Association between Glaucoma Progression in Macular Ganglion Cell Complex and Disc Hemorrhage: Differences between Superior and Inferior Hemiretinas
Abstract
1. Introduction
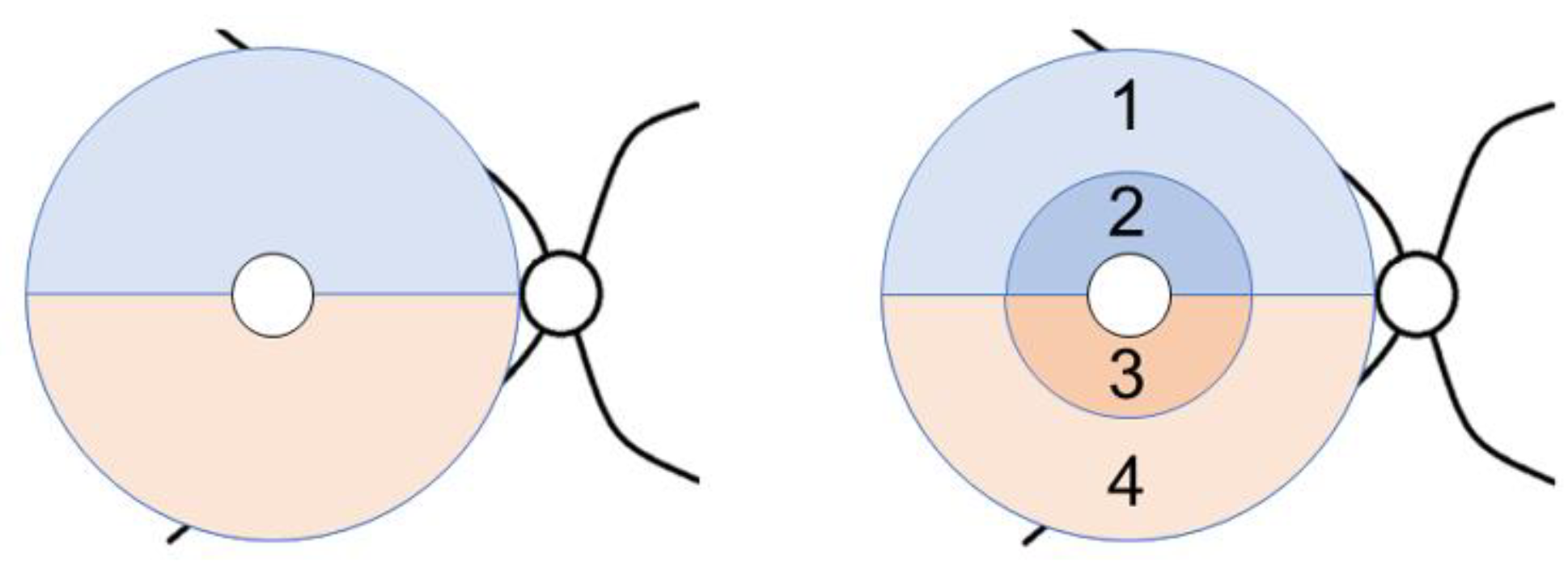
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. GCC Thickness Changes
3.3. TD Changes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The right to sight: An analysis for the global burden of disease study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Hart, W.M.; Becker, B. The onset and evolution of glaucomatous visual field defects. Ophthalmology 1982, 89, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Asman, P.; Heijl, A. Glaucoma Hemifield Test. Automated visual field evaluation. Arch. Ophthalmol. 1992, 110, 812–819. [Google Scholar] [CrossRef]
- Hood, D.C.; Raza, A.S.; de Moraes, C.G.; Liebmann, J.M.; Ritch, R. Glaucomatous damage of the macula. Prog. Retin. Eye Res. 2013, 32, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ernest, P.J.; Schouten, J.S.; Beckers, H.J.; Hendrikse, F.; Prins, M.H.; Webers, C.A. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology 2013, 120, 512–519. [Google Scholar] [CrossRef]
- Drance, S.; Anderson, D.R.; Schulzer, M.; Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am. J. Ophthalmol. 2001, 131, 699–708. [Google Scholar] [CrossRef]
- Bengtsson, B.; Leske, M.C.; Yang, Z.; Heijl, A.; EMGT Group. Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology 2008, 115, 2044–2048. [Google Scholar] [CrossRef]
- Nitta, K.; Sugiyama, K.; Higashide, T.; Ohkubo, S.; Tanahashi, T.; Kitazawa, Y. Does the enlargement of retinal nerve fiber layer defects relate to disc hemorrhage or progressive visual field loss in normal-tension glaucoma? J. Glaucoma 2011, 20, 189–195. [Google Scholar] [CrossRef]
- Kernstock, C.; Dietzsch, J.; Januschowski, K.; Schiefer, U.; Fischer, M.D. Optical coherence tomography shows progressive local nerve fiber loss after disc hemorrhages in glaucoma patients. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 583–587. [Google Scholar] [CrossRef]
- Suh, M.H.; Park, K.H.; Kim, H.; Kim, T.-W.; Kim, S.H.; Kim, S.-Y.; Kim, D.M. Glaucoma progression after the first-detected optic disc hemorrhage by optical coherence tomography. J. Glaucoma 2012, 21, 358–366. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, Y.Y.; Kim, H.K.; Sohn, Y.H. Changes in retinal nerve fiber layer thickness after optic disc hemorrhage in glaucomatous eyes. J. Glaucoma 2014, 23, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Hsia, Y.; Su, C.C.; Wang, T.H.; Huang, J.Y. Clinical characteristics of glaucoma patients with disc hemorrhage in different locations. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Zangwill, L.M.; Saunders, L.J.; Yarmohammadi, A.; Manalastas, P.I.C.; Suh, M.H.; Girkin, C.A.; Liebmann, J.M.; Weinreb, R.N. Rates of Local Retinal Nerve Fiber Layer Thinning before and after Disc Hemorrhage in Glaucoma. Ophthalmology 2017, 124, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Margeta, M.A.; Ratanawongphaibul, K.; Tsikata, E.; Zemplenyi, M.; Ondeck, C.L.; Kim, J.; Coleman, A.L.; Yu, F.; de Boer, J.F.; Chen, T.C. Disc Hemorrhages Are Associated with Localized Three-Dimensional Neuroretinal Rim Thickness Progression in Open-Angle Glaucoma. Am. J. Ophthalmol. 2022, 234, 188–198. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, Y.K.; Park, K.H.; Jeoung, J.W. Evaluation of Ganglion Cell-Inner Plexiform Layer Thinning in Eyes with Optic Disc Hemorrhage: A Trend-Based Progression Analysis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6449–6456. [Google Scholar] [CrossRef]
- Liu, X.; Lau, A.; Hou, H.; Moghimi, S.; Proudfoot, J.A.; Chan, E.; Do, J.; Camp, A.; Welsbie, D.; de Moraes, C.G.; et al. Progressive Thinning of Retinal Nerve Fiber Layer and Ganglion Cell-Inner Plexiform Layer in Glaucoma Eyes with Disc Hemorrhage. Ophthalmol. Glaucoma 2021, 4, 541–549. [Google Scholar] [CrossRef]
- Rao, H.L.; Sreenivasaiah, S.; Dixit, S.; Riyazuddin, M.; Dasari, S.; Venugopal, J.P.; Pradhan, Z.S.; Puttaiah, N.K.; Devi, S.; Mansouri, K.; et al. Choroidal Microvascular Dropout in Primary Open-angle Glaucoma Eyes with Disc Hemorrhage. J. Glaucoma 2019, 28, 181–187. [Google Scholar] [CrossRef]
- Healey, P.R.; Mitchell, P.; Smith, W.; Wang, J.J. Optic disc hemorrhages in a population with and without signs of glaucoma. Ophthalmology 1998, 105, 216–223. [Google Scholar] [CrossRef]
- Furlanetto, R.; De Moraes, C.G.; Teng, C.C.; Liebmann, J.M.; Greenfield, D.S.; Gardiner, S.K.; Ritch, R.; Krupin, T.; Low-Pressure Glaucoma Treatment Study Group. Risk factors for optic disc hemorrhage in the low-pressure glaucoma treatment study. Am. J. Ophthalmol. 2014, 157, 945–952. [Google Scholar] [CrossRef]
- Kim, Y.-D.; Han, S.B.; Park, K.H.; Kim, S.H.; Seong, M.; Kim, T.-W.; Kim, D.M. Risk factors associated with optic disc haemorrhage in patients with normal tension glaucoma. Eye 2010, 24, 567–572. [Google Scholar] [CrossRef]
- Park, H.Y.; Park, S.H.; Oh, Y.S.; Park, C.K. Nail bed hemorrhage: A clinical marker of optic disc hemorrhage in patients with glaucoma. Arch. Ophthalmol. 2011, 129, 1299–1304. [Google Scholar] [CrossRef]
- Sugiyama, K.; Tomita, G.; Kitazawa, Y.; Onda, E.; Shinohara, H.; Park, K.H. The associations of optic disc hemorrhage with retinal nerve fiber layer defect and peripapillary atrophy in normal-tension glaucoma. Ophthalmology 1997, 104, 1926–1933. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, K.H. Lamina cribrosa defects in eyes with glaucomatous disc haemorrhage. Acta Ophthalmol. 2016, 94, e468–e473. [Google Scholar] [CrossRef] [PubMed]
- Drance, S.M. Disc hemorrhages in the glaucomas. Surv. Ophthalmol. 1989, 33, 331–337. [Google Scholar] [CrossRef]
- Razeghinejad, M.R.; Nowroozzadeh, M.H. Optic disk hemorrhage in health and disease. Surv. Ophthalmol. 2017, 62, 784–802. [Google Scholar] [CrossRef]
- Choi, J.A.; Park, H.Y.; Jung, K.I.; Hong, K.H.; Park, C.K. Difference in the properties of retinal nerve fiber layer defect between superior and inferior visual field loss in glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6982–6990. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.C.; Slobodnick, A.; Raza, A.S.; de Moraes, C.G.; Teng, C.C.; Ritch, R. Early glaucoma involves both deep local, and shallow widespread, retinal nerve fiber damage of the macular region. Investig. Ophthalmol. Vis. Sci. 2014, 55, 632–649. [Google Scholar] [CrossRef]
- Higashide, T.; Ohkubo, S.; Udagawa, S.; Sugiyama, K.; Tanihara, H.; Araie, M.; Tomita, G.; Matsumoto, C.; Fukuchi, T.; Tomidokoro, A.; et al. Spatial and Temporal Relationship between Structural Progression and Disc Hemorrhage in Glaucoma in a 3-Year Prospective Study. Ophthalmol. Glaucoma 2020, S2589-4196(20)30220-9, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.W.; Lin, C.; Leung, C.K. Integrating Macular Ganglion Cell Inner Plexiform Layer and Parapapillary Retinal Nerve Fiber Layer Measurements to Detect Glaucoma Progression. Ophthalmology 2018, 125, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Rasker, M.T.; van den Enden, A.; Bakker, D.; Hoyng, P.F. Deterioration of visual fields in patients with glaucoma with and without optic disc hemorrhages. Arch. Ophthalmol. 1997, 115, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yamamoto, T.; Sugiyama, K.; Kitazawa, Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am. J. Ophthalmol. 2000, 129, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, K.H. The relationship between recurrent optic disc hemorrhage and glaucoma progression. Ophthalmology 2006, 113, 598–602. [Google Scholar] [CrossRef]
- de Beaufort, H.C.; De Moraes, C.G.V.; Teng, C.C.; Prata, T.S.; Tello, C.; Ritch, R.; Liebmann, J.M. Recurrent disc hemorrhage does not increase the rate of visual field progression. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Laemmer, R.; Nguyen, T.K.; Horn, F.K.; Mardin, C.Y. Morphologic and functional glaucomatous change after occurrence of single or recurrent optic disc hemorrhages. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1683–1684. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, E.K.; Park, C.K. Clinical Significance of the Location of Recurrent Optic Disc Hemorrhage in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7524–7534. [Google Scholar] [CrossRef]
- Seol, B.R.; Jeoung, J.W.; Park, K.H. Ocular and systemic risk factors associated with recurrent disc hemorrhage in primary open-angle glaucoma. PLoS ONE 2019, 14, e0222166. [Google Scholar] [CrossRef]
- An, D.; House, P.; Barry, C.; Turpin, A.; McKendrick, A.M.; Chauhan, B.C.; Manners, S.; Graham, S.; Yu, D.-Y.; Morgan, W.H. Recurrent Optic Disc Hemorrhage and Its Association with Visual Field Deterioration in Glaucoma. Ophthalmol. Glaucoma 2020, 3, 443–452. [Google Scholar] [CrossRef]
- Kitazawa, Y.; Shirato, S.; Yamamoto, T. Optic disc hemorrhage in low-tension glaucoma. Ophthalmology 1986, 93, 853–857. [Google Scholar] [CrossRef]
- Zeiter, J.H.; Shin, D.H. Diabetes in primary open-angle glaucoma patients with inferior visual field defects. Graefes Arch. Clin. Exp. Ophthalmol. 1994, 232, 205–210. [Google Scholar] [CrossRef]
- Suzuki, J.; Tomidokoro, A.; Araie, M.; Tomita, G.; Yamagami, J.; Okubo, T.; Masumoto, T. Visual field damage in normal-tension glaucoma patients with or without ischemic changes in cerebral magnetic resonance imaging. Jpn. J. Ophthalmol. 2004, 48, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Jansonius, N.M.; Schiefer, J.; Nevalainen, J.; Paetzold, J.; Schiefer, U. A mathematical model for describing the retinal nerve fiber bundle trajectories in the human eye: Average course, variability, and influence of refraction, optic disc size and optic disc position. Exp. Eye Res. 2012, 105, 70–78. [Google Scholar] [CrossRef]
- Nakatani, Y.; Higashide, T.; Ohkubo, S.; Sugiyama, K. Influences of the inner retinal sublayers and analytical areas in macular scans by spectral-domain OCT on the diagnostic ability of early glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7479–7485. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Yang, H.; Lee, T.H.; Lee, K.H. Diagnostic Value of Ganglion Cell-Inner Plexiform Layer Thickness in Glaucoma with Superior or Inferior Visual Hemifield Defects. J. Glaucoma 2016, 25, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.S.; Artes, P.H.; Andreou, P.; Leblanc, R.P.; Chauhan, B.C.; Nicolela, M.T. Factors associated with optic disc hemorrhages in glaucoma. Ophthalmology 2004, 111, 1653–1657. [Google Scholar] [CrossRef]

| No DH (n = 28) | DH (n = 32) | p-Value, between Groups | |
|---|---|---|---|
| Age (year) | 67.9 ± 8.1 | 68.1 ± 10.3 | 0.92 |
| Gender (Male/Female) | 16/12 | 17/15 | 0.76 * |
| Right/left eye | 16/12 | 16/16 | 0.58 * |
| Number of visits with DH in superior/inferior half of the disc † | 0/0 | 0.7 ± 1.3/1.5 ± 1.8 | NA |
| p-Value, between hemiretinas | NA | 0.047 ‡ | |
| Spherical equivalent (D) | −2.8 ± 2.8 | −1.9 ± 3.0 | 0.25 |
| Axial length (mm) | 24.6 ± 1.2 | 24.1 ± 1.1 | 0.11 |
| IOP before treatment (mmHg) | 14.7 ± 2.8 | 14.5 ± 2.5 | 0.80 |
| IOP at the beginning of the study period (mmHg) | 12.1 ± 2.4 | 12.4 ± 1.8 | 0.51 |
| Mean IOP § (mmHg) | 11.7 ± 1.9 | 11.7 ± 1.5 | 0.85 |
| Mean IOP reduction § (%) | 18.6 ± 13.9 | 18.3 ± 11.0 | 0.91 |
| SD of IOP § (mmHg) | 1.35 ± 0.35 | 1.35 ± 0.30 | 0.93 |
| Medication score at the beginning of the study period | 0.82 ± 0.77 | 1.16 ± 0.88 | 0.13 † |
| Medication score at the end of the study period | 0.82 ± 0.67 | 1.03 ± 0.93 | 0.49 † |
| Baseline MD (dB) | −3.6 ± 4.1 | −3.5 ± 3.4 | 0.82 † |
| Number of OCT measurements † | 5.4 ± 1.1 | 6.9 ± 2.2 | 0.002 † || |
| Number of VF measurements † | 7.7 ± 1.2 | 9.0 ± 3.0 | 0.13 † |
| DH | No DH (n = 28) Coef *, p-Value | DH (n = 32) Coef *, p-Value | †p-Value, between Groups | ‡p-Value, Effect of Number of Visits with DH, between Groups | |
|---|---|---|---|---|---|
| Both hemi-retinas combined | + | −1.04 ± 0.19, p < 0.001 (n = 35) | 0.007 § | <0.001 § | |
| − | −0.48 ± 0.11, p < 0.001 (n = 56) | −0.36 ± 0.16, p = 0.028 (n = 29) | 0.400 | NA | |
| Superior hemiretina | + | −1.11 ± 0.32, p = 0.001 (n = 13) | 0.014 § | 0.017 § | |
| − | −0.51 ± 0.13, p < 0.001 (n = 28) | −0.52 ± 0.22, p = 0.016 (n = 19) | 0.951 | NA | |
| Inferior hemiretina | + | −0.95 ± 0.23, p < 0.001 (n = 22) | 0.097 | 0.001 § | |
| − | −0.45 ± 0.18, p = 0.013 (n = 28) | −0.02 ± 0.18, p = 0.915 (n = 10) | 0.143 | NA | |
| Superior outer sector | + | −1.26 ± 0.36, p = 0.001 (n = 13) | 0.018 § | 0.021 § | |
| − | −0.62 ± 0.14, p < 0.001 (n = 28) | −0.65 ± 0.25, p = 0.009 (n = 19) | 0.919 | NA | |
| Superior inner sector | + | −1.16 ± 0.32, p < 0.001 (n = 13) | 0.051 | 0.125 | |
| − | −0.64 ± 0.14, p < 0.001 (n = 28) | −0.54 ± 0.18, p = 0.003 (n = 19) | 0.615 | NA | |
| Inferior outer sector | + | −0.67 ± 0.22, p = 0.003 (n = 22) | 0.668 | 0.041 § | |
| − | −0.51 ± 0.22, p = 0.018 (n = 28) | 0.10 ± 0.19, p = 0.616 (n = 10) | 0.073 | NA | |
| Inferior inner sector | + | −1.76 ± 0.37, p < 0.001 (n = 22) | 0.001 § | <0.001 § | |
| − | −0.36 ± 0.15, p = 0.019 (n = 28) | 0.31 ± 0.29, p = 0.291 (n = 10) | 0.650 | NA |
| DH | Coef *, p-Value | †p-Value, DH+ vs. DH− Hemi-Retinas | ‡p-Value, Effect of Number of Visits with DH | |
|---|---|---|---|---|
| Both hemiretinas combined | + (n = 35) | −1.04 ± 0.19, p < 0.001 | 0.004 § | <0.001 § |
| − (n = 29) | −0.36 ± 0.16, p = 0.028 | |||
| Superior hemiretina | + (n = 13) | −1.11 ± 0.32, p = 0.001 | 0.035 | 0.063 |
| − (n = 19) | −0.52 ± 0.22, p = 0.016 | |||
| Inferior hemiretina | + (n = 22) | −0.95 ± 0.23, p < 0.001 | 0.015 | <0.001 § |
| − (n = 10) | −0.02 ± 0.18, p = 0.915 | |||
| p-value, between hemiretinas (DH+) | 0.383 | |||
| p-value, between hemiretinas (DH−) | 0.133 | |||
| Superior outer sector | + (n = 13) | −1.26 ± 0.36, p = 0.001 | 0.075 | 0.088 |
| − (n = 19) | −0.65 ± 0.25, p = 0.009 | |||
| Superior inner sector | + (n = 13) | −1.16 ± 0.32, p < 0.001 | 0.050 | 0.137 |
| − (n = 19) | −0.54 ± 0.18, p = 0.003 | |||
| Inferior outer sector | + (n = 22) | −0.67 ± 0.22, p = 0.003 | 0.032 | <0.001 § |
| − (n = 10) | 0.10 ± 0.19, p = 0.616 | |||
| Inferior inner sector | + (n = 22) | −1.76 ± 0.37, p < 0.001 | 0.030 | 0.001 § |
| − (n = 10) | −0.31 ± 0.29, p = 0.291 | |||
| DH | No DH (n = 28) Coef *, p-Value | DH (n = 32) Coef *, p-Value | †p-Value, between Groups | ‡p-Value, Effect of Number of Visits with DH, between Groups | |
|---|---|---|---|---|---|
| Both hemifieldscombined | + | −0.44 ± 0.11, p < 0.001 (n = 35) | 0.005 § | 0.138 | |
| − | −0.11 ± 0.06, p = 0.06 (n = 56) | −0.27 ± 0.07, p < 0.001 (n = 29) | 0.112 | NA | |
| Superior hemifield | + | −0.51 ± 0.16, p = 0.001 (n = 22) | 0.008 § | 0.052 | |
| − | −0.07 ± 0.08, p = 0.038 (n = 28) | −0.20 ± 0.18, p = 0.258 (n = 10) | 0.527 | NA | |
| Inferior hemifield | + | −0.30 ± 0.14, p = 0.031 (n = 13) | 0.318 | 0.336 | |
| - | −0.16 ± 0.09, p = 0.061 (n = 28) | −0.29 ± 0.07, p < 0.001 (n = 19) | 0.251 | NA |
| DH | Coef *, p-Value | †p-Value, DH+ vs. DH− Hemi-Fields | ‡p-Value, Effect of Number of Visits with DH | |
|---|---|---|---|---|
| Both hemifields combined | + (n = 35) | −0.44 ± 0.11, p < 0.001 | 0.244 | 0.765 |
| − (n = 29) | −0.27 ± 0.07, p < 0.007 | |||
| Superior hemifield | + (n = 22) | −0.51 ± 0.16, p = 0.001 | 0.321 | 0.494 |
| − (n = 10) | −0.20 ± 0.18, p = 0.258 | |||
| Inferior hemifield | + (n = 13) | −0.30 ± 0.14, p = 0.031 | 0.871 | 0.098 |
| − (n = 19) | −0.29 ± 0.07, p < 0.001 | |||
| p-value, between hemifields (DH+) | 0.392 | |||
| p-value, between hemifields (DH−) | 0.577 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tachibana, G.; Higashide, T.; Nitta, K.; Sugiyama, K. Association between Glaucoma Progression in Macular Ganglion Cell Complex and Disc Hemorrhage: Differences between Superior and Inferior Hemiretinas. J. Clin. Med. 2023, 12, 3996. https://doi.org/10.3390/jcm12123996
Tachibana G, Higashide T, Nitta K, Sugiyama K. Association between Glaucoma Progression in Macular Ganglion Cell Complex and Disc Hemorrhage: Differences between Superior and Inferior Hemiretinas. Journal of Clinical Medicine. 2023; 12(12):3996. https://doi.org/10.3390/jcm12123996
Chicago/Turabian StyleTachibana, Gaku, Tomomi Higashide, Koji Nitta, and Kazuhisa Sugiyama. 2023. "Association between Glaucoma Progression in Macular Ganglion Cell Complex and Disc Hemorrhage: Differences between Superior and Inferior Hemiretinas" Journal of Clinical Medicine 12, no. 12: 3996. https://doi.org/10.3390/jcm12123996
APA StyleTachibana, G., Higashide, T., Nitta, K., & Sugiyama, K. (2023). Association between Glaucoma Progression in Macular Ganglion Cell Complex and Disc Hemorrhage: Differences between Superior and Inferior Hemiretinas. Journal of Clinical Medicine, 12(12), 3996. https://doi.org/10.3390/jcm12123996







