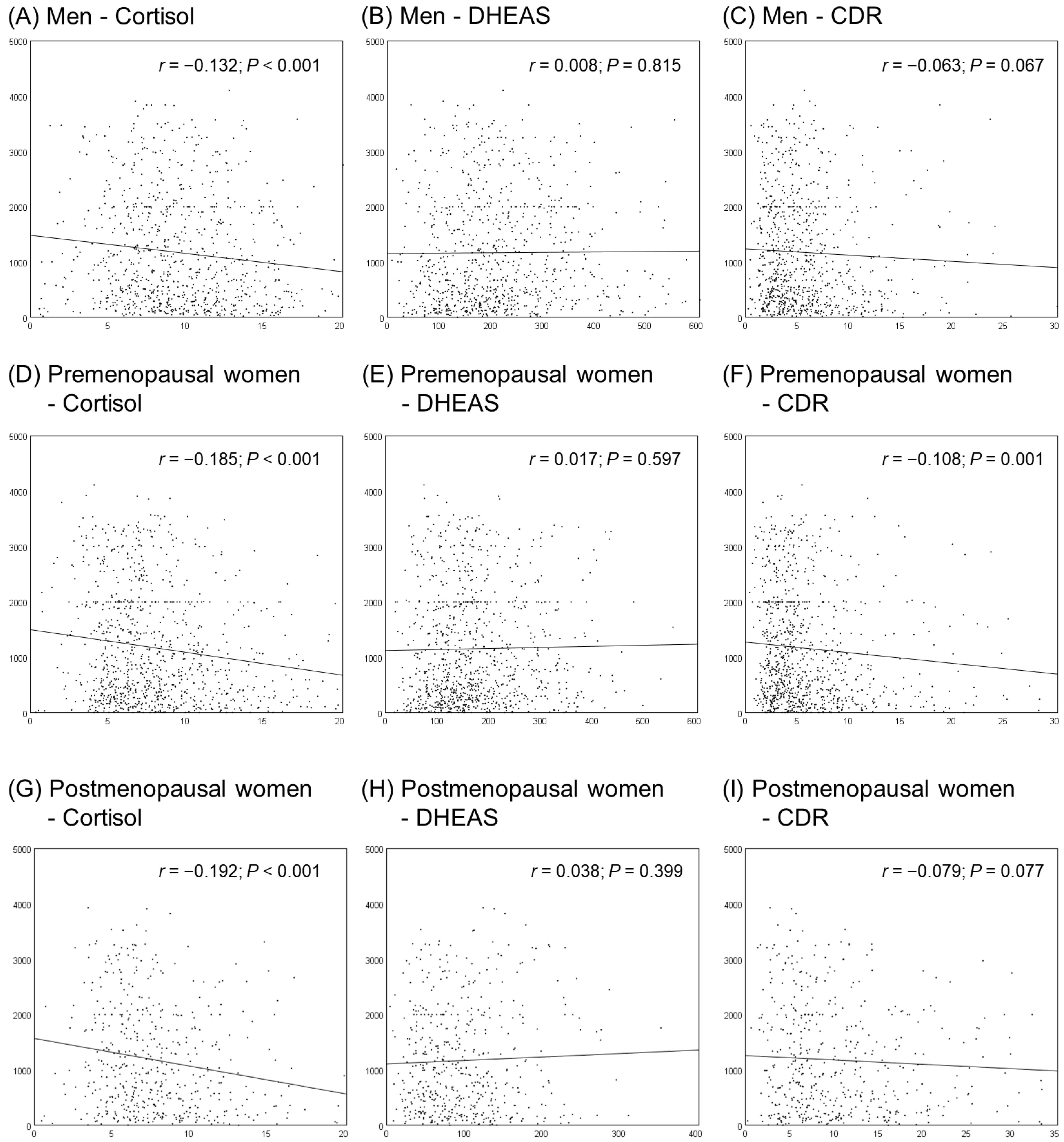
3.2. Correlations between Cortisol, DHEAS, CDR, and NKA
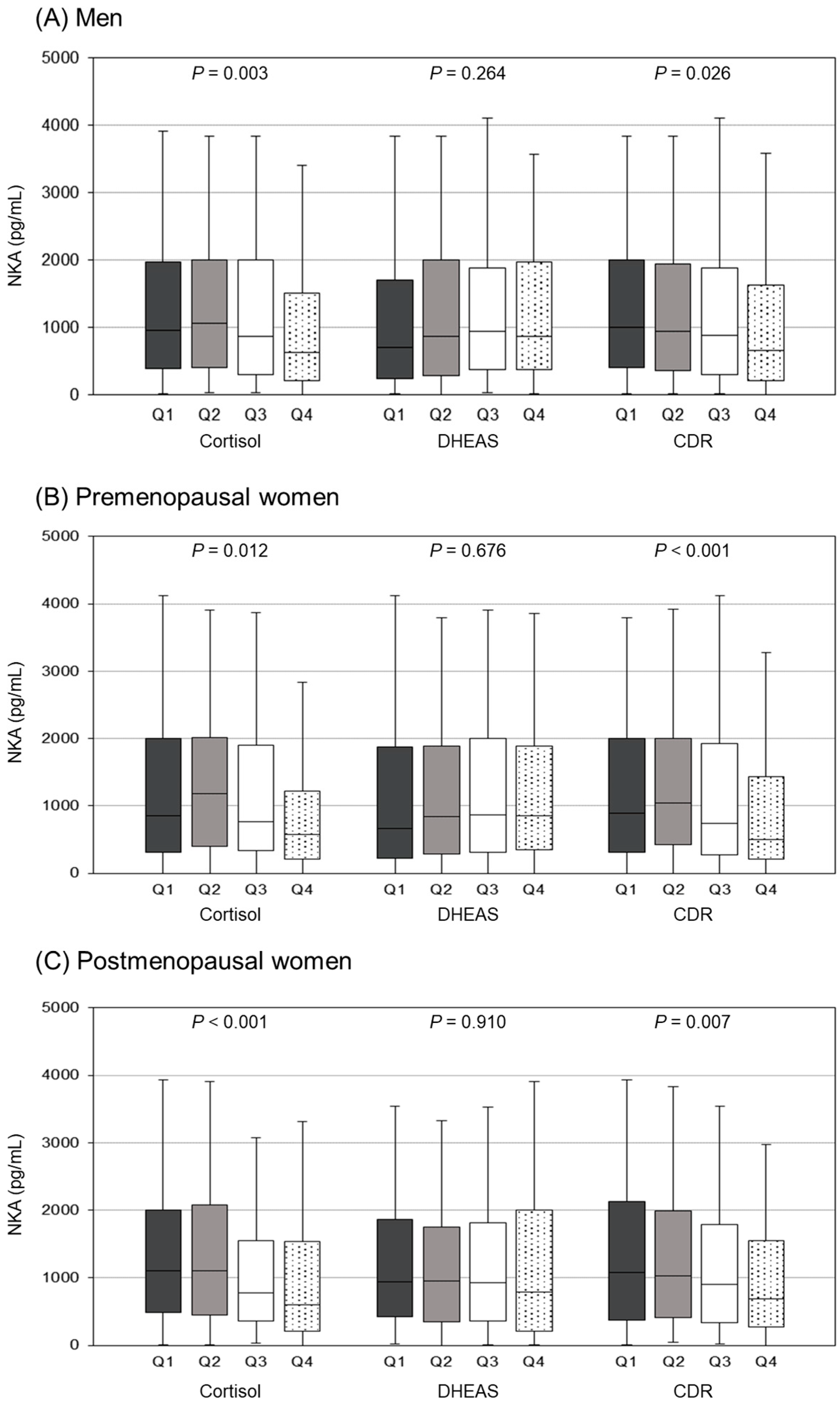
3.3. Comparison of NKA Levels According to Cortisol, DHEAS, and CDR Quartiles
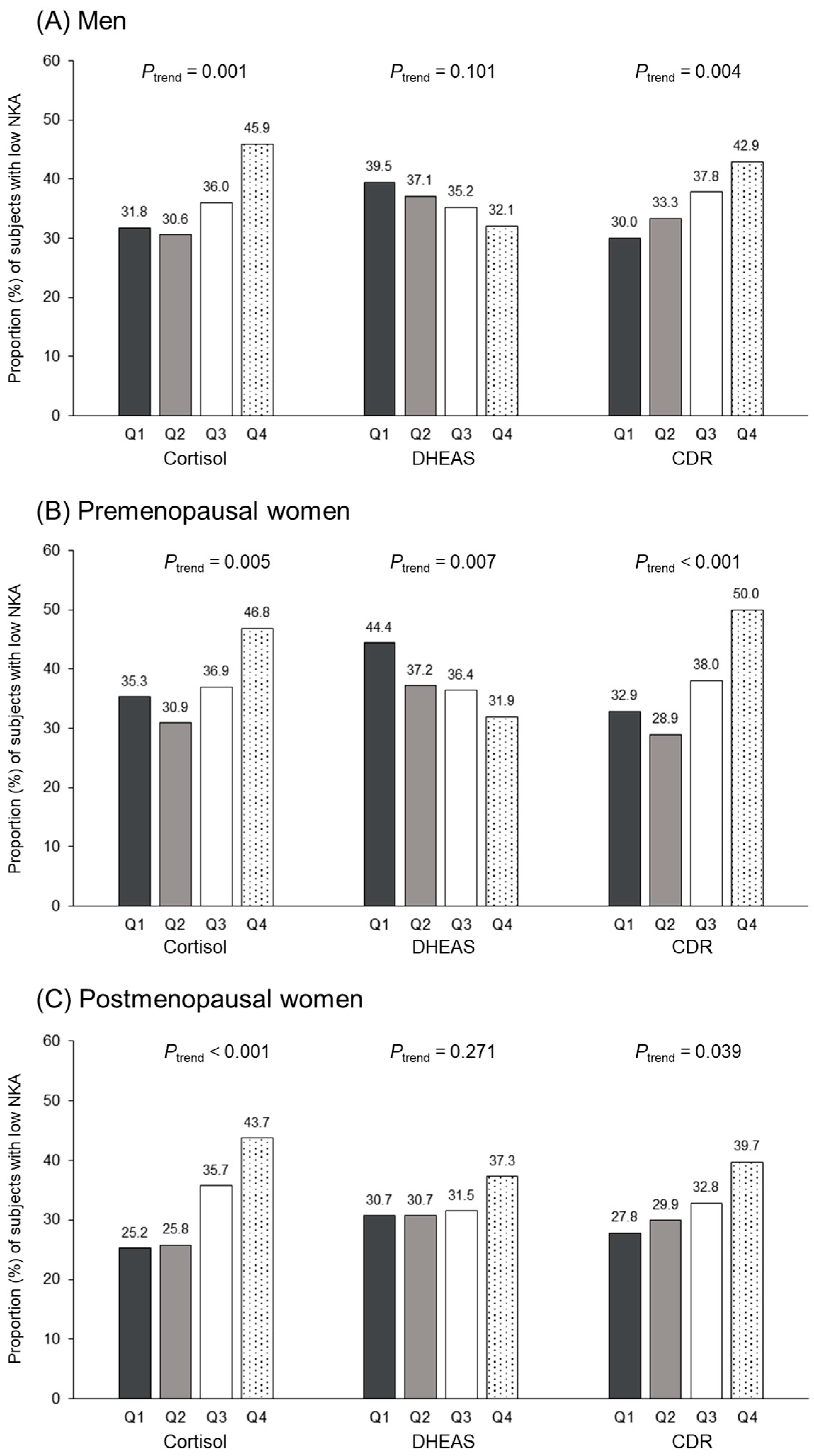
3.4. Relationship between Cortisol, DHEAS, CDR Quartiles, and Low NKA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, M.E. Stress, glucocorticoids and ageing of the immune system. Stress 2005, 8, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Jeckel, C.M.; Luz, C. The role of stress factors during aging of the immune system. Ann. N. Y. Acad. Sci. 2009, 1153, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Cerwenka, A.; Lanier, L.L. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 2001, 1, 41–49. [Google Scholar] [CrossRef]
- Morvan, M.G.; Lanier, L.L. Nk cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; An, E.; Shioi, Y.; Nakamura, K.; Luo, S.; Yokose, N.; Minami, S.; Dan, K. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin. Exp. Immunol. 2001, 124, 392–397. [Google Scholar] [CrossRef]
- Ogata, K.; Yokose, N.; Tamura, H.; An, E.; Nakamura, K.; Dan, K.; Nomura, T. Natural killer cells in the late decades of human life. Clin. Immunol. Immunopathol. 1997, 84, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Jurisic, V.; Srdic, T.; Konjevic, G.; Markovic, O.; Colovic, M. Clinical stage-depending decrease of nk cell activity in multiple myeloma patients. Med. Oncol. 2007, 24, 312–317. [Google Scholar] [CrossRef]
- Konjevic, G.; Vuletic, A.; Mirjacic Martinovic, K.; Colovic, N.; Colovic, M.; Jurisic, V. Decreased cd161 activating and increased cd158a inhibitory receptor expression on nk cells underlies impaired nk cell cytotoxicity in patients with multiple myeloma. J. Clin. Pathol. 2016, 69, 1009–1016. [Google Scholar] [CrossRef]
- Konjevic, G.; Jurisic, V.; Banicevic, B.; Spuzic, I. The difference in nk-cell activity between patients with non-hodgkin’s lymphomas and hodgkin’s disease. Br. J. Haematol. 1999, 104, 144–151. [Google Scholar] [CrossRef]
- van Eeden, C.; Khan, L.; Osman, M.S.; Cohen Tervaert, J.W. Natural killer cell dysfunction and its role in COVID-19. Int. J. Mol. Sci. 2020, 21, 6351. [Google Scholar] [CrossRef]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.H.; Ryu, J.H.; Bae, H.J.; Choi, A.; Lee, H.; Lim, J.; Han, K.; Park, C.H.; Jung, E.S.; et al. Natural killer cell activity for ifn-gamma production as a supportive diagnostic marker for gastric cancer. Oncotarget 2017, 8, 70431–70440. [Google Scholar] [CrossRef] [PubMed]
- Jobin, G.; Rodriguez-Suarez, R.; Betito, K. Association between natural killer cell activity and colorectal cancer in high-risk subjects undergoing colonoscopy. Gastroenterology 2017, 153, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.C.; Shim, D.H.; Yang, C.M.; Lee, S.B.; Kim, S.M.; Shin, T.Y.; Kim, K.H.; Yoon, H.G.; Rha, K.H.; Lee, J.M.; et al. Reduction of the cd16(-)cd56bright nk cell subset precedes nk cell dysfunction in prostate cancer. PLoS ONE 2013, 8, e78049. [Google Scholar] [CrossRef]
- Lee, Y.K.; Haam, J.H.; Cho, S.H.; Kim, Y.S. Cross-sectional and time-dependent analyses on inflammatory markers following natural killer cell activity. Diagnostics 2022, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Park, J.H.; Park, D.I.; Sohn, C.I.; Lee, J.M.; Kim, T.I. Physical inactivity and unhealthy metabolic status are associated with decreased natural killer cell activity. Yonsei Med. J. 2018, 59, 554–562. [Google Scholar] [CrossRef]
- Oh, S.; Chun, S.; Hwang, S.; Kim, J.; Cho, Y.; Lee, J.; Kwack, K.; Choi, S.W. Vitamin d and exercise are major determinants of natural killer cell activity, which is age- and gender-specific. Front. Immunol. 2021, 12, 594356. [Google Scholar] [CrossRef]
- Nair, M.P.; Schwartz, S.A. Immunomodulatory effects of corticosteroids on natural killer and antibody-dependent cellular cytotoxic activities of human lymphocytes. J. Immunol. 1984, 132, 2876–2882. [Google Scholar] [CrossRef]
- Holbrook, N.J.; Cox, W.I.; Horner, H.C. Direct suppression of natural killer activity in human peripheral blood leukocyte cultures by glucocorticoids and its modulation by interferon. Cancer Res. 1983, 43, 4019–4025. [Google Scholar]
- Zhou, J.; Olsen, S.; Moldovan, J.; Fu, X.; Sarkar, F.H.; Moudgil, V.K.; Callewaert, D.M. Glucocorticoid regulation of natural cytotoxicity: Effects of cortisol on the phenotype and function of a cloned human natural killer cell line. Cell. Immunol. 1997, 178, 108–116. [Google Scholar] [CrossRef]
- Chen, L.; Jondal, M.; Yakimchuk, K. Regulatory effects of dexamethasone on nk and t cell immunity. Inflammopharmacology 2018, 26, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, K.; Eddy, J.; Kosik, K.L.; Konley, T.; Janusek, L.W.; Mathews, H.L. Glucocorticoid dysregulation of natural killer cell function through epigenetic modification. Brain Behav. Immun. 2011, 25, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Gatti, G.; Cavallo, R.; Sartori, M.L.; del Ponte, D.; Masera, R.; Salvadori, A.; Carignola, R.; Angeli, A. Inhibition by cortisol of human natural killer (nk) cell activity. J. Steroid Biochem. 1987, 26, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Bodner, G.; Ho, A.; Kreek, M.J. Effect of endogenous cortisol levels on natural killer cell activity in healthy humans. Brain Behav. Immun. 1998, 12, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Hartono, H. Cortisol level decreases natural killer cell activity among women with aircraft noise. Universa Med. 2016, 29, 153–161. [Google Scholar]
- Duggal, N.A.; Upton, J.; Phillips, A.C.; Hampson, P.; Lord, J.M. Nk cell immunesenescence is increased by psychological but not physical stress in older adults associated with raised cortisol and reduced perforin expression. Age 2015, 37, 9748. [Google Scholar] [CrossRef]
- Casson, P.R.; Andersen, R.N.; Herrod, H.G.; Stentz, F.B.; Straughn, A.B.; Abraham, G.E.; Buster, J.E. Oral dehydroepiandrosterone in physiologic doses modulates immune function in postmenopausal women. Am. J. Obstet. Gynecol. 1993, 169, 1536–1539. [Google Scholar] [CrossRef]
- Khorram, O.; Vu, L.; Yen, S.S. Activation of immune function by dehydroepiandrosterone (dhea) in age-advanced men. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, M1-7. [Google Scholar] [CrossRef]
- Valiathan, R.; Lewis, J.E.; Melillo, A.B.; Leonard, S.; Ali, K.H.; Asthana, D. Evaluation of a flow cytometry-based assay for natural killer cell activity in clinical settings. Scand. J. Immunol. 2012, 75, 455–462. [Google Scholar] [CrossRef]
- Lee, S.B.; Cha, J.; Kim, I.K.; Yoon, J.C.; Lee, H.J.; Park, S.W.; Cho, S.; Youn, D.Y.; Lee, H.; Lee, C.H.; et al. A high-throughput assay of nk cell activity in whole blood and its clinical application. Biochem. Biophys. Res. Commun. 2014, 445, 584–590. [Google Scholar] [CrossRef]
- Nederby, L.; Jakobsen, A.; Hokland, M.; Hansen, T.F. Quantification of nk cell activity using whole blood: Methodological aspects of a new test. J. Immunol. Methods 2018, 458, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Nehring, S.M.; Goyal, A.; Patel, B.C. C reactive protein. In Statpearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Buford, T.W.; Willoughby, D.S. Impact of dhea(s) and cortisol on immune function in aging: A brief review. Appl. Physiol. Nutr. Metab. 2008, 33, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Casarotti, D.; Muzzoni, B.; Albertelli, N.; Cravello, L.; Fioravanti, M.; Solerte, S.B.; Magri, F. Age-related changes of the adrenal secretory pattern: Possible role in pathological brain aging. Brain Res. Brain Res. Rev. 2001, 37, 294–300. [Google Scholar] [CrossRef]
- Ginaldi, L.; De Martinis, M.; D’Ostilio, A.; Marini, L.; Loreto, M.F.; Quaglino, D. The immune system in the elderly: Iii. Innate immunity. Immunol. Res. 1999, 20, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Balistreri, C.R.; Candore, G.; Cigna, D.; Colombo, A.; Romano, G.C.; Colucci, A.T.; Gervasi, F.; Listi, F.; Potestio, M.; et al. Granulocyte and natural killer activity in the elderly. Mech. Ageing Dev. 1999, 108, 25–38. [Google Scholar] [CrossRef]
- Solana, R.; Mariani, E. Nk and nk/t cells in human senescence. Vaccine 2000, 18, 1613–1620. [Google Scholar] [CrossRef]
- Kim, B.R.; Chun, S.; Cho, D.; Kim, K.H. Association of neutrophil-to-lymphocyte ratio and natural killer cell activity revealed by measurement of interferon-gamma levels in a healthy population. J. Clin. Lab. Anal. 2019, 33, e22640. [Google Scholar] [CrossRef]
- Parrillo, J.E.; Fauci, A.S. Comparison of the effector cells in human spontaneous cellular cytotoxicity and antibody-dependent cellular cytotoxicity: Differential sensitivity of effector cells to in vivo and in vitro corticosteroids. Scand. J. Immunol. 1978, 8, 99–107. [Google Scholar] [CrossRef]
- Callewaert, D.M.; Moudgil, V.K.; Radcliff, G.; Waite, R. Hormone specific regulation of natural killer cells by cortisol. Direct inactivation of the cytotoxic function of cloned human nk cells without an effect on cellular proliferation. FEBS Lett. 1991, 285, 108–110. [Google Scholar] [CrossRef]
- Vitale, C.; Chiossone, L.; Cantoni, C.; Morreale, G.; Cottalasso, F.; Moretti, S.; Pistorio, A.; Haupt, R.; Lanino, E.; Dini, G.; et al. The corticosteroid-induced inhibitory effect on nk cell function reflects down-regulation and/or dysfunction of triggering receptors involved in natural cytotoxicity. Eur. J. Immunol. 2004, 34, 3028–3038. [Google Scholar] [CrossRef]
- Bush, K.A.; Krukowski, K.; Eddy, J.L.; Janusek, L.W.; Mathews, H.L. Glucocorticoid receptor mediated suppression of natural killer cell activity: Identification of associated deacetylase and corepressor molecules. Cell. Immunol. 2012, 275, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Mavoungou, E.; Bouyou-Akotet, M.K.; Kremsner, P.G. Effects of prolactin and cortisol on natural killer (nk) cell surface expression and function of human natural cytotoxicity receptors (nkp46, nkp44 and nkp30). Clin. Exp. Immunol. 2005, 139, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, M.; Cermakian, N.; Boivin, D.B. Glucocorticoids entrain molecular clock components in human peripheral cells. FASEB J. 2015, 29, 1360–1370. [Google Scholar] [CrossRef]
- Labrecque, N.; Cermakian, N. Circadian clocks in the immune system. J. Biol. Rhythms. 2015, 30, 277–290. [Google Scholar] [CrossRef]
- Bancos, I.; Hazeldine, J.; Chortis, V.; Hampson, P.; Taylor, A.E.; Lord, J.M.; Arlt, W. Primary adrenal insufficiency is associated with impaired natural killer cell function: A potential link to increased mortality. Eur. J. Endocrinol. 2017, 176, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Quatrini, L.; Wieduwild, E.; Guia, S.; Bernat, C.; Glaichenhaus, N.; Vivier, E.; Ugolini, S. Host resistance to endotoxic shock requires the neuroendocrine regulation of group 1 innate lymphoid cells. J. Exp. Med. 2017, 214, 3531–3541. [Google Scholar] [CrossRef]
- Morgan, D.J.; Davis, D.M. Distinct effects of dexamethasone on human natural killer cell responses dependent on cytokines. Front. Immunol. 2017, 8, 432. [Google Scholar] [CrossRef]
- Giefing-Kroll, C.; Berger, P.; Lepperdinger, G.; Grubeck-Loebenstein, B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 2015, 14, 309–321. [Google Scholar] [CrossRef]
- Han, A.; Kim, J.Y.; Kwak-Kim, J.; Lee, S.K. Menopause is an inflection point of age-related immune changes in women. J. Reprod. Immunol. 2021, 146, 103346. [Google Scholar] [CrossRef]

| Men | Premenopausal Women | Postmenopausal Women | p-Value | |
|---|---|---|---|---|
| N | 839 | 929 | 507 | |
| Age (years) | 49.9 ± 12.8 | 36.9 ± 1.5 | 58.6 ± 7.6 | <0.001 |
| BMI (kg/m2) | 25.6 ± 4.0 | 21.3 ± 3.0 | 23.0 ± 3.5 | <0.001 |
| WBC count (cells/μL) | 5.80 ± 1.51 | 5.61 ± 1.51 | 5.31 ± 1.40 | <0.001 |
| NLR | 1.69 ± 0.77 | 1.84 ± 0.89 | 1.55 ± 0.70 | <0.001 |
| CRP (mg/dL) | 0.13 ± 0.15 | 0.11 ± 0.15 | 0.14 ± 0.17 | <0.001 |
| Cortisol (μg/dL) | 9.7 ± 4.0 | 8.5 ± 4.6 | 8.0 ± 3.7 | <0.001 |
| DHEAS (μg/dL) | 213.9 ± 115.4 | 175.8 ± 91.7 | 99.0 ± 58.2 | <0.001 |
| CDR | 6.2 ± 5.6 | 6.3 ± 5.7 | 11.3 ± 9.6 | <0.001 |
| NKA (pg/mL) | 1168.2 ± 997.6 | 1154.6 ± 1009.4 | 1168.7 ± 960.9 | 0.948 |
| Hypertension, n (%) | 125 (14.9) | 11 (1.2) | 101 (19.9) | <0.001 |
| Diabetes mellitus, n (%) | 55 (6.5) | 2 (0.2) | 35 (7.0) | <0.001 |
| Dyslipidemia, n (%) | 148 (17.6) | 20 (2.1) | 138 (27.3) | <0.001 |
| Cortisol Quartiles | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Men | ||||
| Unadjusted | 1.00 (Reference) | 0.95 (0.63–1.43) | 1.21 (0.81–1.81) | 1.82 (1.22–2.71) |
| Adjusted | 1.00 (Reference) | 0.95 (0.62–1.45) | 1.21 (0.80–1.84) | 1.66 (1.09–2.51) |
| Premenopausal women | ||||
| Unadjusted | 1.00 (Reference) | 0.82 (0.56–1.20) | 1.07 (0.73–1.56) | 1.61 (1.11–2.33) |
| Adjusted | 1.00 (Reference) | 0.85 (0.57–1.27) | 1.03 (0.70–1.52) | 1.58 (1.07–2.33) |
| Postmenopausal women | ||||
| Unadjusted | 1.00 (Reference) | 1.03 (0.59–1.81) | 1.65 (0.96–2.84) | 2.30 (1.35– 3.92) |
| Adjusted | 1.00 (Reference) | 1.01 (0.56–1.82) | 1.62 (0.92–2.82) | 2.23 (1.28–3.87) |
| DHEAS quartiles | ||||
| Q1 | Q2 | Q3 | Q4 | |
| Men | ||||
| Unadjusted | 1.00 (Reference) | 0.90 (0.61–1.34) | 0.83 (0.56–1.24) | 0.72 (0.48–1.08) |
| Adjusted | 1.00 (Reference) | 0.97 (0.64–1.45) | 0.93 (0.62–1.39) | 0.68 (0.45–1.03) |
| Premenopausal women | ||||
| Unadjusted | 1.00 (Reference) | 0.74 (0.51–1.07) | 0.72 (0.49–1.04) | 0.59 (0.40–0.86) |
| Adjusted | 1.00 (Reference) | 0.74 (0.51–1.08) | 0.64 (0.44–0.94) | 0.51 (0.35–0.76) |
| Postmenopausal women | ||||
| Unadjusted | 1.00 (Reference) | 1.00 (0.59–1.70) | 1.04 (0.61–1.77) | 1.34 (0.80–2.26) |
| Adjusted | 1.00 (Reference) | 1.09 (0.63–1.88) | 1.03 (0.59–1.77) | 1.26 (0.73–2.16) |
| CDR quartiles | ||||
| Q1 | Q2 | Q3 | Q4 | |
| Men | ||||
| Unadjusted | 1.00 (Reference) | 1.17 (0.78–1.76) | 1.42 (0.94–2.13) | 1.75 (1.17–2.62) |
| Adjusted | 1.00 (Reference) | 1.22 (0.80–1.87) | 1.47 (0.96–2.23) | 1.68 (1.11–2.55) |
| Premenopausal women | ||||
| Unadjusted | 1.00 (Reference) | 0.83 (0.56–1.23) | 1.25 (0.86–1.83) | 2.04 (1.40–2.97) |
| Adjusted | 1.00 (Reference) | 0.92 (0.62–1.38) | 1.33 (0.89–1.96) | 2.33 (1.58–3.46) |
| Postmenopausal women | ||||
| Unadjusted | 1.00 (Reference) | 1.11 (0.64–1.91) | 1.27 (0.74–2.17) | 1.71 (1.01–2.90) |
| Adjusted | 1.00 (Reference) | 1.15 (0.65–2.02) | 1.36 (0.78–2.38) | 1.85 (1.07–3.21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, E.; Cho, A.-R.; Haam, J.-H.; Gil, M.; Lee, Y.-K.; Kim, Y.-S. Relationship between Serum Cortisol, Dehydroepiandrosterone Sulfate (DHEAS) Levels, and Natural Killer Cell Activity: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 4027. https://doi.org/10.3390/jcm12124027
Suh E, Cho A-R, Haam J-H, Gil M, Lee Y-K, Kim Y-S. Relationship between Serum Cortisol, Dehydroepiandrosterone Sulfate (DHEAS) Levels, and Natural Killer Cell Activity: A Cross-Sectional Study. Journal of Clinical Medicine. 2023; 12(12):4027. https://doi.org/10.3390/jcm12124027
Chicago/Turabian StyleSuh, Eunkyung, A-Ra Cho, Ji-Hee Haam, Minchan Gil, Yun-Kyong Lee, and Young-Sang Kim. 2023. "Relationship between Serum Cortisol, Dehydroepiandrosterone Sulfate (DHEAS) Levels, and Natural Killer Cell Activity: A Cross-Sectional Study" Journal of Clinical Medicine 12, no. 12: 4027. https://doi.org/10.3390/jcm12124027
APA StyleSuh, E., Cho, A.-R., Haam, J.-H., Gil, M., Lee, Y.-K., & Kim, Y.-S. (2023). Relationship between Serum Cortisol, Dehydroepiandrosterone Sulfate (DHEAS) Levels, and Natural Killer Cell Activity: A Cross-Sectional Study. Journal of Clinical Medicine, 12(12), 4027. https://doi.org/10.3390/jcm12124027





