Metabolism of Acetaminophen by Enteric Epithelial Cells Mitigates Hepatocellular Toxicity In Vitro
Abstract
:1. Introduction
2. Materials and Methods
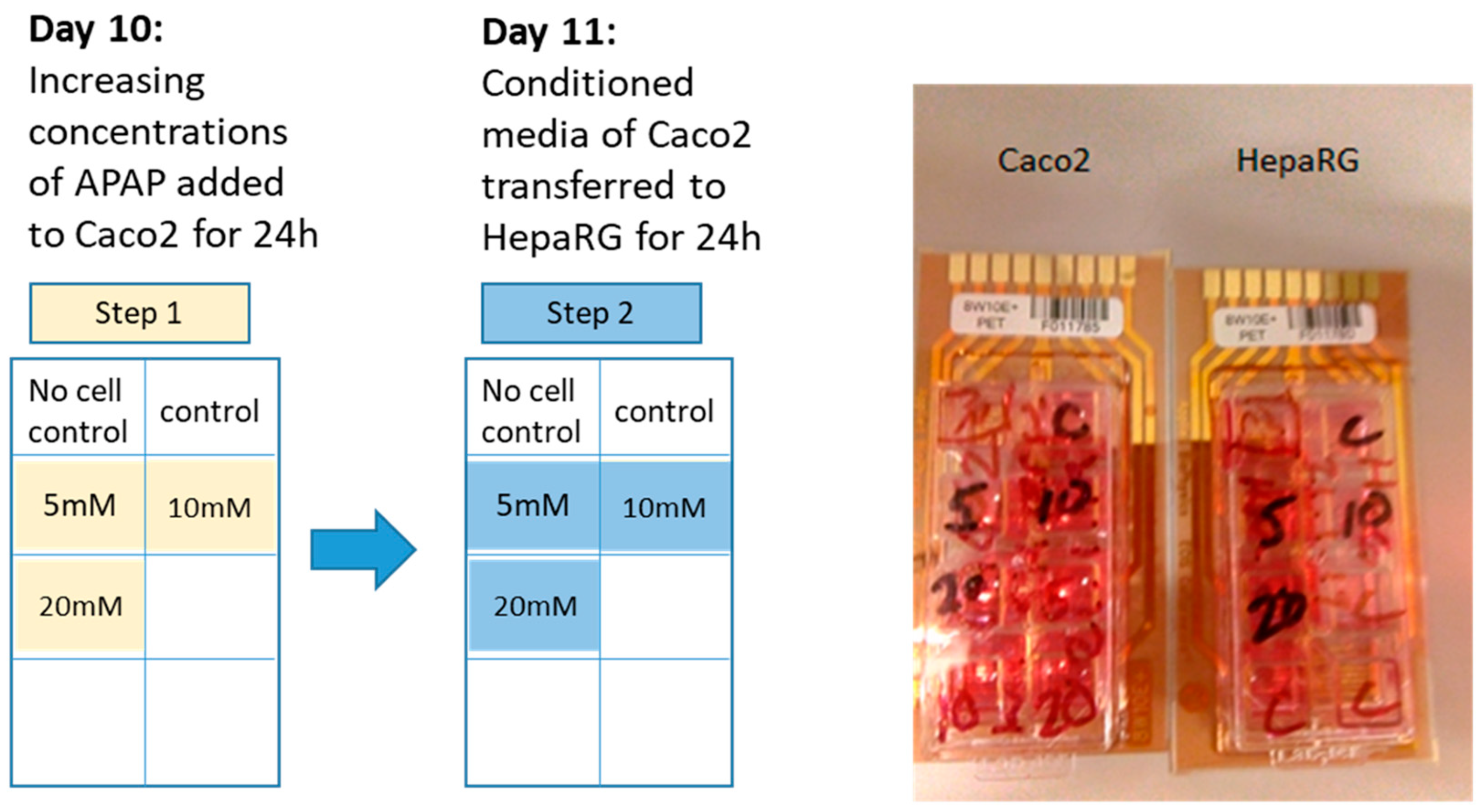
2.1. Cell Culture
2.2. Drugs
2.3. PrestoBlue® Live-Cell Viability and CellTiter-Glo® ATP-Depletion Endpoint Hepatocellular Toxicity Assays
2.4. Electric Cell-Substrate Impedance Sensing® (ECIS®) Impedance-Based Assay
2.5. APAP and Metabolite Analysis by Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS)
Sample Preparation and Analysis of APAP and Its Metabolites by LC-MS/MS
2.6. Statistical Analysis of Data
2.6.1. Cell Viability Data Analysis
2.6.2. Impedance Data Analysis
2.6.3. LC-MS/MS Data Analysis
3. Results
3.1. Cell Viability
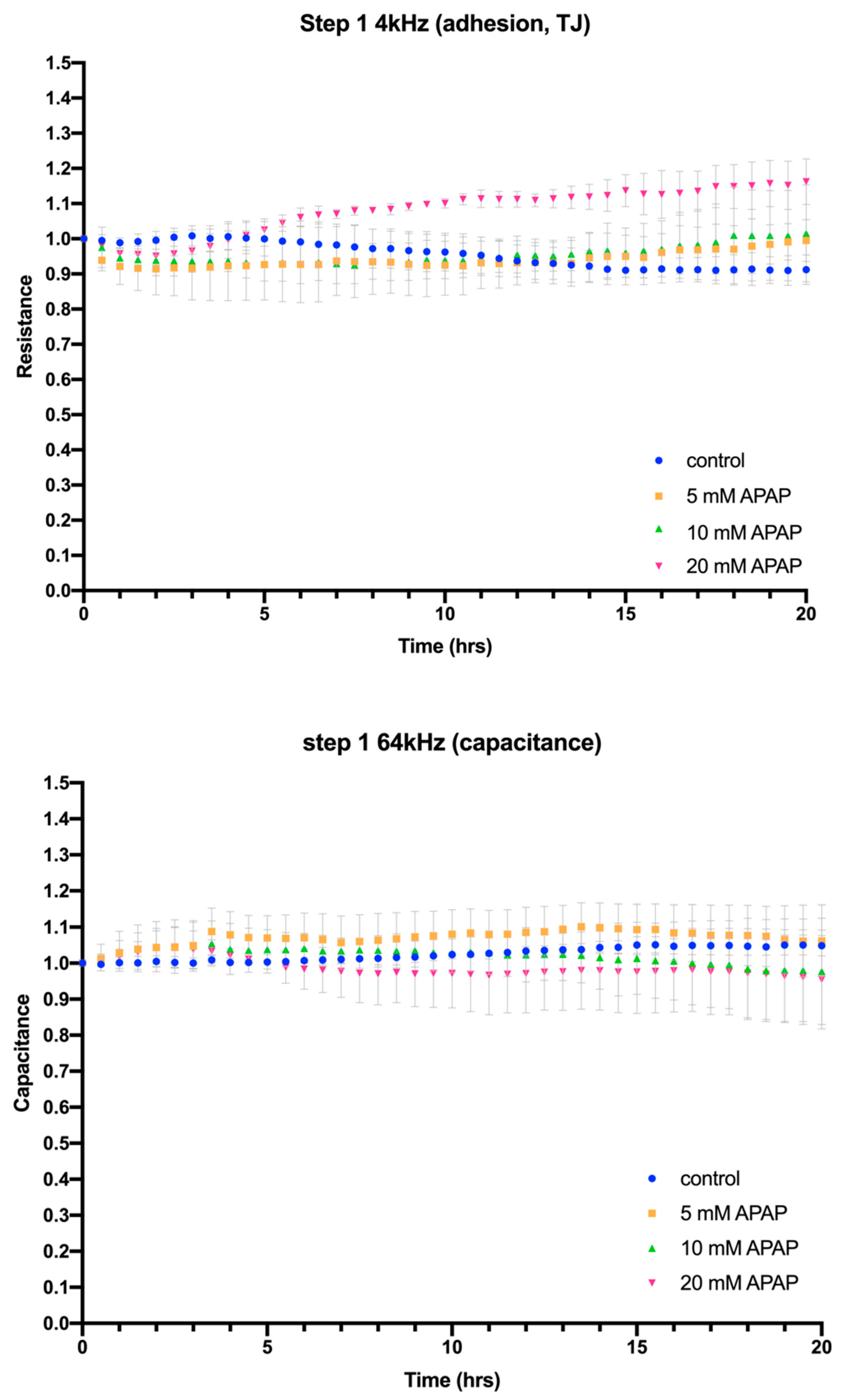
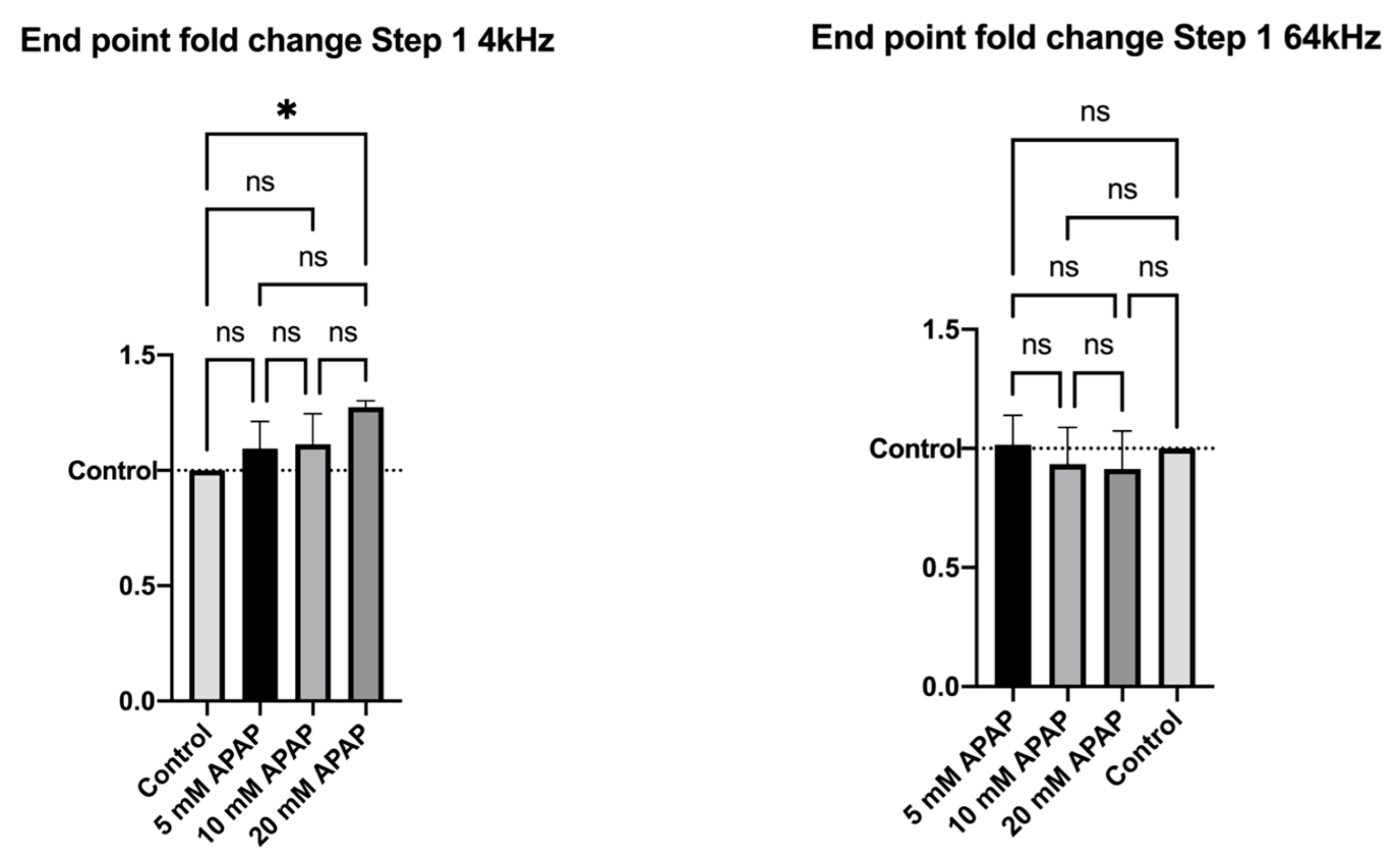
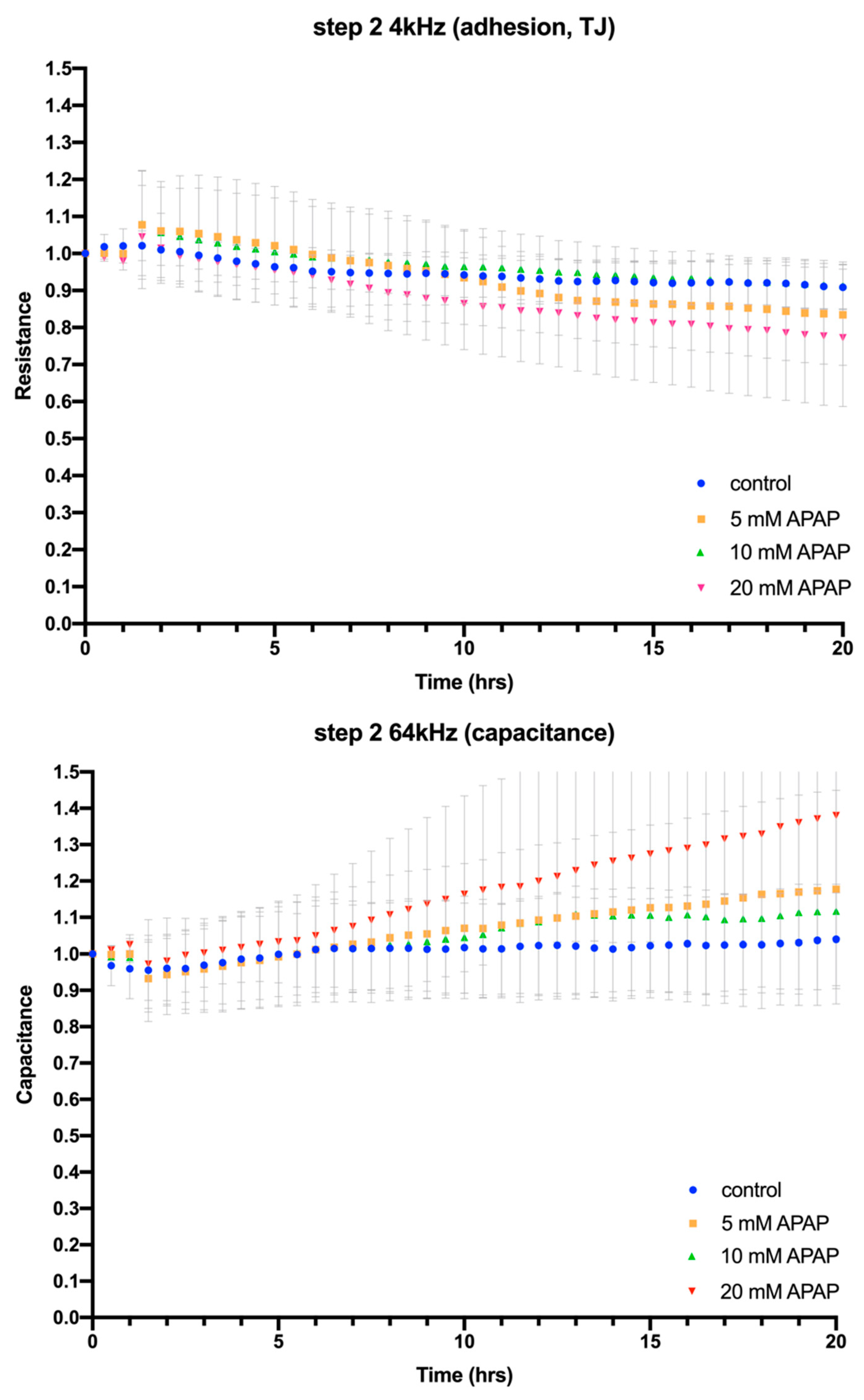
3.2. Cellular Impedance Assay of Cell Adhesion, Cell–Cell Tight Junctions and Membrane Integrity
3.3. LC-MS/MS Analysis of APAP Metabolism during Caco-2 Cell Culture
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martín-Mateos, R.; Albillos, A. The Role of the Gut-Liver Axis in Metabolic Dysfunction-Associated Fatty Liver Disease. Front. Immunol. 2021, 12, 660179. [Google Scholar] [CrossRef]
- Konturek, P.C.; Harsch, I.A.; Konturek, K.; Schink, M.; Konturek, T.; Neurath, M.F.; Zopf, Y. Gut−Liver Axis: How Do Gut Bacteria Influence the Liver? Med. Sci. 2018, 6, 79. [Google Scholar] [CrossRef] [Green Version]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [Green Version]
- Turner, J. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, A.; Nelson, W.J. Adherens and Tight Junctions: Structure, Function and Connections to the Actin Cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2001, 46, 27–43. [Google Scholar] [CrossRef]
- Kulo, A.; Peeters, M.Y.; Allegaert, K.; Smits, A.; de Hoon, J.; Verbesselt, R.; Lewi, L.; van de Velde, M.; Knibbe, C.A. Pharmacokinetics of paracetamol and its metabolites in women at delivery and post-partum. Br. J. Clin. Pharmacol. 2013, 75, 850–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oude Elferink, R.P.; Bakker, C.T.; Jansen, P.L. Glutathione-conjugate transport by human colon adenocarcinoma cells (Caco-2 cells). Biochem. J. 1993, 290, 759–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prot, J.M.; Maciel, L.; Bricks, T.; Merlier, F.; Cotton, J.; Paullier, P.; Bois, F.Y.; Leclerc, E. First pass intestinal and liver metabolism of paracetamol in a microfluidic platform coupled with a mathematical modelling as a means of evaluating ADME processes in humans. Biotechnol. Bioeng. 2014, 111, 2027–2040. [Google Scholar] [CrossRef] [PubMed]
- Thelen, K.; Dressman, J.B. Cytochrome P450-mediated metabolism in the human gut wall. J. Pharm. Pharmacol. 2009, 61, 541–558. [Google Scholar] [CrossRef]
- Morgan, K.; Martucci, N.; Kozlowska, A.; Gamal, W.; Brzeszczyński, F.; Treskes, P.; Samuel, K.; Hayes, P.; Nelson, L.; Bagnaninchi, P.; et al. Chlorpromazine toxicity is associated with disruption of cell membrane integrity and initiation of a pro-inflammatory response in the HepaRG hepatic cell line. Biomed. Pharmacother. 2019, 111, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.J.; Morgan, K.; Treskes, P.; Samuel, K.; Henderson, C.J.; LeBled, C.; Homer, N.; Grant, M.H.; Hayes, P.C.; Plevris, J.N. Human hepatic HepaRG cells maintain an organotypic phenotype with high intrinsic CYP450 activity/metabolism and significantly outperform standard HepG2/C3A cells for pharmaceutical and therapeutic applications. Basic. Clin. Pharmacol. Toxicol. 2017, 120, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerec, V.; Glaise, D.; Garnier, D.; Morosan, S.; Turlin, B.; Drenou, B.; Gripon, P.; Kremsdorf, D.; Guguen-Guillouzo, C.; Corlu, A. Transdifferentiation of hepatocyte-like cells from the human hepatoma HepaRG cell line through bipotent progenitor. Hepatology 2007, 45, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Gamal, W.; Treskes, P.; Samuel, K.; Sullivan, G.J.; Siller, R.; Srsen, V.; Morgan, K.; Bryans, A.; Kozlowska, A.; Koulovasilopoulos, A.; et al. Low-dose acetaminophen induces early disruption of cell-cell tight junctions in human hepatic cells and mouse liver. Sci. Rep. 2017, 7, 37541. [Google Scholar] [CrossRef] [Green Version]
- Bagnall, W.E.; Kelleher, J.; Walker, B.E.; Losowsky, M.S. The gastrointestinal absorption of paracetamol in the rat. J. Pharm. Pharmacol. 1979, 31, 157–160. [Google Scholar] [CrossRef]
- Schäfer, C.; Schröder, K.R.; Höglinger, O.; Tollabimazraehno, S.; Lornejad-Schäfer, M.R. Acetaminophen Changes Intestinal Epithelial Cell Membrane Properties, Subsequently Affecting Absorption Processes. Cell Physiol. Biochem. 2013, 32, 431–447. [Google Scholar] [CrossRef]
- Marin, T.M.; de Carvalho Indolfo, N.; Rocco, S.A.; Basei, F.L.; de Carvalho, M.; de Almeida Gonçalves, K.; Pagani, E. Acetaminophen absorption and metabolism in an intestine/liver microphysiological system. Chem. Biol. Interact. 2019, 299, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Lornejad-Schäfer, M.R.; Schäfer, C.; Riethmueller, C.; Schroder, K.R. Reduced bioavaibility of acetaminophen and of co-administerd substances across an intestinal barrier model through different mechanisms. Acta Physiol. 2012, 204, 134. [Google Scholar]
- Fedi, A.; Vitale, C.; Ponschin, G.; Ayehunie, S.; Fato, M.; Scaglione, S. In vitro models replicating the human intestinal epithelium for absorption and metabolism studies: A systematic review. J. Control Release 2021, 335, 247–268. [Google Scholar] [CrossRef]
- Rai, M.; Kon, K. (Eds.) Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases; Academic Press: London, UK; Elsevier: London, UK, 2015. [Google Scholar]
- Osakwe, O.; Rizvi, S.A. Social Aspects of Drug Discovery, Development and Commercialization; Academic Press: London, UK; Elsevier: London, UK, 2016. [Google Scholar]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Morgan, K.; Gamal, W.; Samuel, K.; Morley, S.D.; Hayes, P.C.; Bagnaninchi, P.; Plevris, J.N. Application of impedance-based techniques in hepatology research. J. Clin. Med. 2020, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Alexander, C.; Sadiku, M. Fundamentals of Electric Circuits; McGraw-Hill: Boston, MA, USA, 2020. [Google Scholar]
- Herman, T.F.; Santos, C. First Pass Effect. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551679/ (accessed on 6 April 2023).
- Feldmüller, M.; Höchel, J. Metabolism in the Gastrointestinal Tract: Relevance for Drug Development. Drug Res. 2021, 71, S8–S10. [Google Scholar] [CrossRef]
- Dichamp, J.; Cellière, G.; Ghallab, A.; Hassan, R.; Boissier, N.; Hofmann, U.; Reinders, J.; Sezgin, S.; Zühlke, S.; Hengstler, J.G.; et al. In vitro to in vivo acetaminophen hepatotoxicity extrapolation using classical schemes, pharmacodynamic models and a multiscale spatial-temporal liver twin. Front. Bioeng. Biotechnol. 2023, 11, 1049564. [Google Scholar] [CrossRef]
- Filippi, C.; Keatch, S.A.; Rangar, D.; Nelson, L.J.; Hayes, P.C.; Plevris, J.N. Improvement of C3A cell metabolism for usage in bioartificial liver support systems. J. Hepatol. 2004, 41, 599–605. [Google Scholar] [CrossRef]
- Nelson, L.J.; Navarro, M.; Treskes, P.; Samuel, K.; Tura-Ceide, O.; Morley, S.D.; Hayes, P.C.; Plevris, J.N. Acetaminophen cytotoxicity is ameliorated in a human liver organotypic co-culture model. Sci. Rep. 2015, 5, 17455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhtar, I.; Anwar, H.; Mirza, O.A.; Al, Q.; Ijaz, M.U.; Hume, M.; Prabhala, B.K.; Iftikhar, A.; Hussain, G.; Sohail, M.U.; et al. Sulpiride Serves, a Substrate for the Gut Microbiome. Dose-Response 2021, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, I.; Anwar, H.; Hussain, G.; Rasul, A.; Naqvi, S.A.; Faisal, M.N.; Mustafa, I.; Malik, S.; Shaukat, A.; Mirza, O.A.; et al. Detection of paracetamol as substrate of the gut microbiome. Pak. J. Pharm. Sci. 2019, 32, 751–757. [Google Scholar]
- Uzoigwe, C.E. Rapid Response to: Where are we now with paracetamol. BMJ 2015, 351, h3705. [Google Scholar]
- Mallama, M.; Valencia, A.; Rijs, K.; Rietdijk, W.J.R.; Klimek, M.; Calvache, J.A. A systematic review and trial sequential analysis of intravenous vs. oral peri-operative paracetamol. Anaesthesia 2021, 76, 270–276. [Google Scholar] [CrossRef]
- Procter, N.J.; Lamacraft, G.; Joubert, G. Intravenous paracetamol—Waste not, want not: A retrospective audit on the appropriate use of intravenous paracetamol at universitas academic hospital complex—Bloemfontein. South. Afr. J. Anaesth. Analg. 2018, 24, 22–28. [Google Scholar] [CrossRef] [Green Version]


| Total APAP ng/Sample | Remaining APAP ng/Sample | Remaining APAP Percent/Sample | Remaining APAP mM | |
|---|---|---|---|---|
| 5 mM | 116.11 | 19.36 | 16.67% | 0.834 mM |
| 10 mM | 233.87 | 36.56 | 15.63% | 1.56 mM |
| 20 mM | 378.00 | 87.72 | 23.21% | 4.64 mM |
| APAP-Cys ng/mL | APAP-Sul ng/mL | APAP-Glu ng/mL | |
|---|---|---|---|
| 5 mM | 129.9 | 1021 | 222 |
| 10 mM | 220.95 | 1693 | 505.5 |
| 20 mM | 480 | 926 | 732 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, K.; Morley, S.D.; Raja, A.K.; Vandeputte, M.; Samuel, K.; Waterfall, M.; Homer, N.Z.M.; Hayes, P.C.; Fallowfield, J.A.; Plevris, J.N. Metabolism of Acetaminophen by Enteric Epithelial Cells Mitigates Hepatocellular Toxicity In Vitro. J. Clin. Med. 2023, 12, 3995. https://doi.org/10.3390/jcm12123995
Morgan K, Morley SD, Raja AK, Vandeputte M, Samuel K, Waterfall M, Homer NZM, Hayes PC, Fallowfield JA, Plevris JN. Metabolism of Acetaminophen by Enteric Epithelial Cells Mitigates Hepatocellular Toxicity In Vitro. Journal of Clinical Medicine. 2023; 12(12):3995. https://doi.org/10.3390/jcm12123995
Chicago/Turabian StyleMorgan, Katie, Steven D. Morley, Arslan K. Raja, Martin Vandeputte, Kay Samuel, Martin Waterfall, Natalie Z. M. Homer, Peter C. Hayes, Jonathan A. Fallowfield, and John N. Plevris. 2023. "Metabolism of Acetaminophen by Enteric Epithelial Cells Mitigates Hepatocellular Toxicity In Vitro" Journal of Clinical Medicine 12, no. 12: 3995. https://doi.org/10.3390/jcm12123995
APA StyleMorgan, K., Morley, S. D., Raja, A. K., Vandeputte, M., Samuel, K., Waterfall, M., Homer, N. Z. M., Hayes, P. C., Fallowfield, J. A., & Plevris, J. N. (2023). Metabolism of Acetaminophen by Enteric Epithelial Cells Mitigates Hepatocellular Toxicity In Vitro. Journal of Clinical Medicine, 12(12), 3995. https://doi.org/10.3390/jcm12123995









