The Promise of Single-Cell RNA Sequencing to Redefine the Understanding of Crohn’s Disease Fibrosis Mechanisms
Abstract
:1. Introduction
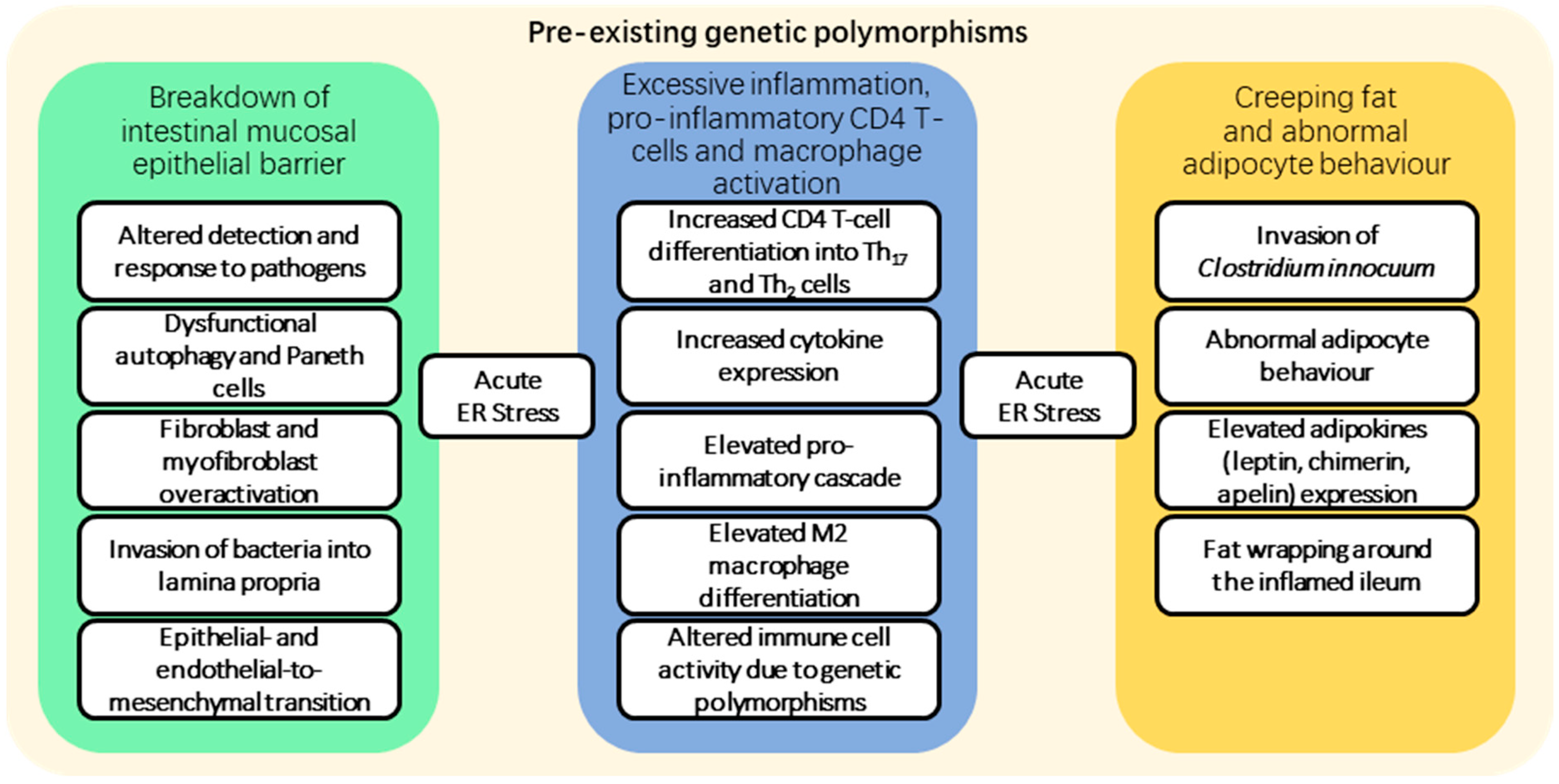
2. Fibrosis Pathogenesis
3. Current Treatment Strategies
| Intervention | Indications | Technical Success Rates | Complications | Recurrence |
|---|---|---|---|---|
| Endoscopic balloon dilatation | - Short, straight strictures <5 cm - No penetrating complications or deep ulceration - No evidence of malignancy | - >80% (the ability of the endoscope to pass stricture following dilatation) [52] | - 3% perforation risk (increases by 8% for every 1 cm increase in stricture length) [52] | - 52% require repeat dilatation and 30% require surgical intervention at 12 months [50] - 73% require repeat dilatation and 42% require surgery at 24 months [52] |
| Endoscopic stricturotomy | - Not yet widely practiced | - >90% immediate technical success rate in retrospective studies [58,59,60] | - Lower perforation rate but higher bleeding rate than balloon dilatation [53] | - Small retrospective studies report a 9–15% subsequent need for surgical intervention [59,60] |
| Endoscopic self-expanding metal stent | - Not yet widely practiced | - >90% technical success rate in small retrospective studies - with 60–80% initial symptomatic improvement [61,62] | - Safety profile similar to EBD in one randomised trial; further studies needed [54] | - 49% of patients required further intervention at 12 months in one randomised trial; further studies needed [54] |
| Surgical stricturoplasty | - Long strictures to minimise the risk of short bowel - Ileo-colonic anastomotic strictures | NA | - Peri-operative complication rate averages 13% [63] | - Low site-specific recurrence rate (2–5% at 10 years) [55] |
| Surgical resection | - Complicated disease with perforation or abscess, or concern regarding malignancy - Localised ileo-caecal disease | NA | - Dependent on multiple factors including the extent of surgery and approach required - Risk of short bowel with multiple/extensive resections | - 25% recurrence rate in a meta-analysis of six studies [64] |
4. Single-Cell Sequencing
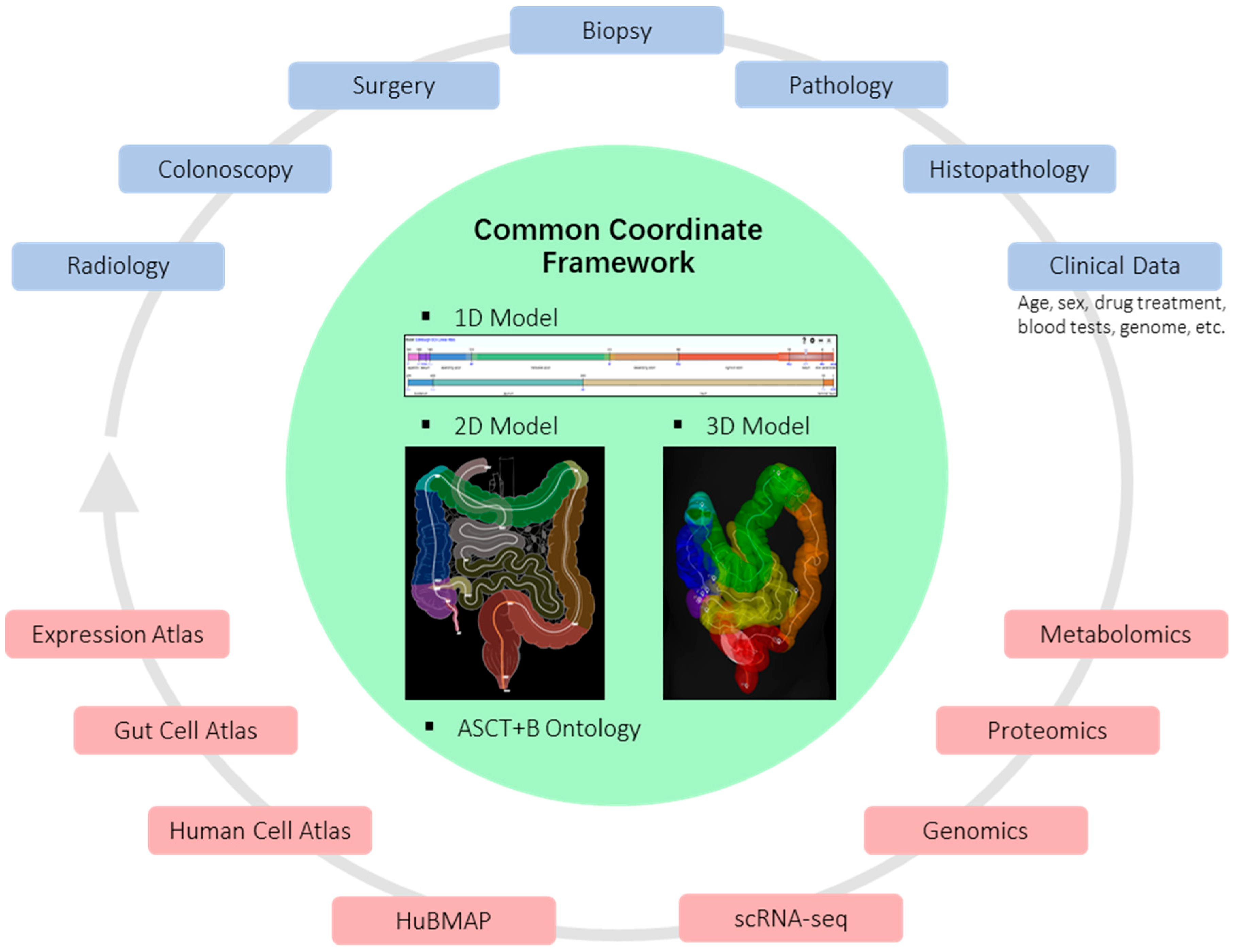
5. Spatial Analysis
6. Collaborative Approaches
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.R.; Lyons, M.; Plevris, N.; Jenkinson, P.W.; Bisset, C.; Burgess, C.; Din, S.; Fulforth, J.; Henderson, P.; Ho, G.T.; et al. IBD prevalence in Lothian, Scotland, derived by capture-recapture methodology. Gut 2019, 68, 1953–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Vasudevan, A.; Bruining, D.H.; Loftus, E.V.; Faubion, W.; Ehman, E.C.; Raffals, L. Approach to medical therapy in perianal Crohn’s disease. World J. Gastroenterol. 2021, 27, 3693–3704. [Google Scholar] [CrossRef]
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Barreiro-de Acosta, M.; Boberg, K.M.; Burisch, J.; De Vos, M.; De Vries, A.-M.; Dick, A.D.; et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J. Crohns Colitis 2016, 10, 239–254. [Google Scholar] [CrossRef]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.P.W.; Mourad, F.; Leong, R.W. Crohn’s disease associated strictures: Crohn’s disease associated strictures. J. Gastroenterol. Hepatol. 2018, 33, 998–1008. [Google Scholar] [CrossRef]
- Rieder, F.; Latella, G.; Magro, F.; Yuksel, E.S.; Higgins, P.D.R.; Di Sabatino, A.; de Bruyn, J.R.; Rimola, J.; Brito, J.; Bettenworth, D.; et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J. Crohns Colitis 2016, 10, 873–885. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, P.W.; Plevris, N.; Siakavellas, S.; Lyons, M.; Arnott, I.D.; Wilson, D.; Watson, A.J.M.; Jones, G.-R.; Lees, C.W. Temporal Trends in Surgical Resection Rates and Biologic Prescribing in Crohn’s Disease: A Population-based Cohort Study. J. Crohns Colitis 2020, 14, 1241–1247. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [Green Version]
- Boyapati, R.; Satsangi, J.; Tzer Ho, G. Pathogenesis of Crohn’s disease. Prime Rep. 2015, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Bettenworth, D.; Bokemeyer, A.; Baker, M.; Mao, R.; Parker, C.E.; Nguyen, T.; Ma, C.; Panés, J.; Rimola, J.; Fletcher, J.G.; et al. Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: A systematic review. Gut 2019, 68, 1115–1126. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Alfredsson, J.; Wick, M.J. Mechanism of fibrosis and stricture formation in Crohn’s disease. Scand. J. Immunol. 2020, 92, e12990. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [Green Version]
- Eckmann, L.; Karin, M. NOD2 and Crohn’s disease: Loss or gain of function? Immunity 2005, 22, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Henderson, P.; Stevens, C. The Role of Autophagy in Crohn’s Disease. Cells 2012, 1, 492–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hold, G.L.; Berry, S.; Saunders, K.A.; Drew, J.; Mayer, C.; Brookes, H.; Gay, N.J.; El-Omar, E.M.; Bryant, C.E. The TLR4 D299G and T399I SNPs Are Constitutively Active to Up-Regulate Expression of Trif-Dependent Genes. PLoS ONE 2014, 9, e111460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cario, E. Toll-like receptors in inflammatory bowel diseases: A decade later. Inflamm. Bowel Dis. 2010, 16, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhu, Y.; Huang, X.; Zhang, W.; Han, Z.; Liu, S. Association between TLR2 and TLR4 gene polymorphisms and the susceptibility to inflammatory bowel disease: A meta-analysis. PLoS ONE 2015, 10, e0126803. [Google Scholar] [CrossRef] [Green Version]
- Ha, C.W.Y.; Martin, A.; Sepich-Poore, G.D.; Shi, B.; Wang, Y.; Gouin, K.; Humphrey, G.; Sanders, K.; Ratnayake, Y.; Chan, K.S.L.; et al. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell 2020, 183, 666–683.e17. [Google Scholar] [CrossRef]
- Weidinger, C.; Ziegler, J.F.; Letizia, M.; Schmidt, F.; Siegmund, B. Adipokines and their role in intestinal inflammation. Front. Immunol. 2018, 9, 1974. [Google Scholar] [CrossRef] [Green Version]
- Zabel, B.A.; Kwitniewski, M.; Banas, M.; Zabieglo, K.; Murzyn, K.; Cichy, J. Chemerin regulation and role in host defense. Am. J. Clin. Exp. Immunol. 2014, 3, 1–19. [Google Scholar]
- Andreoli, M.F.; Donato, J.; Cakir, I.; Perello, M. Leptin resensitisation: A reversion of leptin-resistant states. J. Endocrinol. 2019, 241, R81–R96. [Google Scholar] [CrossRef] [Green Version]
- Kredel, L.I.; Jödicke, L.J.; Scheffold, A.; Gröne, J.; Glauben, R.; Erben, U.; Kühl, A.A.; Siegmund, B. T-cell composition in ileal and colonic creeping fat—Separating ileal from colonic Crohn’s disease. J. Crohns Colitis 2019, 13, 79–91. [Google Scholar] [CrossRef]
- Lewy, T.G.; Grabowski, J.M.; Bloom, M.E. BiP: Master regulator of the unfolded protein response and crucial factor in flavivirus biology. Yale J. Biol. Med. 2017, 90, 291–300. [Google Scholar]
- Alsereihi, R.; Schulten, H.J.; Bakhashab, S.; Saini, K.; Al-Hejin, A.M.; Hussein, D. Leveraging the role of the metastatic associated protein Anterior Gradient Homologue 2 in unfolded protein degradation: A novel therapeutic biomarker for cancer. Cancers 2019, 11, 890. [Google Scholar] [CrossRef] [Green Version]
- Maurel, M.; Obacz, J.; Avril, T.; Ding, Y.; Papadodima, O.; Treton, X.; Daniel, F.; Pilalis, E.; Hörberg, J.; Hou, W.; et al. Control of anterior GR adient 2 ( AGR 2) dimerization links endoplasmic reticulum proteostasis to inflammation. EMBO Mol. Med. 2019, 11, e10120. [Google Scholar] [CrossRef]
- Cao, S.S. Epithelial ER stress in Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2016, 22, 984–993. [Google Scholar] [CrossRef]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [Green Version]
- Neary, R.; Watson, C.J.; Baugh, J.A. Epigenetics and the overhealing wound: The role of DNA methylation in fibrosis. Fibrogenesis Tissue Repair 2015, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Shen, J.; Ran, Z. Epithelial-mesenchymal transition in Crohn’s disease. Mucosal Immunol. 2018, 11, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Rout-Pitt, N.; Farrow, N.; Parsons, D.; Donnelley, M. Epithelial mesenchymal transition (EMT): A universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir. Res. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Salton, F.; Volpe, M.C.; Confalonieri, M. Epithelial-mesenchymal transition in the pathogenesis of idiopathic pulmonary fibrosis. Med. Lith. 2019, 55, 83. [Google Scholar] [CrossRef] [Green Version]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.G.; Lee, A.; Chang, W.; Lee, M.S.; Kim, J. Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front. Immunol. 2018, 9, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Platel, V.; Faure, S.; Corre, I.; Clere, N. Endothelial-to-Mesenchymal Transition (EndoMT): Roles in Tumorigenesis, Metastatic Extravasation and Therapy Resistance. J. Oncol. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, G.; Rodriguez-Justo, M.; Higginson, A.; Bassett, P.; Windsor, A.; Cohen, R.; Halligan, S.; Taylor, S.A. Inflammation and fibrosis in Crohn’s disease: Location-matched histological correlation of small bowel ultrasound features. Abdom. Radiol. 2021, 46, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Pellegrino, R.; Romeo, M.; Palladino, G.; Cipullo, M.; Iadanza, G.; Olivieri, S.; Zagaria, G.; De Gennaro, N.; Santonastaso, A.; et al. Quality of bowel preparation in patients with inflammatory bowel disease undergoing colonoscopy: What factors to consider? World J. Gastrointest. Endosc. 2023, 15, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 2019, 13, 144–164K. [Google Scholar] [CrossRef] [Green Version]
- Shaban, N.; Hoad, C.L.; Naim, I.; Alshammari, M.; Radford, S.J.; Clarke, C.; Marciani, L.; Moran, G. Imaging in inflammatory bowel disease: Current and future perspectives. Frontline Gastroenterol. 2022, 13, e28–e34. [Google Scholar] [CrossRef]
- Bouhnik, Y.; Carbonnel, F.; Laharie, D.; Stefanescu, C.; Hébuterne, X.; Abitbol, V.; Nachury, M.; Brixi, H.; Bourreille, A.; Picon, L.; et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: A multicentre, prospective, observational cohort (CREOLE) study. Gut 2018, 67, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Colombel, J.-F.; Narula, N.; Peyrin-Biroulet, L. Management Strategies to Improve Outcomes of Patients With Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 351–361.e5. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, B.; Jin, T.; Ocansey, D.K.W.; Jiang, J.; Mao, F. Intestinal Fibrosis in Inflammatory Bowel Disease and the Prospects of Mesenchymal Stem Cell Therapy. Front. Immunol. 2022, 13, 835005. [Google Scholar] [CrossRef]
- Li, L.; Shapiro, R.L.; Joo, M.K.; Josyula, A.; Hsueh, H.T.; Gutierrez, O.B.; Halpert, G.; Akshintala, V.; Chen, H.; Curtis, S.; et al. Injectable, Drug-Eluting Nanocrystals Prevent Fibrosis and Stricture Formation In Vivo. Gastroenterology 2023, 164, 937–952.e13. [Google Scholar] [CrossRef]
- Ismail, M.S.; Charabaty, A. Management of Crohn’s stricture: Medical, endoscopic and surgical therapies. Frontline Gastroenterol. 2022, 13, 524–530. [Google Scholar] [CrossRef]
- Paine, E.; Shen, B. Endoscopic therapy in inflammatory bowel diseases (with videos). Gastrointest. Endosc. 2013, 78, 819–835. [Google Scholar] [CrossRef]
- Bettenworth, D.; Gustavsson, A.; Atreja, A.; Lopez, R.; Tysk, C.; van Assche, G.; Rieder, F. A Pooled Analysis of Efficacy, Safety, and Long-term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 133–142. [Google Scholar] [CrossRef]
- Gu, Y.B.; Zhong, J. Chinese IBD Endoscopic Club Endoscopic management of stricturing Crohn’s disease. J. Dig. Dis. 2020, 21, 351–354. [Google Scholar] [CrossRef]
- Loras, C.; Andújar, X.; Gornals, J.B.; Sanchiz, V.; Brullet, E.; Sicilia, B.; Martín-Arranz, M.D.; Naranjo, A.; Barrio, J.; Dueñas, C.; et al. Self-expandable metal stents versus endoscopic balloon dilation for the treatment of strictures in Crohn’s disease (ProtDilat study): An open-label, multicentre, randomised trial. Lancet Gastroenterol. Hepatol. 2022, 7, 332–341. [Google Scholar] [CrossRef]
- Bemelman, W.A.; Warusavitarne, J.; Sampietro, G.M.; Serclova, Z.; Zmora, O.; Luglio, G.; de Buck van Overstraeten, A.; Burke, J.P.; Buskens, C.J.; Francesco, C.; et al. ECCO-ESCP Consensus on Surgery for Crohn’s Disease. J. Crohns Colitis 2017, 12, 1–16. [Google Scholar] [CrossRef]
- Bossuyt, P.; Debeuckelaere, C.; Ferrante, M.; de Buck van Overstraeten, A.; Vanbeckevoort, D.; Billiet, T.; Wolthuis, A.; Cleynen, I.; Van Assche, G.; D’Hoore, A.; et al. Risk Stratification for Surgery in Stricturing Ileal Crohn’s Disease: The BACARDI Risk Model. J. Crohns Colitis 2018, 12, 32–38. [Google Scholar] [CrossRef]
- Lin, S.-N.; Mao, R.; Qian, C.; Bettenworth, D.; Wang, J.; Li, J.; Bruining, D.H.; Jairath, V.; Feagan, B.G.; Chen, M.-H.; et al. Development of antifibrotic therapy for stricturing Crohn’s disease: Lessons from randomized trials in other fibrotic diseases. Physiol. Rev. 2022, 102, 605–652. [Google Scholar] [CrossRef]
- Mohy-ud-din, N.; Kochhar, G.S. Endoscopic Stricturotomy Is an Efficacious Option for Management of Strictures in Patients With Inflammatory Bowel Disease. Crohns Colitis 360 2020, 2, otaa069. [Google Scholar] [CrossRef]
- Lan, N.; Stocchi, L.; Delaney, C.P.; Hull, T.L.; Shen, B. Endoscopic stricturotomy versus ileocolonic resection in the treatment of ileocolonic anastomotic strictures in Crohn’s disease. Gastrointest. Endosc. 2019, 90, 259–268. [Google Scholar] [CrossRef]
- Pokala, A.; Shen, B. Update of endoscopic management of Crohn’s disease strictures. Intest. Res. 2020, 18, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Loras, C.; Pérez-Roldan, F.; Gornals, J.B.; Barrio, J.; Igea, F.; González-Huix, F.; González-Carro, P.; Pérez-Miranda, M.; Espinós, J.C.; Fernández-Bañares, F.; et al. Endoscopic treatment with self-expanding metal stents for Crohn’s disease strictures. Aliment. Pharmacol. Ther. 2012, 36, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Singh, R.; Din, S.; Lund, J.; Krishnamoorthy, R.; Hearing, S.; Norton, B.; Williams, J.; Fraser, C.; Goddard, A.; et al. Therapeutic resolution of focal, predominantly anastomotic Crohn’s disease strictures using removable stents: Outcomes from a single-center case series in the United Kingdom. Gastrointest. Endosc. 2020, 92, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Ambe, R.; Campbell, L.; Cagir, B. A Comprehensive Review of Strictureplasty Techniques in Crohn’s Disease: Types, Indications, Comparisons, and Safety. J. Gastrointest. Surg. 2012, 16, 209–217. [Google Scholar] [CrossRef]
- Butt, W.T.; Ryan, É.J.; Boland, M.R.; McCarthy, E.M.; Omorogbe, J.; Hazel, K.; Bass, G.A.; Neary, P.C.; Kavanagh, D.O.; McNamara, D.; et al. Strictureplasty versus bowel resection for the surgical management of fibrostenotic Crohn’s disease: A systematic review and meta-analysis. Int. J. Colorectal Dis. 2020, 35, 705–717. [Google Scholar] [CrossRef]
- Picelli, S. Single-cell RNA-sequencing: The future of genome biology is now. RNA Biol. 2017, 14, 637–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashima, Y.; Sakamoto, Y.; Kaneko, K.; Seki, M.; Suzuki, Y.; Suzuki, A. Single-cell sequencing techniques from individual to multiomics analyses. Exp. Mol. Med. 2020, 52, 1419–1427. [Google Scholar] [CrossRef]
- Rao, A.; Barkley, D.; França, G.S.; Yanai, I. Exploring tissue architecture using spatial transcriptomics. Nature 2021, 596, 211–220. [Google Scholar] [CrossRef]
- Longo, S.K.; Guo, M.G.; Ji, A.L.; Khavari, P.A. Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat. Rev. Genet. 2021, 22, 627–644. [Google Scholar] [CrossRef]
- Tavakoli, P.; Vollmer-Conna, U.; Hadzi-Pavlovic, D.; Grimm, M.C. A Review of Inflammatory Bowel Disease: A Model of Microbial, Immune and Neuropsychological Integration. Public Health Rev. 2021, 42, 1603990. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, S.; Yao, J.; Lin, X.; Li, Y.; Wang, W.; Weng, J.; Zou, Y.; Zhu, L.; Zhi, M. Correlation between altered gut microbiota and elevated inflammation markers in patients with Crohn’s disease. Front. Immunol. 2022, 13, 947313. [Google Scholar] [CrossRef]
- Mayorga, L.; Serrano-Gómez, G.; Xie, Z.; Borruel, N.; Manichanh, C. Intercontinental Gut Microbiome Variances in IBD. Int. J. Mol. Sci. 2022, 23, 10868. [Google Scholar] [CrossRef]
- Rosati, E.; Rios Martini, G.; Pogorelyy, M.V.; Minervina, A.A.; Degenhardt, F.; Wendorff, M.; Sari, S.; Mayr, G.; Fazio, A.; Dowds, C.M.; et al. A novel unconventional T cell population enriched in Crohn’s disease. Gut 2022, 71, 2194–2204. [Google Scholar] [CrossRef]
- Globig, A.-M.; Mayer, L.S.; Heeg, M.; Andrieux, G.; Ku, M.; Otto-Mora, P.; Hipp, A.V.; Zoldan, K.; Pattekar, A.; Rana, N.; et al. Exhaustion of CD39-Expressing CD8+ T Cells in Crohn’s Disease Is Linked to Clinical Outcome. Gastroenterology 2022, 163, 965–981.e31. [Google Scholar] [CrossRef]
- Jaeger, N.; Gamini, R.; Cella, M.; Schettini, J.L.; Bugatti, M.; Zhao, S.; Rosadini, C.V.; Esaulova, E.; Di Luccia, B.; Kinnett, B.; et al. Single-cell analyses of Crohn’s disease tissues reveal intestinal intraepithelial T cells heterogeneity and altered subset distributions. Nat. Commun. 2021, 12, 2–13. [Google Scholar] [CrossRef]
- Casalegno Garduño, R.; Däbritz, J. New Insights on CD8+ T Cells in Inflammatory Bowel Disease and Therapeutic Approaches. Front. Immunol. 2021, 12, 738762. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.M.; Wulff, L.; Jones, G.-R.; Vandamme, J.; Jørgensen, P.B.; Bain, C.C.; Lee, J.; Izarzugaza, J.M.G.; Belling, K.G.; Ho, G.-T.; et al. Single-cell characterisation of mononuclear phagocytes in the human intestinal mucosa. Immunology, 2021; Preprint. [Google Scholar]
- Mukherjee, P.K.; Nguyen, Q.T.; Li, J.; Zhao, S.; Christensen, S.M.; West, G.A.; Chandra, J.; Gordon, I.O.; Lin, S.; Wang, J.; et al. Stricturing Crohn’s disease single-cell RNA sequencing reveals fibroblast heterogeneity and intercellular interactions. Immunology, 2023; Preprint. [Google Scholar]
- Martin, J.C.; Chang, C.; Boschetti, G.; Ungaro, R.; Giri, M.; Grout, J.A.; Gettler, K.; Chuang, L.; Nayar, S.; Greenstein, A.J.; et al. Single-Cell Analysis of Crohn’s Disease Lesions Identifies a Pathogenic Cellular Module Associated with Resistance to Anti-TNF Therapy. Cell 2019, 178, 1493–1508.e20. [Google Scholar] [CrossRef]
- Elmentaite, R.; Kumasaka, N.; Roberts, K.; Fleming, A.; Dann, E.; King, H.W.; Kleshchevnikov, V.; Dabrowska, M.; Pritchard, S.; Bolt, L.; et al. Cells of the human intestinal tract mapped across space and time. Nature 2021, 597, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Elmentaite, R.; Ross, A.D.B.; Roberts, K.; James, K.R.; Ortmann, D.; Gomes, T.; Nayak, K.; Tuck, L.; Pritchard, S.; Bayraktar, O.A.; et al. Single-Cell Sequencing of Developing Human Gut Reveals Transcriptional Links to Childhood Crohn’s Disease. Dev. Cell 2020, 55, 771–783.e5. [Google Scholar] [CrossRef]
- Xu, C.; Lopez, R.; Mehlman, E.; Regier, J.; Jordan, M.I.; Yosef, N. Probabilistic harmonization and annotation of single-cell transcriptomics data with deep generative models. Mol. Syst. Biol. 2021, 17, e9620. [Google Scholar] [CrossRef] [PubMed]
- Finak, G.; McDavid, A.; Yajima, M.; Deng, J.; Gersuk, V.; Shalek, A.K.; Slichter, C.K.; Miller, H.W.; McElrath, M.J.; Prlic, M.; et al. MAST: A flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 2015, 16, 278. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Guo, F.R. BSDE: Barycenter single-cell differential expression for case–control studies. Bioinformatics 2022, 38, 2765–2772. [Google Scholar] [CrossRef] [PubMed]
- Dann, E.; Henderson, N.C.; Teichmann, S.A.; Morgan, M.D.; Marioni, J.C. Differential abundance testing on single-cell data using k-nearest neighbor graphs. Nat. Biotechnol. 2022, 40, 245–253. [Google Scholar] [CrossRef]
- Saelens, W.; Cannoodt, R.; Todorov, H.; Saeys, Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 2019, 37, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Schiebinger, G.; Shu, J.; Tabaka, M.; Cleary, B.; Subramanian, V.; Solomon, A.; Gould, J.; Liu, S.; Lin, S.; Berube, P.; et al. Optimal-Transport Analysis of Single-Cell Gene Expression Identifies Developmental Trajectories in Reprogramming. Cell 2019, 176, 928–943.e22. [Google Scholar] [CrossRef] [Green Version]
- Pellino, G.; Pallante, P.; Selvaggi, F. Novel biomarkers of fibrosis in Crohn’s disease. World J. Gastrointest. Pathophysiol. 2016, 7, 266. [Google Scholar] [CrossRef]
- Haniffa, M.; Taylor, D.; Linnarsson, S.; Aronow, B.J.; Bader, G.D.; Barker, R.A.; Camara, P.G.; Camp, J.G.; Chédotal, A.; Copp, A.; et al. A roadmap for the Human Developmental Cell Atlas. Nature 2021, 597, 196–205. [Google Scholar] [CrossRef]
- Burger, A.; Baldock, R.A.; Adams, D.J.; Din, S.; Papatheodorou, I.; Glinka, M.; Hill, B.; Houghton, D.; Sharghi, M.; Wicks, M.; et al. Towards a clinically-based common coordinate framework for the human gut cell atlas: The gut models. BMC Med. Inform. Decis. Mak. 2023, 23, 36. [Google Scholar] [CrossRef]
- Zilbauer, M.; Kylie, J.; Kaur, M.; Pott, S.; Li, Z.; Burger, A.; Thiagarajah, J.; Burclaff, J.; Jahnsen, F.; Perrone, F.; et al. A Roadmap for the Human Gut Cell Atlas. Nat. Rev. Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef]
- Arnott, I.D.R. Crohn’s disease or Crohn’s diseases? Gut 2003, 52, 460–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, I.; Glinka, M.; Shaban, F.; Kirkwood, K.J.; Nadalin, F.; Adams, D.; Papatheodorou, I.; Burger, A.; Baldock, R.A.; Arends, M.J.; et al. The Promise of Single-Cell RNA Sequencing to Redefine the Understanding of Crohn’s Disease Fibrosis Mechanisms. J. Clin. Med. 2023, 12, 3884. https://doi.org/10.3390/jcm12123884
Campbell I, Glinka M, Shaban F, Kirkwood KJ, Nadalin F, Adams D, Papatheodorou I, Burger A, Baldock RA, Arends MJ, et al. The Promise of Single-Cell RNA Sequencing to Redefine the Understanding of Crohn’s Disease Fibrosis Mechanisms. Journal of Clinical Medicine. 2023; 12(12):3884. https://doi.org/10.3390/jcm12123884
Chicago/Turabian StyleCampbell, Iona, Michael Glinka, Fadlo Shaban, Kathryn J. Kirkwood, Francesca Nadalin, David Adams, Irene Papatheodorou, Albert Burger, Richard A. Baldock, Mark J. Arends, and et al. 2023. "The Promise of Single-Cell RNA Sequencing to Redefine the Understanding of Crohn’s Disease Fibrosis Mechanisms" Journal of Clinical Medicine 12, no. 12: 3884. https://doi.org/10.3390/jcm12123884
APA StyleCampbell, I., Glinka, M., Shaban, F., Kirkwood, K. J., Nadalin, F., Adams, D., Papatheodorou, I., Burger, A., Baldock, R. A., Arends, M. J., & Din, S. (2023). The Promise of Single-Cell RNA Sequencing to Redefine the Understanding of Crohn’s Disease Fibrosis Mechanisms. Journal of Clinical Medicine, 12(12), 3884. https://doi.org/10.3390/jcm12123884







