Rate of Postoperative Urinary Retention after Anterior Compartment Prolapse Surgery: A Randomized Controlled Trial Comparing Early versus Conventional Transurethral Catheter Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Objectives
2.3. Participating Hospitals and Interventions
2.4. Outcomes
2.5. Sample Size Calculation and Power Estimation
2.6. Statistical Analysis
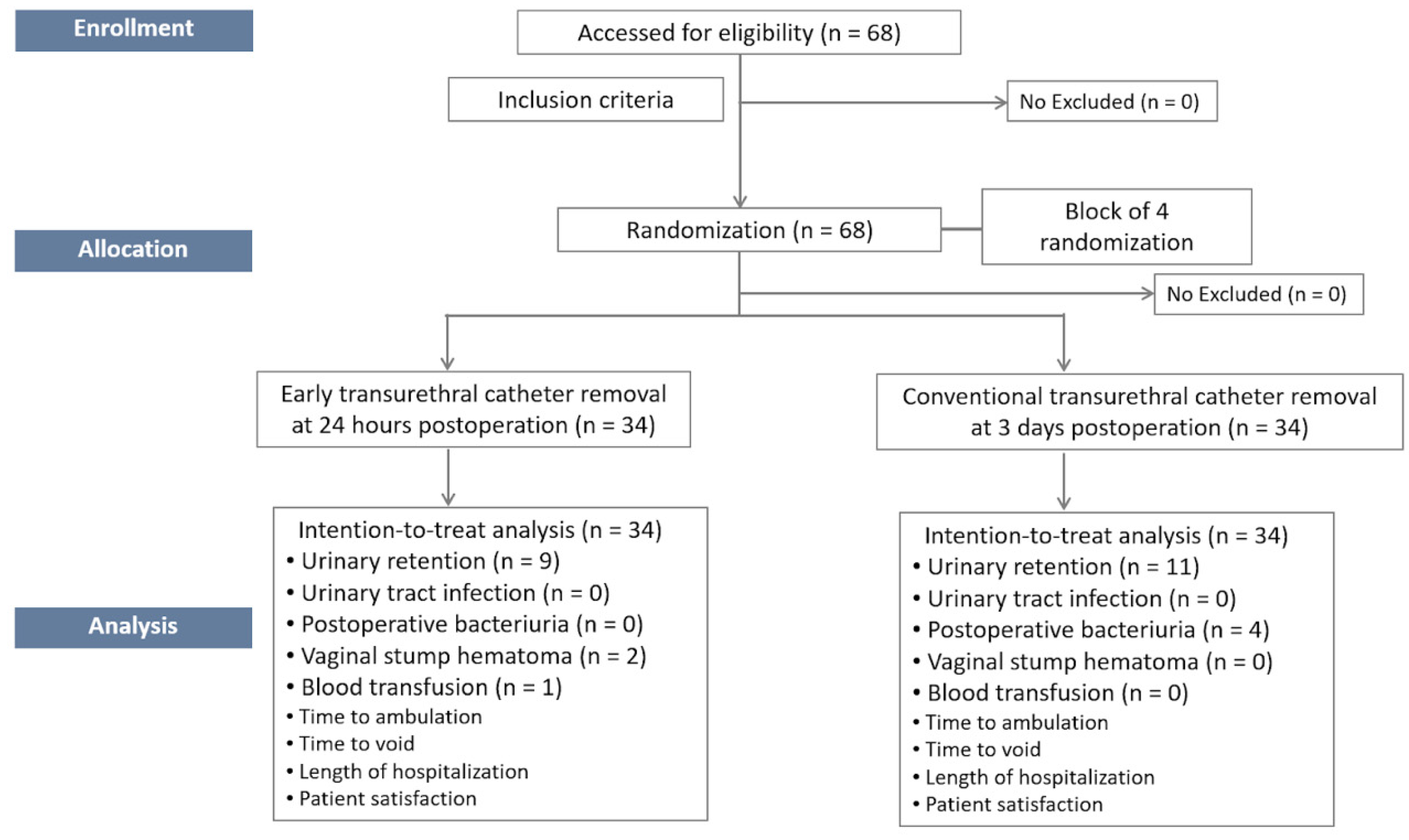
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, B.; Chen, Y.; Zhu, X.; Wang, T.; Li, M.; Huang, Y.; Xue, L.; Zhu, Q.; Gao, X.; Wu, M. Global burden and trends of pelvic organ prolapse associated with aging women: An observational trend study from 1990 to 2019. Front. Public Health 2022, 10, 975829. [Google Scholar] [CrossRef] [PubMed]
- Aboseif, C.; Liu, P. Pelvic Organ Prolapse. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chow, D.; Rodríguez, L.V. Epidemiology and prevalence of pelvic organ prolapse. Curr. Opin. Urol. 2013, 23, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.P. Systematic review of lower urinary tract symptoms occurring with pelvic organ prolapse. Arab. J. Urol. 2019, 17, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Wein, A.J.; Kavoussi, L.R.; Partin, A.W.; Peters, C.A. Campbell-Walsh Urology 2016, 4-Volume Set, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Thanagumtorn, K. Accuracy of Post-Void Residual Urine Volume Measurement Using an Ultrasound Bladder Scanner among Postoperative Radical Hysterectomy Patients. J. Med. Assoc. Thai. 2016, 99, 1061–1066. [Google Scholar] [PubMed]
- Anglim, B.C.; Tomlinson, G.; Paquette, J.; McDermott, C.D. A risk calculator for postoperative urinary retention (POUR) following vaginal pelvic floor surgery: Multivariable prediction modelling. BJOG 2022, 129, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Bohlin, K.S.; Ankardal, M.; Nüssler, E.; Lindkvist, H.; Milsom, I. Factors influencing the outcome of surgery for pelvic organ prolapse. Int. Urogynecol. J. 2017, 29, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Hu, Z.; Ye, Z.; Xu, Q.; Chen, J.; Lin, Y. A systematic review comparing early with late removal of indwelling urinary catheters after pelvic organ prolapse surgery. Int. Urogynecol. J. 2020, 32, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Geller, E.J. Prevention and management of postoperative urinary retention after urogynecologic surgery. Int. J. Women’s Health 2014, 6, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Hakvoort, R.; Elberink, R.; Vollebregt, A.; Ploeg, T.; Emanuel, M. How long should urinary bladder catheterisation be continued after vaginal prolapse surgery? A randomised controlled trial comparing short term versus long term catheterisation after vaginal prolapse surgery. BJOG 2004, 111, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Kamilya, G.; Seal, S.L.; Mukherji, J.; Bhattacharyya, S.K.; Hazra, A. A randomized controlled trial comparing short versus long-term catheterization after uncomplicated vaginal prolapse surgery. J. Obstet. Gynaecol. Res. 2010, 36, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Prapaspongsa, T.; Manonai, J. Transvaginal Sonographic Assessment of Postvoid Residual Urine Volumes in Women with Pelvic Floor Dysfunction. Ramathibodi Med. J. 2017, 40, 34–41. [Google Scholar]
- Chinthakanan, O.; Petcharopas, A.; Wattanayingcharoenchai, R.; Manonai, J.; Aimjirakul, K. Prevalence of Postoperative Urinary Retention and the Optimal Duration of Transurethral Urinary Catheterization after Pelvic Floor Surgery. J. Med. Assoc. Thai. 2018, 101, 1569–1573. [Google Scholar]
- Haya, N.; Feiner, B.; Baessler, K.; Christmann-Schmid, C.; Maher, C. Perioperative interventions in pelvic organ prolapse surgery. Cochrane Database Syst. Rev. 2018, 2018, CD013105. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, S.; Robinson, D.; Cardozo, L. Validation of the Patient Global Impression of Improvement (PGI-I) for urogenital prolapse. Int. Urogynecol. J. 2009, 21, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Givler, D.N.; Givler, A. Asymptomatic Bacteriuria. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tan, C.; Chlebicki, M. Urinary tract infections in adults. Singap. Med. J. 2016, 57, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalez, S.; Franco, E.M.; Martínez-Cumplido, R.; Coll, C.M.; González, F.O.; Roig, M.D.G.; Tardiu, L.A. Reducing postoperative catheterisation after anterior colporrhaphy from 48 to 24 h: A randomised controlled trial. Int. Urogynecol. J. 2018, 30, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A. Campbell-Walsh Urology 11th Edition Review, E-Book ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
| Conventional Transurethral | Early Transurethral | p Value | ||
|---|---|---|---|---|
| Catheter Removal (n = 34) | Catheter Removal (n = 34) | |||
| Age (y), mean ± SD | 65.7 ± 10.7 | 68.1 ± 9.4 | 0.33 a | |
| BMI (kg/m2), mean ± SD | 25.0 ± 4.0 | 24.6 ± 3.6 | 0.71 a | |
| Vaginal parity, n (%) | ||||
| 0 | 1 (2.9%) | 1 (2.9%) | 1.00 c | |
| 3 (8.8%) | 4 (11.8%) | |||
| ≥2 | 30 (88.2%) | 29 (85.2%) | ||
| Menopause, n (%) | 32 (94.1%) | 34 (100%) | 0.49 c | |
| Medical comorbidity, n (%) | ||||
| Respiratory disease | 4 (11.6%) | 0 | 0.11 c | |
| Metabolic disease | 26 (76.5%) | 28 (82.4%) | 0.55 b | |
| CVD | 1 (2.9%) | 6 (17.7%) | 0.11 c | |
| Current drug use, n (%) | ||||
| Alpha-blocker | 1 (2.94%) | 0 | 1.00 c | |
| Beta-blocker | 2 (5.9%) | 4 (11.8%) | 0.67 c | |
| Prior surgery, n (%) | ||||
| Hysterectomy | 5 (14.7%) | 5 (14.7%) | 1.00 b | |
| Anti-incontinence surgery | 1 (2.9%) | 0 | 1.00 c | |
| POP surgery | ||||
| AC | 1 (2.9%) | 3 (8.8%) | 0.61 c | |
| PC | 1 (2.9%) | 1 (2.9%) | 1.00 c | |
| Baseline anterior vaginal prolapse, n (%) | ||||
| POP-Q stage 2 | 5 (14.7%) | 9 (26.5%) | 0.49 b | |
| POP-Q stage 3 | 15 (44.1%) | 13 (38.2%) | ||
| POP-Q stage 4 | 14 (41.2%) | 12 (35.3%) | ||
| Preoperative PVR (mL), median (range) | 42.95 (0.45–149) | 37.5 (0.97–121) | 0.94 d | |
| Preoperative asymptomatic bacteriuria, n (%) | 5 (14.7%) | 6 (17.7%) | 1.00 c | |
| Conventional Transurethral | Early Transurethral | p Value | |
|---|---|---|---|
| Catheter Removal (n = 34) | Catheter Removal (n = 34) | ||
| Main operation, n (%) | 0.45 c | ||
| Anterior colporrhaphy | 20 (58.8%) | 24 (70.6%) | |
| Colpocleisis | 14 (41.1%) | 10 (29.4%) | |
| Total colpocleisis | 13 (38.2%) | 10 (29.4%) | |
| Lefort | 1 (2.9%) | 0 | |
| Concomitant surgery, n (%) | |||
| Vaginal hysterectomy | 26 (76.5%) | 25 (73.5%) | 0.78 b |
| McCall culdoplasty | 1 (2.9%) | 6 (17.6%) | 0.11 c |
| Sacrospinous ligament fixation | 5 (14.7%) | 2 (5.9%) | 0.43 c |
| Laparoscopic sacrocolpopexy | 0 | 1 (2.9%) | 1.00 c |
| Manchester operation | 0 | 1 (2.9%) | 1.00 c |
| Perineoplasty | 9 (26.5%) | 2 (5.9%) | 0.02 b |
| Posterior colporrhaphy | 12 (35.3%) | 11 (34.2%) | 0.80 b |
| Uterosacral ligament fixation | 1 (3.13%) | 0 | 1.00 c |
| Intraoperative factors | |||
| Operative time (min), mean ± SD | 108.1 ± 37.1 | 90.6 ± 33.7 | 0.05 a |
| Blood loss (mL), median (range) | 50 (10–300) | 50 (10–200) | 1.00 d |
| Intraoperative IV fluid (mL), median (range) | 1002.9 ± 342.0 | 869.7 ± 379.3 | 0.13 d |
| Intraoperative urine output (mL), median (range) | 300 (0–900) | 300 (50–900) | 1.00 d |
| Anesthetic modality, n (%) | |||
| General anesthesia | 9 (26.5%) | 13 (38.2%) | 0.05 c |
| SB without intrathecal morphine | 17 (50.0%) | 20 (58.8%) | |
| SB with intrathecal morphine | 8 (23.5%) | 1 (2.9%) | |
| Opioid use (equianalgesic to IV morphine) | |||
| Intraoperative use, median (range) | 0 (0–10) | 0 (0–10) | 1.00 d |
| Postoperative use, median (range) | 6 (0–30) | 4 (0–37.3) | 0.61 d |
| Complications, n (%) | |||
| Vaginal hematoma | 0 | 2 (5.9%) | 0.49 c |
| Blood transfusion | 0 | 1 (2.9%) | 1.00 c |
| Conventional Transurethral | Early Transurethral | p Value | |
|---|---|---|---|
| Catheter Removal (n = 34) | Catheter Removal (n = 34) | ||
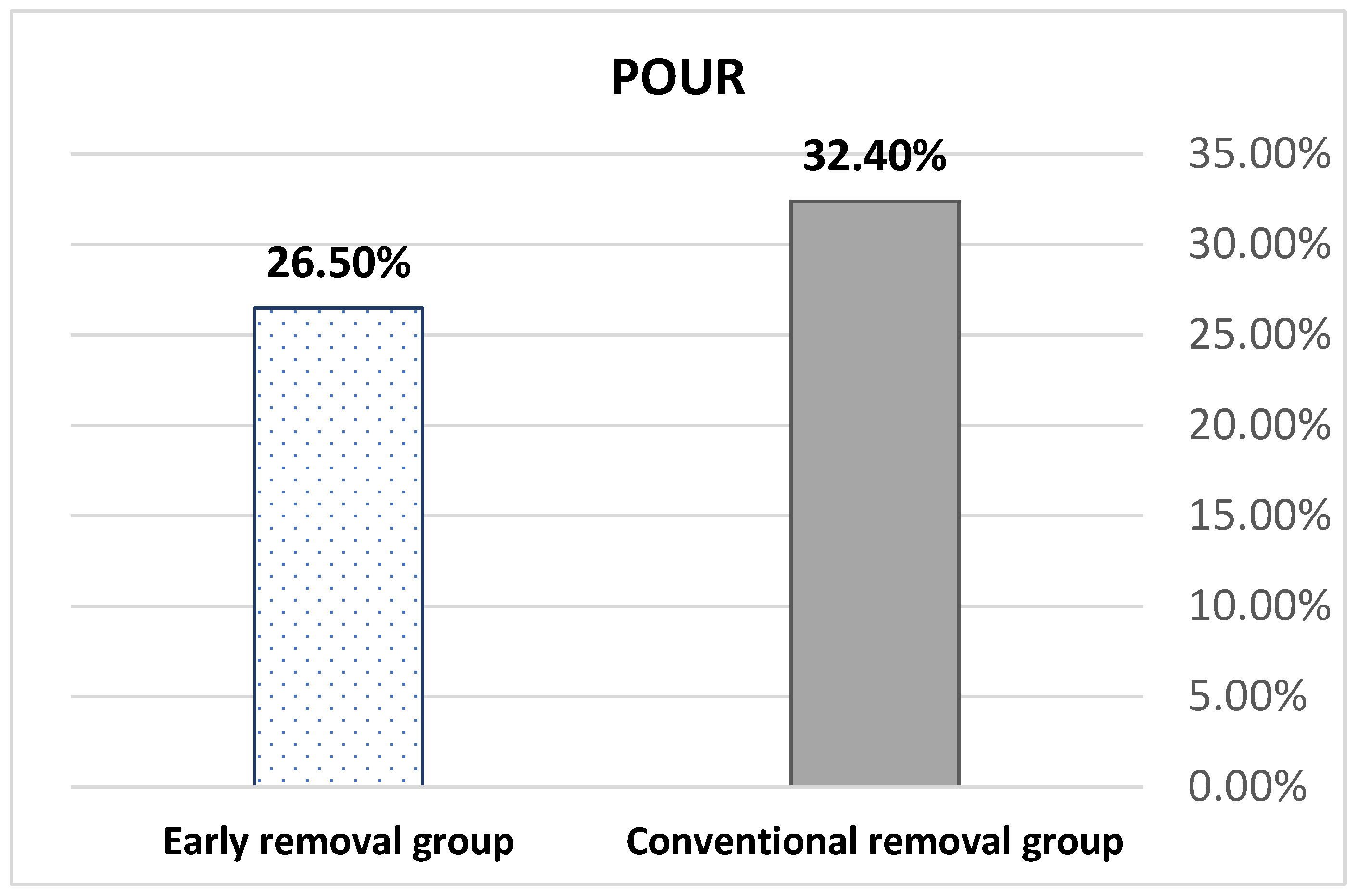
| POUR, n (%) | 11 (32.4%) | 9 (26.5%) | 0.60 a |
| Time to ambulation (h), median (range) | 28 (6–60) | 24.4 (15.3–28) | 0.20 c |
| Time to spontaneous voiding (h), median (range) | 1.5 (0.5–4.3) | 2 (0.5–5.75) | 0.06 c |
| Length of hospitalization (d), median (range) | 3 (3–4) | 1 (1–5) | <0.001 d |
| Patient satisfaction (PGI-I), median (range) | 2 (1–4) | 2 (1–3) | 0.58 c |
| Postoperative asymptomatic bacteriuria, n (%) | 5 (14.7%) | 0 | 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chansriniyom, N.; Saraluck, A.; Kijmanawat, A.; Wattanayingcharoenchai, R.; Aimjirakul, K.; Manonai Bartlett, J.; Chinthakanan, O. Rate of Postoperative Urinary Retention after Anterior Compartment Prolapse Surgery: A Randomized Controlled Trial Comparing Early versus Conventional Transurethral Catheter Removal. J. Clin. Med. 2023, 12, 3436. https://doi.org/10.3390/jcm12103436
Chansriniyom N, Saraluck A, Kijmanawat A, Wattanayingcharoenchai R, Aimjirakul K, Manonai Bartlett J, Chinthakanan O. Rate of Postoperative Urinary Retention after Anterior Compartment Prolapse Surgery: A Randomized Controlled Trial Comparing Early versus Conventional Transurethral Catheter Removal. Journal of Clinical Medicine. 2023; 12(10):3436. https://doi.org/10.3390/jcm12103436
Chicago/Turabian StyleChansriniyom, Nareenun, Apisith Saraluck, Athasit Kijmanawat, Rujira Wattanayingcharoenchai, Komkrit Aimjirakul, Jittima Manonai Bartlett, and Orawee Chinthakanan. 2023. "Rate of Postoperative Urinary Retention after Anterior Compartment Prolapse Surgery: A Randomized Controlled Trial Comparing Early versus Conventional Transurethral Catheter Removal" Journal of Clinical Medicine 12, no. 10: 3436. https://doi.org/10.3390/jcm12103436
APA StyleChansriniyom, N., Saraluck, A., Kijmanawat, A., Wattanayingcharoenchai, R., Aimjirakul, K., Manonai Bartlett, J., & Chinthakanan, O. (2023). Rate of Postoperative Urinary Retention after Anterior Compartment Prolapse Surgery: A Randomized Controlled Trial Comparing Early versus Conventional Transurethral Catheter Removal. Journal of Clinical Medicine, 12(10), 3436. https://doi.org/10.3390/jcm12103436





