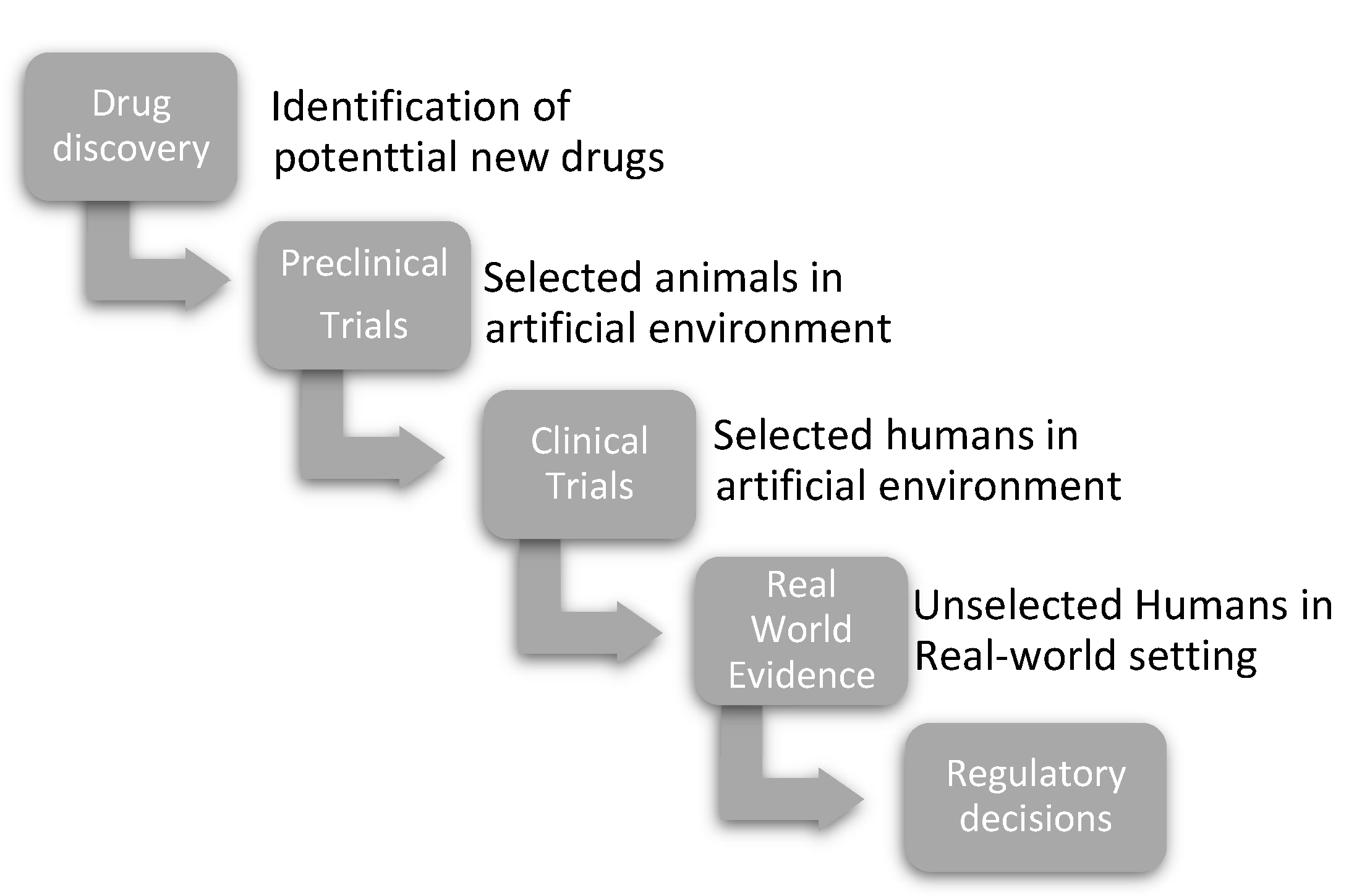
The Disruptive Force of Real-World Evidence
1. Effectiveness and Efficacy
2. RCTs and RWE Can Complement Each Other
3. Conclusions
Conflicts of Interest
References
- Davidoff, F.; DeAngelis, C.D.; Drazen, J.M.; Hoey, J.; Hojgaard, L.; Horton, R.; Kotzin, S.; Nicholls, M.G.; Nylenna, M.; Overbeke, A.J.; et al. Sponsorship, authorship, and accountability. Lancet 2001, 358, 854–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokka, T.; Pincus, T. Most patients receiving routine care for rheumatoid arthritis in 2001 did not meet inclusion criteria for most recent clinical trials or american college of rheumatology criteria for remission. J. Rheumatol. 2003, 30, 1138–1146. [Google Scholar] [PubMed]
- Oishi, K.; Hamada, K.; Murata, Y.; Matsuda, K.; Ohata, S.; Yamaji, Y.; Asami-Noyama, M.; Edakuni, N.; Kakugawa, T.; Hirano, T.; et al. A Real-World Study of Achievement Rate and Predictive Factors of Clinical and Deep Remission to Biologics in Patients with Severe Asthma. J. Clin. Med. 2023, 12, 2900. [Google Scholar] [CrossRef] [PubMed]
- Asai, N.; Shibata, Y.; Hirai, J.; Ohashi, W.; Sakanashi, D.; Kato, H.; Hagihara, M.; Suematsu, H.; Mikamo, H. A Gap of Patients with Infective Endocarditis between Clinical Trials and the Real World. J. Clin. Med. 2023, 12, 1566. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, S.; Konig, A.; Gerger, V.; Rummenigge, C.; Muller, A.C.; Jung, T.; Frank, A.; Tassopoulos, G.; Laurent, E.; Kaufmann, R.; et al. Efficacy and Treatment Satisfaction of Different Systemic Therapies in Children and Adolescents with Moderate-to-Severe Atopic Dermatitis: A Real-World Study. J. Clin. Med. 2023, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Wang, Y.; Mei, F.; Zou, K.; Li, L.; Sun, X. Methods for the Inclusion of Real-World Evidence in a Rare Events Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 1690. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.; Philipp, J.; Wilfer, J.; Kostev, K. Predictive Attributes for Developing Long COVID-A Study Using Machine Learning and Real-World Data from Primary Care Physicians in Germany. J. Clin. Med. 2023, 12, 3511. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt-Egenolf, M. The Disruptive Force of Real-World Evidence. J. Clin. Med. 2023, 12, 4026. https://doi.org/10.3390/jcm12124026
Schmitt-Egenolf M. The Disruptive Force of Real-World Evidence. Journal of Clinical Medicine. 2023; 12(12):4026. https://doi.org/10.3390/jcm12124026
Chicago/Turabian StyleSchmitt-Egenolf, Marcus. 2023. "The Disruptive Force of Real-World Evidence" Journal of Clinical Medicine 12, no. 12: 4026. https://doi.org/10.3390/jcm12124026
APA StyleSchmitt-Egenolf, M. (2023). The Disruptive Force of Real-World Evidence. Journal of Clinical Medicine, 12(12), 4026. https://doi.org/10.3390/jcm12124026




