Cycle Biodynamics of Women’s Microbiome in the Urinary and Reproductive Systems
Abstract
:1. Introduction
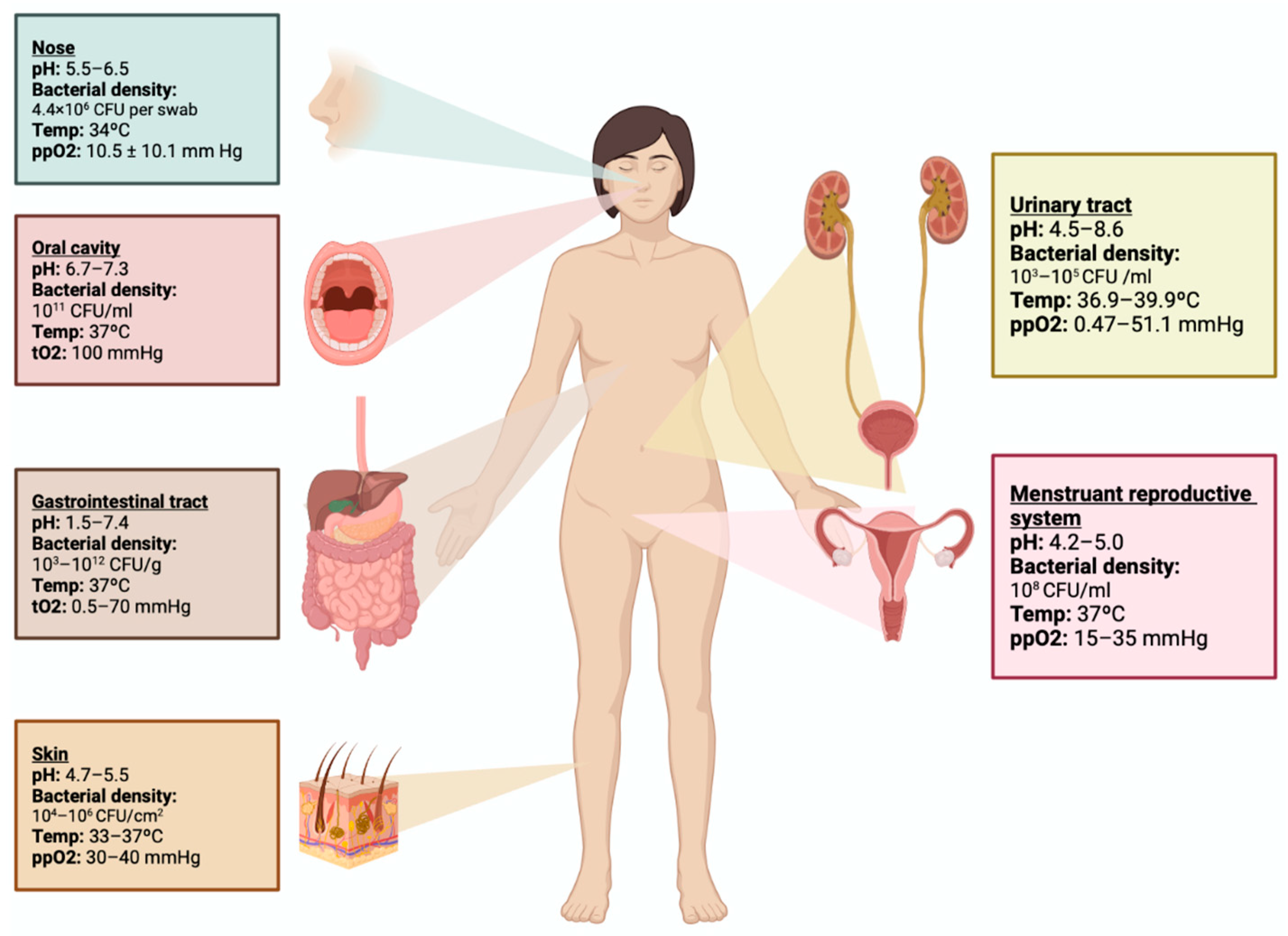
2. Genitourinary Tract Microbiome
3. Genitourinary Microbiome during a Woman’s Lifetime
4. Menstrual Cycle and Genitourinary Microbiome
5. Hormonal Contraceptives and Genitourinary Microbiome
6. Reproductive Health and the Genitourinary Microbiome
6.1. Pregnancy
6.2. Infertility
7. Urinary Tract Infections and Urinary Microbiome
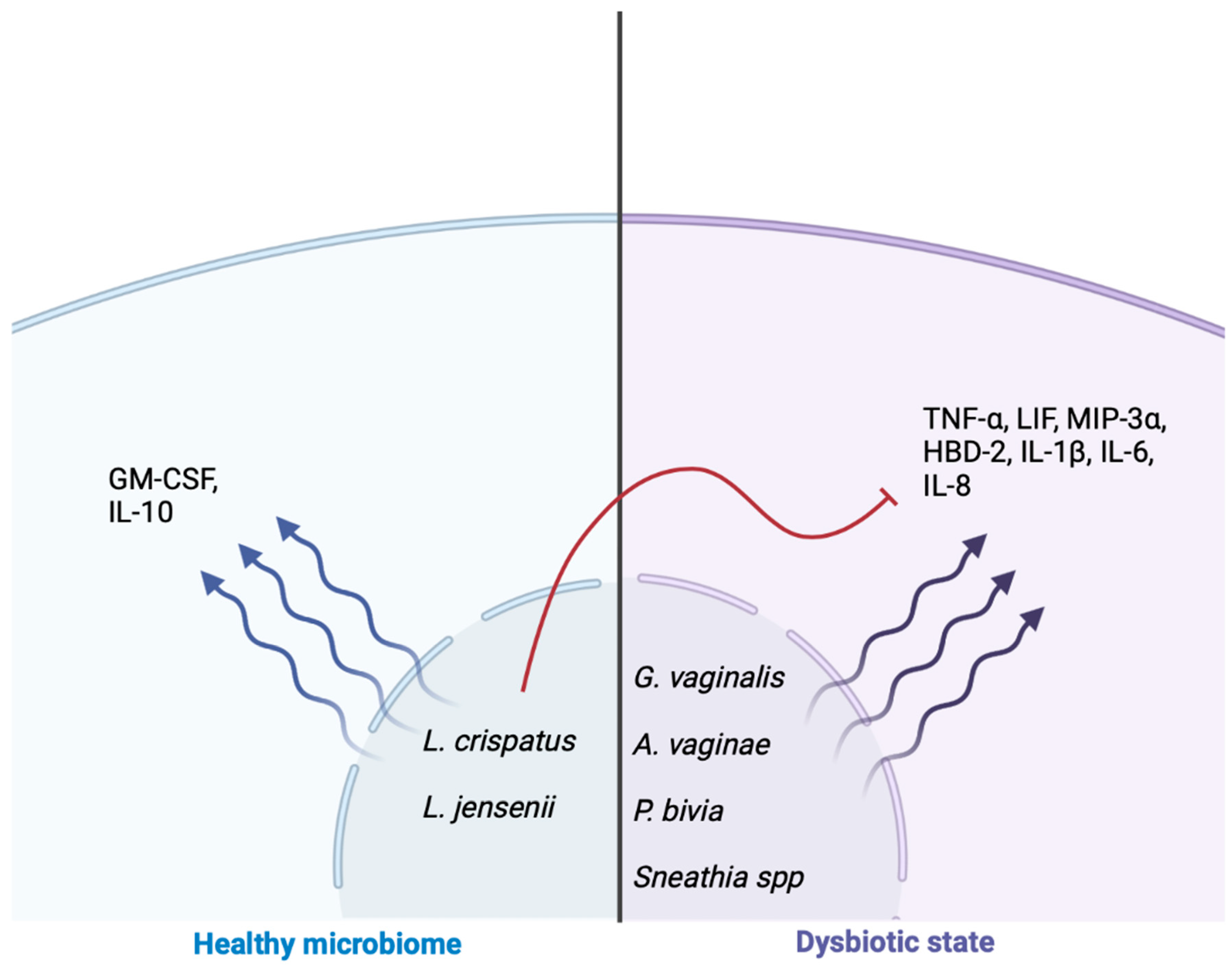
8. Genitourinary Microbiome and Inflammatory Cascade
9. Probiotics and Diet for the Establishment of the Genitourinary Microbiome
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno, I.; Franasiak, J.M. Endometrial microbiota-new player in town. Fertil. Steril. 2017, 108, 32–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the social brain. Science 2019, 366, eaar2016. [Google Scholar] [CrossRef] [PubMed]
- Neugent, M.L.; Hulyalkar, N.V.; Nguyen, V.H.; Zimmern, P.E.; De Nisco, N.J. Advances in Understanding the Human Urinary Microbiome and Its Potential Role in Urinary Tract Infection. MBio 2020, 11, e00218-20. [Google Scholar] [CrossRef]
- Mtshali, A.; San, J.E.; Osman, F.; Garrett, N.; Balle, C.; Giandhari, J.; Onywera, H.; Mngomezulu, K.; Mzobe, G.; de Oliveira, T.; et al. Temporal Changes in Vaginal Microbiota and Genital Tract Cytokines Among South African Women Treated for Bacterial Vaginosis. Front. Immunol. 2021, 12, 730986. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rodriguez, C.; Romero-Gonzalez, R.; Albani-Campanario, M.; Figueroa-Damian, R.; Meraz-Cruz, N.; Hernandez-Guerrero, C. Vaginal microbiota of healthy pregnant Mexican women is constituted by four Lactobacillus species and several vaginosis-associated bacteria. Infect. Dis. Obstet. Gynecol. 2011, 2011, 851485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, S.S.; Berry, A.; Camenga, D.R.; Fitzgerald, C.M.; Gahagan, S.; Hardacker, C.T.; Harlow, B.L.; Hebert-Beirne, J.; LaCoursiere, D.Y.; Lewis, J.B.; et al. Applying concepts of life course theory and life course epidemiology to the study of bladder health and lower urinary tract symptoms among girls and women. Neurourol. Urodyn. 2020, 39, 1185–1202. [Google Scholar] [CrossRef]
- Kalinderi, K.; Delkos, D.; Kalinderis, M.; Athanasiadis, A.; Kalogiannidis, I. Urinary tract infection during pregnancy: Current concepts on a common multifaceted problem. J. Obstet. Gynaecol. 2018, 38, 448–453. [Google Scholar] [CrossRef]
- Petricevic, L.; Domig, K.J.; Nierscher, F.J.; Sandhofer, M.J.; Fidesser, M.; Krondorfer, I.; Husslein, P.; Kneifel, W.; Kiss, H. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci. Rep. 2014, 4, 5136. [Google Scholar] [CrossRef] [PubMed]
- Punzon-Jimenez, P.; Labarta, E. The impact of the female genital tract microbiome in women health and reproduction: A review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541. [Google Scholar] [CrossRef] [PubMed]
- Wolff, B.J.; Price, T.K.; Joyce, C.J.; Wolfe, A.J.; Mueller, E.R. Oral probiotics and the female urinary microbiome: A double-blinded randomized placebo-controlled trial. Int. Urol. Nephrol. 2019, 51, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Fouts, D.E.; Pieper, R.; Szpakowski, S.; Pohl, H.; Knoblach, S.; Suh, M.J.; Huang, S.T.; Ljungberg, I.; Sprague, B.M.; Lucas, S.K.; et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl. Med. 2012, 10, 174. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, H.; Nederbragt, A.J.; Lagesen, K.; Jeansson, S.L.; Jakobsen, K.S. Assessing diversity of the female urine microbiota by high throughput sequencing of 16S rDNA amplicons. BMC Microbiol. 2011, 11, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curley, T.; Forster, C.S. Recurrent UTIs in Girls: What Is the Role of the Microbiome? Urology 2021, 151, 94–97. [Google Scholar] [CrossRef]
- Hickey, R.J.; Zhou, X.; Settles, M.L.; Erb, J.; Malone, K.; Hansmann, M.A.; Shew, M.L.; Van Der Pol, B.; Fortenberry, J.D.; Forney, L.J. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. MBio 2015, 6, e00097-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storm, D.W.; Copp, H.L.; Halverson, T.M.; Du, J.; Juhr, D.; Wolfe, A.J. A Child’s urine is not sterile: A pilot study evaluating the Pediatric Urinary Microbiome. J. Pediatr. Urol. 2022, 18, 383–392. [Google Scholar] [CrossRef]
- Rostok, M.; Hutt, P.; Roop, T.; Smidt, I.; Stsepetova, J.; Salumets, A.; Mandar, R. Potential vaginal probiotics: Safety, tolerability and preliminary effectiveness. Benef. Microbes 2019, 10, 385–393. [Google Scholar] [CrossRef]
- Javan Balegh Marand, A.; Van Koeveringe, G.A.; Janssen, D.; Vahed, N.; Vogeli, T.A.; Heesakkers, J.; Hajebrahimi, S.; Rahnama’I, M.S. Urinary Microbiome and its Correlation with Disorders of the Genitourinary System. Urol. J. 2021, 18, 259–270. [Google Scholar]
- Saraf, V.S.; Sheikh, S.A.; Ahmad, A.; Gillevet, P.M.; Bokhari, H.; Javed, S. Vaginal microbiome: Normalcy vs. dysbiosis. Arch. Microbiol. 2021, 203, 3793–3802. [Google Scholar] [CrossRef]
- Neugent, M.L.; Kumar, A.; Hulyalkar, N.V.; Lutz, K.C.; Nguyen, V.H.; Fuentes, J.L.; Zhang, C.; Nguyen, A.; Sharon, B.M.; Kuprasertkul, A.; et al. Recurrent urinary tract infection and estrogen shape the taxonomic ecology and function of the postmenopausal urogenital microbiome. Cell Rep. Med. 2022, 3, 100753. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Ma, N.; Mitchell, A.J.; Wu, M.C.; Valint, D.J.; Proll, S.; Reed, S.D.; Guthrie, K.A.; Lacroix, A.Z.; Larson, J.C.; et al. Association between postmenopausal vulvovaginal discomfort, vaginal microbiota, and mucosal inflammation. Am. J. Obstet. Gynecol. 2021, 225, 159.e1–159.e15. [Google Scholar] [CrossRef]
- van de Wijgert, J.; Jespers, V. The global health impact of vaginal dysbiosis. Res. Microbiol. 2017, 168, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef] [Green Version]
- Song, S.D.; Acharya, K.D.; Zhu, J.E.; Deveney, C.M.; Walther-Antonio, M.R.S.; Tetel, M.J.; Chia, N. Daily Vaginal Microbiota Fluctuations Associated with Natural Hormonal Cycle, Contraceptives, Diet, and Exercise. MSphere 2020, 5, e00593-20. [Google Scholar] [CrossRef] [PubMed]
- Krog, M.C.; Hugerth, L.W.; Fransson, E.; Bashir, Z.; Nyboe Andersen, A.; Edfeldt, G.; Engstrand, L.; Schuppe-Koistinen, I.; Nielsen, H.S. The healthy female microbiome across body sites: Effect of hormonal contraceptives and the menstrual cycle. Hum. Reprod. 2022, 37, 1525–1543. [Google Scholar] [CrossRef]
- Chaban, B.; Links, M.G.; Jayaprakash, T.P.; Wagner, E.C.; Bourque, D.K.; Lohn, Z.; Albert, A.Y.; van Schalkwyk, J.; Reid, G.; Hemmingsen, S.M. Characterization of the vaginal microbiota of healthy Canadian women through the menstrual cycle. Microbiome 2014, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Van den Veyver, I.; Milosavljevic, A.; et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef]
- Cobo, T.; Vergara, A.; Collado, M.C.; Herreros, E.; Bosch, J.; Sanchez-Garcia, A.B.; Lopez-Parellada, R.; Ponce, J.; Gratacos, E. Characterization of vaginal microbiota in women with preterm labor with intra-amniotic inflammation. Sci. Rep. 2019, 9, 18963. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.; Zhang, X.; Liang, Y.; Lin, S.; Qian, W.; Fan, S. Alterations in Vaginal Microbiota and Associated Metabolome in Women with Recurrent Implantation Failure. MBio 2020, 11, e03242-19. [Google Scholar] [CrossRef]
- Tomaiuolo, R.; Veneruso, I.; Cariati, F.; D’Argenio, V. Microbiota and Human Reproduction: The Case of Female Infertility. High Throughput 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Mrozikiewicz, A.E.; Ozarowski, M.; Jedrzejczak, P. Biomolecular Markers of Recurrent Implantation Failure-A Review. Int. J. Mol. Sci. 2021, 22, 10082. [Google Scholar] [CrossRef]
- Gholiof, M.; Adamson-De Luca, E.; Wessels, J.M. The female reproductive tract microbiotas, inflammation, and gynecological conditions. Front. Reprod. Health 2022, 4, 963752. [Google Scholar] [CrossRef] [PubMed]
- Wee, B.A.; Thomas, M.; Sweeney, E.L.; Frentiu, F.D.; Samios, M.; Ravel, J.; Gajer, P.; Myers, G.; Timms, P.; Allan, J.A.; et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust. New Zealand J. Obstet. Gynaecol. 2018, 58, 341–348. [Google Scholar] [CrossRef]
- Riganelli, L.; Iebba, V.; Piccioni, M.; Illuminati, I.; Bonfiglio, G.; Neroni, B.; Calvo, L.; Gagliardi, A.; Levrero, M.; Merlino, L.; et al. Structural Variations of Vaginal and Endometrial Microbiota: Hints on Female Infertility. Front. Cell Infect. Microbiol. 2020, 10, 350. [Google Scholar] [CrossRef]
- Haahr, T.; Jensen, J.S.; Thomsen, L.; Duus, L.; Rygaard, K.; Humaidan, P. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: A prospective study in IVF patients. Hum. Reprod. 2016, 31, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Sehring, J.; Beltsos, A.; Jeelani, R. Human implantation: The complex interplay between endometrial receptivity, inflammation, and the microbiome. Placenta 2022, 117, 179–186. [Google Scholar] [CrossRef]
- Miller-Ensminger, T.; Garretto, A.; Brenner, J.; Thomas-White, K.; Zambom, A.; Wolfe, A.J.; Putonti, C. Bacteriophages of the Urinary Microbiome. J. Bacteriol. 2018, 200, e00738-17. [Google Scholar] [CrossRef] [Green Version]
- Morand, A.; Cornu, F.; Dufour, J.C.; Tsimaratos, M.; Lagier, J.C.; Raoult, D. Human Bacterial Repertoire of the Urinary Tract: A Potential Paradigm Shift. J. Clin. Microbiol. 2019, 57, e00675-18. [Google Scholar] [CrossRef] [Green Version]
- Price, T.K.; Hilt, E.E.; Thomas-White, K.; Mueller, E.R.; Wolfe, A.J.; Brubaker, L. The urobiome of continent adult women: A cross-sectional study. BJOG 2020, 127, 193–201. [Google Scholar] [CrossRef]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Brubaker, L.; Wolfe, A.J.; Mueller, E.R.; Schreckenberger, P.C. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustafa, A.; Li, W.; Singh, H.; Moncera, K.J.; Torralba, M.G.; Yu, Y.; Manuel, O.; Biggs, W.; Venter, J.C.; Nelson, K.E.; et al. Microbial metagenome of urinary tract infection. Sci. Rep. 2018, 8, 4333. [Google Scholar] [CrossRef] [PubMed]
- Magistro, G.; Stief, C.G. The Urinary Tract Microbiome: The Answer to All Our Open Questions? Eur. Urol. Focus 2019, 5, 36–38. [Google Scholar] [CrossRef]
- Govender, Y.; Gabriel, I.; Minassian, V.; Fichorova, R. The Current Evidence on the Association Between the Urinary Microbiome and Urinary Incontinence in Women. Front. Cell Infect. Microbiol. 2019, 9, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragon, I.M.; Herrera-Imbroda, B.; Queipo-Ortuno, M.I.; Castillo, E.; Del Moral, J.S.; Gomez-Millan, J.; Yucel, G.; Lara, M.F. The Urinary Tract Microbiome in Health and Disease. Eur. Urol. Focus 2018, 4, 128–138. [Google Scholar] [CrossRef]
- Villa, P.; Cipolla, C.; D’Ippolito, S.; Amar, I.D.; Shachor, M.; Ingravalle, F.; Scaldaferri, F.; Puca, P.; Di Simone, N.; Scambia, G. The interplay between immune system and microbiota in gynecological diseases: A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5676–5690. [Google Scholar]
- Doerflinger, S.Y.; Throop, A.L.; Herbst-Kralovetz, M.M. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J. Infect. Dis. 2014, 209, 1989–1999. [Google Scholar] [CrossRef] [Green Version]
- Dabee, S.; Passmore, J.S.; Heffron, R.; Jaspan, H.B. The Complex Link between the Female Genital Microbiota, Genital Infections, and Inflammation. Infect. Immun. 2021, 89, e00487-20. [Google Scholar] [CrossRef]
- Raglan, O.; MacIntyre, D.A.; Mitra, A.; Lee, Y.S.; Smith, A.; Assi, N.; Nautiyal, J.; Purkayastha, S.; Gunter, M.J.; Gabra, H.; et al. The association between obesity and weight loss after bariatric surgery on the vaginal microbiota. Microbiome 2021, 9, 124. [Google Scholar] [CrossRef]
- Masson, L.; Passmore, J.A.; Liebenberg, L.J.; Werner, L.; Baxter, C.; Arnold, K.B.; Williamson, C.; Little, F.; Mansoor, L.E.; Naranbhai, V.; et al. Genital inflammation and the risk of HIV acquisition in women. Clin. Infect. Dis. 2015, 61, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Dabee, S.; Tanko, R.F.; Brown, B.P.; Bunjun, R.; Balle, C.; Feng, C.; Konstantinus, I.N.; Jaumdally, S.Z.; Onono, M.; Nair, G.; et al. Comparison of Female Genital Tract Cytokine and Microbiota Signatures Induced by Initiation of Intramuscular DMPA and NET-EN Hormonal Contraceptives—A Prospective Cohort Analysis. Front. Immunol. 2021, 12, 760504. [Google Scholar] [CrossRef]
- Jean, S.; Huang, B.; Parikh, H.I.; Edwards, D.J.; Brooks, J.P.; Kumar, N.G.; Sheth, N.U.; Koparde, V.; Smirnova, E.; Huzurbazar, S.; et al. Multi-omic Microbiome Profiles in the Female Reproductive Tract in Early Pregnancy. Infect. Microbes Dis. 2019, 1, 49–60. [Google Scholar] [CrossRef]
- Husain, S.; Allotey, J.; Drymoussi, Z.; Wilks, M.; Fernandez-Felix, B.M.; Whiley, A.; Dodds, J.; Thangaratinam, S.; McCourt, C.; Prosdocimi, E.M.; et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: A randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG 2020, 127, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Li, Z.; Tye, K.D.; Chen, Y.; Luo, H.; Xiao, X. The effect of probiotic supplementation during pregnancy on the interaction network of vaginal microbiome. J. Obstet. Gynaecol. Res. 2021, 47, 103–113. [Google Scholar] [CrossRef]
- Skoracka, K.; Ratajczak, A.E.; Rychter, A.M.; Dobrowolska, A.; Krela-Kazmierczak, I. Female Fertility and the Nutritional Approach: The Most Essential Aspects. Adv. Nutr. 2021, 12, 2372–2386. [Google Scholar] [CrossRef] [PubMed]
- Akoh, C.C.; Pressman, E.K.; Cooper, E.; Queenan, R.A.; Pillittere, J.; O’Brien, K.O. Low Vitamin D is Associated With Infections and Proinflammatory Cytokines During Pregnancy. Reprod. Sci. 2018, 25, 414–423. [Google Scholar] [CrossRef]
- Sun, H.; Yamada, P.; Paetow, A.; Chan, M.; Arslan, A.; Landberg, R.; Dominguez-Bello, M.G.; Young, B.K. A randomized controlled trial of the effects of whole grains versus refined grains diets on the microbiome in pregnancy. Sci. Rep. 2022, 12, 7509. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet. Gynecol. 2007, 110, 1050–1058. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, Y.; Yang, J.; Xie, X.; Chen, H. The immune responses to different Uropathogens call individual interventions for bladder infection. Front. Immunol. 2022, 13, 953354. [Google Scholar] [CrossRef]
- Bruyere, F.; Azzouzi, A.R.; Lavigne, J.P.; Droupy, S.; Coloby, P.; Game, X.; Karsenty, G.; Issartel, B.; Ruffion, A.; Misrai, V.; et al. A Multicenter, Randomized, Placebo-Controlled Study Evaluating the Efficacy of a Combination of Propolis and Cranberry (Vaccinium macrocarpon) (DUAB(R)) in Preventing Low Urinary Tract Infection Recurrence in Women Complaining of Recurrent Cystitis. Urol. Int. 2019, 103, 41–48. [Google Scholar] [CrossRef]
- Botto, H.; Dreyfus, J.F. The Results Given in the Paper by Bruyere et al. “Multicenter, Randomized, Placebo-Controlled Study [of] the Efficacy of a Combination of Propolis and Cranberry... (DUAB(R)) in Preventing Low Urinary Tract Infection Recurrence in Women... [with] Recurrent Cystitis” Should Be Used with Great Caution. Urol. Int. 2020, 104, 497–498. [Google Scholar]
- Maki, K.C.; Kaspar, K.L.; Khoo, C.; Derrig, L.H.; Schild, A.L.; Gupta, K. Consumption of a cranberry juice beverage lowered the number of clinical urinary tract infection episodes in women with a recent history of urinary tract infection. Am. J. Clin. Nutr. 2016, 103, 1434–1442. [Google Scholar] [CrossRef] [Green Version]
- Kaspar, K.L.; Howell, A.B.; Khoo, C. A randomized, double-blind, placebo-controlled trial to assess the bacterial anti-adhesion effects of cranberry extract beverages. Food Funct. 2015, 6, 1212–1217. [Google Scholar] [CrossRef]
- Beerepoot, M.; Geerlings, S. Non-Antibiotic Prophylaxis for Urinary Tract Infections. Pathogens 2016, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Hassuna, N.A.; Rabie, E.M.; Mahd, W.K.M.; Refaie, M.M.M.; Yousef, R.K.M.; Abdelraheem, W.M. Antibacterial effect of vitamin C against uropathogenic E. coli in vitro and in vivo. BMC Microbiol. 2023, 23, 112. [Google Scholar] [CrossRef]
- Cerezo, A.B.; Catunescu, G.M.; Gonzalez, M.M.; Hornedo-Ortega, R.; Pop, C.R.; Rusu, C.C.; Chirila, F.; Rotar, A.M.; Garcia-Parrilla, M.C.; Troncoso, A.M. Anthocyanins in Blueberries Grown in Hot Climate Exert Strong Antioxidant Activity and May Be Effective against Urinary Tract Bacteria. Antioxidants 2020, 9, 478. [Google Scholar] [CrossRef]
- Archana, K.M.; Rajalakshmi, S.; Kumar, P.S.; Krishnaswamy, V.G.; Rajagopal, R.; Kumar, D.T.; Priya Doss, C.G. Effect of shape and anthocyanin capping on antibacterial activity of CuI particles. Environ. Res. 2021, 200, 111759. [Google Scholar] [CrossRef]
- Bauckman, K.A.; Matsuda, R.; Higgins, C.B.; DeBosch, B.J.; Wang, C.; Mysorekar, I.U. Dietary restriction of iron availability attenuates UPEC pathogenesis in a mouse model of urinary tract infection. Am. J. Physiol. Renal. Physiol. 2019, 316, F814–F822. [Google Scholar] [CrossRef]
- Patras, K.A.; Ha, A.D.; Rooholfada, E.; Olson, J.; Ramachandra Rao, S.P.; Lin, A.E.; Nizet, V. Augmentation of Urinary Lactoferrin Enhances Host Innate Immune Clearance of Uropathogenic Escherichia coli. J. Innate Immun. 2019, 11, 481–495. [Google Scholar] [CrossRef]
- Murtha, M.J.; Eichler, T.; Bender, K.; Metheny, J.; Li, B.; Schwaderer, A.L.; Mosquera, C.; James, C.; Schwartz, L.; Becknell, B.; et al. Insulin receptor signaling regulates renal collecting duct and intercalated cell antibacterial defenses. J. Clin. Investig. 2018, 128, 5634–5646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.H.; Liu, S.P.; Fan, C.K.; Tzou, K.Y.; Wu, C.C.; Cheng, P.C. Insulin Downregulated the Infection of Uropathogenic Escherichia coli (UPEC) in Bladder Cells in a High-Glucose Environment through JAK/STAT Signaling Pathway. Microorganisms 2021, 9, 2421. [Google Scholar] [CrossRef]
- Huttner, A.; Hatz, C.; van den Dobbelsteen, G.; Abbanat, D.; Hornacek, A.; Frolich, R.; Dreyer, A.M.; Martin, P.; Davies, T.; Fae, K.; et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: A randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect. Dis. 2017, 17, 528–537. [Google Scholar] [CrossRef]
- Eldridge, G.R.; Hughey, H.; Rosenberger, L.; Martin, S.M.; Shapiro, A.M.; D’Antonio, E.; Krejci, K.G.; Shore, N.; Peterson, J.; Lukes, A.S.; et al. Safety and immunogenicity of an adjuvanted Escherichia coli adhesin vaccine in healthy women with and without histories of recurrent urinary tract infections: Results from a first-in-human phase 1 study. Hum. Vaccines Immunother. 2021, 17, 1262–1270. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grobeisen-Duque, O.; Mora-Vargas, C.D.; Aguilera-Arreola, M.G.; Helguera-Repetto, A.C. Cycle Biodynamics of Women’s Microbiome in the Urinary and Reproductive Systems. J. Clin. Med. 2023, 12, 4003. https://doi.org/10.3390/jcm12124003
Grobeisen-Duque O, Mora-Vargas CD, Aguilera-Arreola MG, Helguera-Repetto AC. Cycle Biodynamics of Women’s Microbiome in the Urinary and Reproductive Systems. Journal of Clinical Medicine. 2023; 12(12):4003. https://doi.org/10.3390/jcm12124003
Chicago/Turabian StyleGrobeisen-Duque, Orly, Carlos Daniel Mora-Vargas, Ma. Guadalupe Aguilera-Arreola, and Addy Cecilia Helguera-Repetto. 2023. "Cycle Biodynamics of Women’s Microbiome in the Urinary and Reproductive Systems" Journal of Clinical Medicine 12, no. 12: 4003. https://doi.org/10.3390/jcm12124003
APA StyleGrobeisen-Duque, O., Mora-Vargas, C. D., Aguilera-Arreola, M. G., & Helguera-Repetto, A. C. (2023). Cycle Biodynamics of Women’s Microbiome in the Urinary and Reproductive Systems. Journal of Clinical Medicine, 12(12), 4003. https://doi.org/10.3390/jcm12124003





