Abstract
Red wine is a rich source of nutrients whose biological properties have inspired numerous scientific studies. Indeed, it has been widely reported that there is a correlation between the positive health effects of moderate consumption of red wine and its phenolic content, which, due to its antioxidant activity, has proved to be useful in the improvement of various diseases, such as cardiovascular diseases, metabolic syndrome, cognitive disorders, depression, and cancer. It is a common opinion that the antioxidant activity of red wine is to be ascribed to its entire content of polyphenols, which act synergistically and not as a single component. Furthermore, this health-promoting effect of red wine can also be linked to its ethanol content, which has shown a wide array of biological properties. Beyond this evidence, very little is known about a possible correlation between moderate consumption of red wine and male sexual function. This brief review aimed to evaluate the effects of moderate consumption of red wine on erectile function. To accomplish this, Pubmed and Google Scholar databases were searched to retrieve the most relevant studies on this topic. The evidence so far collected has shown that red wine, if consumed in moderation, can be potentially beneficial for patients with erectile dysfunction as well as can positively influence reproductive function through mechanisms that depend on the vasorelaxant properties of red wine and its antioxidant properties.
1. Introduction
Red wine is an alcoholic beverage obtained from the fermentation of dark-colored grapes that are rich in phytonutrients to which the organoleptic properties and beneficial health effects on the health of red wine have been attributed [1]. Chemically red wine is a complex mixture of organic compounds, including water, ethanol (EtOH), glycerol, sugars, vitamins, organic acids, aldehydes, ketones, esters, minerals, lipids, and phenolics [2]. The beneficial health effects of red wine consumption have been mainly associated with its polyphenolic content, whose total amount ranges between 2000 and 6000 mg/L [3] and consists of flavonoids and non-flavonoids. Flavonoids include flavonols (quercetin and myricetin), flavanols (catechin and epicatechin), and anthocyanins. Stilbenes (resveratrol), hydroxycinnamates (caftaric, caffeic, and coutaric acids), and hydroxybenzoates belong to the class of non-flavonoid compounds [4].
While it was widely accepted that moderate intake of red wine induces positive health effects [5], the real properties of its components on cellular systems and the mechanism underlying their molecular interactions with organic systems remain to be elucidated. A major issue concerns the alcohol content of red wine and the threshold between the amount of alcohol that can adversely affect biological functions and the amount of alcohol that can have beneficial effects on human health [6]. It was observed that EtOH present in red wine produces a broad spectrum of biological activities, such as influencing the composition of cholesterol and the activity of its metabolizing enzymes, which include the cytochrome p450 family, the glutathione S-transferase superfamily, and N-acetyl transferases [7]. Moderate alcohol consumption (15–30 g/day) has been associated with a cardioprotective effect due to an increase in plasma levels of high-density lipoprotein (HDL), cholesterol, and a reduction in platelet adhesiveness, all responsible for atherosclerosis [8]. As shown by meta-analysis studies, the daily intake of 1 to 3 glasses of standard drinks containing about 10–30 g of alcohol reduced cardiovascular risk [9,10,11]. To the same extent, the pro-oxidant effect [12,13] exerted by EtOH seems to be neutralized by the high content of polyphenol in red wine, which exhibits clear antioxidant activity [14]. Chemical analysis of red wine has revealed that it contains 10 times more polyphenols than other types of wine due to fermentation of darker red or black grapes, including skins and seeds [15].
It is commonly believed that the antioxidant activity of red wine can be attributed to its entire polyphenol content [16]. It can therefore be hypothesized that the total antioxidant capacity of red wine may not depend on a single compound but rather results from the synergistic antioxidant effect of other phytochemicals present in it [17]. Polyphenols show also an anti-inflammatory activity that derives from two different functions: the radical scavenging properties that can block oxidative stress signaling or the modulation of pro-inflammatory signaling transduction [18]. These findings underlie the concept known as the “French paradox”, which reported a low prevalence of coronary heart disease in France in both male and female populations despite eating a diet characterized by a high content of saturated fat [19]. In fact, the authors of this study ascribed this discrepancy to the free radical scavenging activity of polyphenolic compounds contained in red wine. Since then, much attention has been paid to the French situation, inspiring further studies on various aspects of the properties of red wine [20]. Much more is currently known about the phenolic composition of red wine, and several in vitro experiments have been carried out to support findings on the biological properties of red wine flavonoids [21,22,23]. Improvement of clinical outcomes of several diseases, such as cardiovascular disease, metabolic syndrome, cognitive disorders, depression, and cancer, has been mainly attributed to the abundance of antioxidants [24,25]. These include resveratrol, anthocyanins, catechins, and flavonoids in red wine, which can counteract the action of a wide array of oxidant molecules, such as reactive oxygen species (ROS), nitrogen oxide, and chlorine, and also suppress the synthesis of these reactive species. The consumption of red wine has also been seen as a contributing factor in improving blood pressure outcomes in hypertensive patients [25]. Evidence from epidemiological studies [26,27,28] has shown that moderate daily consumption (20–30 g) of red wine could positively influence cardiovascular health and minimize the risk related to all-cause mortality among middle-aged adults and elderly people. Although numerous pieces of evidence exist on the cardiovascular protection associated with moderate consumption of red wine [29,30,31,32,33], there are still few studies on the possible effect of red wine on male gonadal function.
In this brief review, the effects of moderate consumption of red wine (as a complex mixture of ethanol and phytocompounds) on erectile function were evaluated. Pubmed and Google Scholar were searched using the following keywords: “red wine and sexual function”, “red wine and sexual desire”, “red wine and male infertility”, and “red wine and spermatozoa” to find the most relevant studies that indicate a correlation between regular red wine intake and sexual function. Table 1 reports studies performed in vitro, in vivo, and in humans. These later were described in Table 2.

Table 1.
Studies performed in vitro, in vivo, and in humans.

Table 2.
Studies in humans.
2. Red Wine and Gonadal Function
Regular intake of different types of red wine appears to affect serum follicle-stimulating hormone (FSH), testosterone, 17β-estradiol, and prolactin serum levels in young adult male rats, playing a role in modulating reproductive outcomes. These effects appear to be dose-dependent and influenced by wine characteristics [14]. In the same way, the beneficial effect of red wine on the vascular system could also be supposed for erectile dysfunction (ED), currently considered the first manifestation of atherosclerosis and therefore a marker of systemic vascular disease rather than an age-related complication of heart disease [51]. However, in this context, the role of alcohol and polyphenol content in red wine remains still to be clarified [52,53].
3. Red Wine Polyphenols and Erectile Dysfunction
Penile erection is a neuro-vascular event characterized by smooth muscle relaxation and increased blood flow to the penile cavernous level leading to a veno-occlusive mechanism [42]. It is widely recognized that nitric oxide (NO) increases cGMP production by cavernous smooth muscle cells, which, through activation of protein kinase G, causes increased calcium efflux with smooth muscle relaxation. In ED, the small vessels of the penis undergo structural and functional changes in the endothelium [46], resulting in decreased NO bioavailability as a consequence of a reduced synthesis or increased NO degradation in perivascular smooth muscle [54]. Red wine polyphenols are thought to act directly through the modulation of the NO system [34], increasing the production of endothelial NO synthase and reducing the synthesis of endothelin-1, a potent peptide with vasoconstrictor activity, the overexpression of which is considered a fundamental factor in the development of vascular disease and atherosclerosis [55]. NO from neuronal and endothelial cells in the corpora cavernosa (CC) of the penis actives soluble guanylyl cyclase (sGC), which catalyzes the conversion of guanosine triphosphate to cyclic guanosine monophosphate (cGMP). Intracellular cyclic GMP is a biochemical mediator that interferes with the activity of calcium channels, providing a reduction of the amounts of cytosolic calcium and regulating the activity of contractile proteins. This dual action allows for relaxation of the smooth muscle of the CC, causing penile vasodilation and therefore its erection [56].
In in vitro experiments, red wine polyphenols exhibited relaxant effects in the arteries of different vascular beds mainly via a NO/sGC-dependent mechanism [35,36]. However, there is some controversy regarding the possible involvement of the NO system in achieving the relaxing activity of red wine and whether this effect could result from a combined action of polyphenols and alcohol contained in it. The in vitro study by Boydens and colleagues evaluated the relaxant capacity of two red wine polyphenols on isolated mouse aorta and CCs along with their contribution to oxidative stress-induced ED [37]. From the results obtained, it was hypothesized that two polyphenols, quercetin, and resveratrol act as potent vasodilators of the mouse aorta. However, the relaxant effect observed in the CC was probably due to resveratrol alone, suggesting that the experimental results are not in line with a NO/sGC-dependent mechanism. According to the author, this disparity in the literature data could be ascribed to species differences and different experimental models. Alternatively, given the structural diversity between quercetin and resveratrol, the authors hypothesized that quercetin could act through a target-specific activation or that its receptor could have a higher affinity/selectivity for the mouse aorta than the CC. Furthermore, in the latter, resveratrol showed a greater antioxidant activity than quercetin, manifesting a pronounced reduction in neuronal NO responses. Endothelial NO reduces cardiovascular risk through vasoprotective effects in the development of atherosclerosis [57].
Polyphenols have antiatherosclerotic properties [58] also by reducing the expression of adhesion molecules and growth factors responsible for the migration and proliferation of vascular smooth muscle cells. To the same extent, EtOH present in varying amounts in wine has been shown to exert antiatherogenic effects [59]. Data from animal and human studies attributed to EtOH antithrombotic effects and the ability to increase high-density lipoprotein levels [60,61]. In this context, this may explain the results of epidemiological studies reporting a relationship between moderate consumption of red wine and a reduced risk of coronary heart disease [33,62,63]. The effects of chronic red wine intake on the expression of angiogenetic factors, such as the vascular endothelial growth factor (VEGF), angiopoietin 1, angiopoietin 2, and its receptors in rat erectile tissue were studied [38]. In fact, NO seems to play an important role in the development of ED thanks to its involvement in the angiogenic process. Based on mounting evidence, NO regulates VEGF-induced angiogenesis [64]. Results from experimental rat models of ED have revealed that intracavernous administration of VEGF can have positive effects on erectile function [43,44]. Angiogenic factors angiopoietin1 (Ang1) and angiopoietin2 (Ang2) crosstalk with VEGF to modulate angiogenesis. Both Ang1 and Ang2 act on vascular development and maturation by interacting with endothelial-specific tyrosine kinase 2 with immunoglobulin-like and epidermal growth factor homology domains (Tie2). The results from Neves’ study showed that red wine-treated rats had reduced VEGF expression, which is consistent with a decrease in the angiogenesis process in the CC of these animals. This effect could be attributed to the antiatherosclerotic properties of red wine on plaque formation. However, unlike the EtOH group, the obtained data revealed an increase in the expression of Ang1 and Ang2 at CC levels in the red wine group, which may balance the loss of VEGF. Thus, it is likely that this protective effect on the vascular system can be ascribed to the antioxidants present in the red wine that interfere with angiopoietin/Tie2-dependent mechanisms for maintaining cavernous tissue vascularization.
Yetik-Anacak and coworkers [39] suggested an alternative hypothesis that provided an underlying rationale for the protective effect of red wine on ED. In this study, resveratrol is believed to relax the CC in mice by inducing hydrogen sulfide (H2S) formation in a NO pathway-independent manner. H2S is an endogenous gas transmitter endowed with vasorelaxant and pro-erectile activity, produced in the CC in response to neural excitation from L-cysteine through the intervention of two enzymes: cystathionine-gamma-lyase (CSE) and cystathionine-β-synthase (CBS) [65]. Although crosstalk between the two gas transmitters NO and H2S in angiogenesis and vascular relaxation can be hypothesized, in this study, the effect of resveratrol on H2S production in penile tissues was consistent with a direct action on CBS and/or CSE. The data obtained, in fact, showed that resveratrol significantly increased the relaxation induced by L-cysteine, and H2S inhibitors inhibited this effect.
4. Red Wine Polyphenols and Gonad-Related Hormones
The antioxidant properties of polyphenols found in red wine appear to be beneficial for the male reproductive system, suggesting a positive correlation between red wine consumption and testosterone serum levels. Altered physiological hormone levels in the blood, including lower testosterone or testosterone/luteinizing hormone ratio, higher 17β-estradiol levels, or hyperprolactinemia, may adversely affect male fertility. As demonstrated by an in vitro study, red wine has been found to increase testosterone levels by suppressing the activity of the aromatase enzyme CYP19A1 responsible for its conversion to 17β-estradiol [40]. Furthermore, red wine has been proven to block testosterone metabolism through glucuronidation, which inhibits its excretion [41]. A study by Oczkowski and colleagues explored the effects of regular consumption of different red wines on hormonal reproductive profiles and total antioxidant status in rats [14]. Dry red wine (D-RW) showed the highest content of phenolic compounds and the highest antioxidant activity in comparison to semi-sweet (SD-RW), sweet (S-RW), and semi-sweet (SS-RW) red wines considered in this analysis. Furthermore, the investigators reported that the intake of red wine did not alter the serum hormone levels in rats, and the antioxidant status showed no difference even compared to the control group. These observations led the authors to hypothesize that the pro-oxidative effects of wine EtOH on the reproductive system could be partly reduced by the antioxidant activity of phenolic compounds naturally present in red wines. Furthermore, the hypothesis of a threshold relationship between the consumption of red wine, intended as a fraction of EtOH consumed, and hormones measured could explain why in the D-RW, SD-RW, and SS-RW groups, where consumption was low, reproductive hormone levels did not change compared to the control groups. In contrast, the differences in the analyzed hormones (FSH, 17β-estradiol, and prolactin) were observed in the S-RW group characterized by a higher intake of wine than the other groups due to the presence of a greater quantity of sugar. These data are supported by evidence that in humans and rats, chronic intake of EtOH increases prolactin levels and aromatase expression in the rat adipose tissue chronically exposed to red wine or alcohol. Furthermore, the authors attributed these high serum estrogen levels in male animals chronically treated with red wine to their phytoestrogen content and/or the absence of their excretion by the liver. However, higher FSH levels in rats consuming S-RW compared to controls and D-RW could be a consequence of the highest EtOH consumption in this group. In this study, despite the higher consumption of red wine and alcohol in the S-RW group, testosterone levels did not decrease, probably due to the protective effect of caffeic acid, a polyphenol present in large quantities in this type of wine. Moreover, rats given dry red wine showed very low testosterone levels, although their alcohol intake was the lowest, and this wine was characterized by the highest amount of phenols. According to the authors, phytoestrogens may influence steroidogenesis, particularly in dry red wine. A report on the effect of regular red wine consumption on testicular profiles in male rats revealed increased perfusion of the testicular tissue without morphological differences in the seminiferous tubules containing germ cells at different stages of development [45].
5. Red Wine and Female Sexual Function
In addition to investigating a positive correlation between moderate red wine consumption and sexual health in men, Mondaini and colleagues also investigated the potential role of red wine intake in women’s sexual function [47]. As in men, endothelial dysfunction is believed to be part of the pathophysiology of female sexual dysfunction, although this relationship needs to be further elucidated. The NO pathway has also been recognized as crucial for female sexuality, although the female sexual response cycle appears to encompass factors different from that of men, such as psychological factors that include motivation and availability to initiate sex. In this study, the authors evaluated the effects of red wine assumption on several organic sexual responses, such as vulvar swelling, vaginal lubrication, and engorgement-inducing endothelium-dependent dilation of blood vessels. A total of 798 women living in the Chianti area (Tuscany, Italy) were enrolled in this study and were asked to complete the Female Sexual Function Index (FSFI) questionnaire. Fascinatingly, women consuming moderate (one–two glasses a day) red wine (group 1) showed higher FSFI scores for sexual desire and lubrication with an overall improvement in sexual function compared to teetotaler women (group 2) or “occasional drinkers” who consumed less than one glass per day of any type of wine or other alcoholic beverages. Considering that age usually correlates inversely with sexual function, these findings appear to be very interesting, as the women in group 1 were older than in groups 2 and 3. According to the authors, these findings could be due to a synergistic effect of both polyphenols and alcohol content in red wine. In this study, red wine intake resulted in an overall improvement of sexual function in group 1 compared to group 3, which was composed of occasional drinkers of other types of alcoholic beverages, including white wine and to a lesser extent red wine. Red wine polyphenols, through an improvement of NO-dependent endothelial function, provide a rationale for the improvement of peripheral arterial vasodilatory properties. This improvement translates into the positive effects of red wine on metabolic syndrome and arterial-mediated phases of female sexual function. Thus, the NO/cGMP pathway appears to be a key factor exerting local vasodilatory activity of female sexual organs, such as the vagina and clitoris, a sine qua non for an adequate female sexual response [66].
6. Effects of Alcohol
Although moderate consumption of alcohol has been shown to have a protective effect on ED [48], possibly due to the long-term benefits of alcohol on increasing NO levels [67], alcohol affects sexual health in a different manner depending on the frequency and amount of consumption. While the consumption of alcohol in low doses promotes sexual behavior and desire, in higher doses, it is detrimental to sexuality. At the central level, alcohol acts as a depressant, reducing brain function, respiration, and circulation [68]. This depressive effect of alcohol appears to be related to the development of sexual dysfunction, mainly ED in men and lack of vaginal lubrication in women [67]. Chronic alcohol intake reduces testosterone levels, impairs spermatogenesis [69], and decreases testicular volume as well as can lead to feminization with increased ED severity, testicular atrophy, and gynecomastia [70]. Furthermore, in women, high alcohol consumption is harmful to sexual health. Indeed, it was associated with low sexual desire, anorgasmia, and dissatisfaction with one’s sexual functions [49]. In recent times, given the widespread awareness of the negative effects produced by alcohol abuse, there has been a growing interest in dealcoholized drinks. The dealcoholization of wine consists of the partial or total removal of alcohol using various methods. Membrane separation and thermal distillation are the most commonly used methods for commercial production [71]. The elimination of ethanol affects the quality of the wine but maintains its beneficial effects on health [50] due to the presence of phytonutrients as well as not causing negative effects related to the alcohol content. Furthermore, a greater number of people, including youngsters, pregnant women, drivers, and teetotalers, can consume dealcoholized wine (Figure 1).
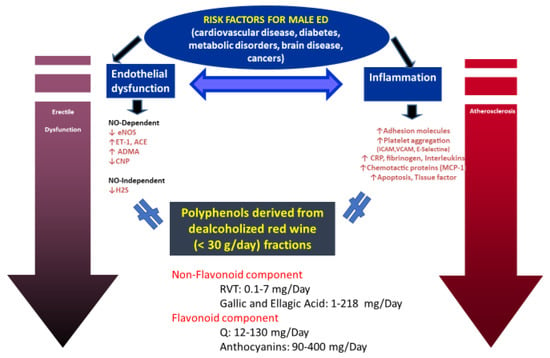
Figure 1.
Red wine (intact or dealcoholized) contains high concentrations of polyphenolic compounds, such as flavonoids (catechin, epicatechin, quercetin, anthocyanins, and procyanidins), resveratrol [3,5,40–trihydroxystilbene (RVT)], and polymeric tannins. RVT and quercetin (Q) may protect against male erectile dysfunction and may induce penile smooth muscle relaxation (corpus cavernosum) through nitric oxide (NO)-dependent (eNOS-endothelin1 reduction) and independent (hydrogen dysulphide-H2S) mechanisms. Furthermore, the anti-inflammatory effects of red wine also protect against platelet aggregation and thus the progression of atherosclerosis (adapted from reference [29]).
7. Limitations of the Study and Caution in Data Interpretation
The effects of alcohol use are mainly harmful (based on daily consumption) on different organs and systems (diabetes, hypertension, liver, heart, etc.). This aspect is beyond the scope of this article. To this aspect, it should be added that there is insufficient evidence to support that antioxidant therapy improves sexual function in clinical practice, for example, only in the study by Shirai et al. Resveratrol (300 mg/d) within a combination with testofen, 600 mg/d; L-citrullines, 800 mg/d; and caffeine, 40 mg/d was evaluated by applying the IIEF (International Index of Erectile Function) for the evaluation of efficacy on male sexual function with good results [72]. The practical clinical message regarding the improvement of erectile dysfunction does not concern the possibility of improving sexual function with the consumption of red wines but only underlines the potential aspects of interest of this nutritional habit on sexual and reproductive health, which appears to be a field of interest for future clinical trials. Finally, the aspect relating to the usefulness of mobile applications regarding the most appropriate nutritional choices for male reproductive health also appears to be little explored and worthy of further investigation through well-conducted clinical studies [73].
8. Concluding Remarks
In conclusion, it is commonly believed that moderate consumption of red wine plays a valuable role in human health due to the co-presence of flavonoids and EtOH. Although many studies have explored the beneficial effects of red wine through in vitro and in vivo experimental models, there is a paucity of clinical trials supporting a relationship between moderate red wine consumption and male fertility. Based on the literature, it can be concluded that the antioxidant properties of polyphenols present in red wine appear to be beneficial for the reproductive system. Furthermore, the data reveal that the polyphenolic fraction in red wine can counteract the effects of EtOH or act directly on male reproduction. The effects of red wine intake on the hormonal regulation of the male reproductive system appear to depend on the type and amount of red wine. However, further investigations are necessary to confirm the precise role of both EtOH and polyphenols in the field of male and female sexual response, as well as to elucidate the specific mechanisms of their action.
Author Contributions
L.B. and S.L.V. designed the study; R.A.C., R.C., F.B. and A.C. did a bibliographic search; A.E.C. and A.A. revised the text. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fernandes, L.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Wine Flavonoids in Health and Disease Prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Chemical Composition and Polyphenolic Compounds of Red Wines: Their Antioxidant Activities and Effects on Human Health—A Review. Beverages 2022, 8, 1. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, more than just simple natural antioxidants: Oxidative stress, aging and age-related diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Guo, H.; Fang, Y.; Zhou, G. The mechanisms of wine phenolic compounds for preclinical anticancer therapeutics. Food Nutr. Res. 2021, 65, 6507. [Google Scholar] [CrossRef]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular Properties of Red Wine Compounds and Cardiometabolic Benefits. Nutr. Metab. Insights 2016, 9, 51–57. [Google Scholar] [CrossRef]
- Cordova, A.C.; Sumpio, B.E. Polyphenols are medicine: Is it time to prescribe red wine for our patients? Int. J. Angiol. 2009, 18, 111–117. [Google Scholar] [CrossRef]
- Bell, J.; Donnovan, J.; Wong, R.; Waterhouse, A.L.; German, J.B.; Walzem, R.L.; Kasim-Karakas, S.E. (+)-catechin in human plasma after ingestion of a single serving of reconstituted red wine. Am. J. Clin. Nutr. 2000, 71, 103–108. [Google Scholar] [CrossRef]
- Di-Castelnuovo, A.; Rotondo, S.; Lacoviello, L.; Donati, M.B.; De Gaetano, G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation 2002, 105, 2836–2844. [Google Scholar] [CrossRef]
- Corrao, G.; Rubbiati, L.; Bagnardi, V.; Zambon, A.; Poikolamen, C. Alcohol and coronary heart disease: A meta-analysis. Addiction 2000, 95, 1505–1523. [Google Scholar] [CrossRef]
- Maclure, M. Demonstration of deductive meta-analysis: Ethanol intake and risk of myocardiac infarction. Epidemiol. Rev. 1993, 15, 328–351. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Aviram, M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr. Opin. Lipidol. 2001, 12, 41–48. [Google Scholar] [CrossRef]
- Nigdikar, S.V.; Williams, N.R.; Griffin, B.A.; Howard, A.N. Consumption of red wine polyphenols reduces the susceptibility of low-density lipoproteins to oxidation in vivo. Am. J. Clin. Nutr. 1998, 68, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Oczkowski, M.; Średnicka-Tober, D.; Stachon, M.; Kołota, A.; Wolińska-Witort, E.; Malik, A.; Hallmann, E.; Rusaczonek, A.; Gromadzka-Ostrowska, J. The effect of red wine consumption on hormonal reproductive parameters and total antioxidant status in young adult male rats. Food Funct. 2014, 5, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Artero, A.; Artero, A.; Tarín, J.J.; Cano, A. The impact of moderate wine consumption on health. Maturitas 2015, 80, 3–13. [Google Scholar] [CrossRef]
- Tekos, F.; Makri, S.; Skaperda, Z.-V.; Patouna, A.; Kalliroi, T.; Kyriazis, I.D.; Kotseridis, Y.; Mikropoulou, E.V.; Papaefstathiou, G.; Halabalaki, M.; et al. Assessment of Antioxidant and Antimutagenic Properties of Red and White Wine Extracts In Vitro. Metabolites 2021, 11, 436. [Google Scholar] [CrossRef]
- Xiang, L.; Xiao, L.; Wang, Y.; Li, H.; Huang, Z.; He, X. Health benefits of wine: Don’t expect resveratrol too much. Food Chem. 2014, 156, 258–263. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Llorach, R.; Rotches-Ribalta, M.; Guillen, M.; Casas, R.; Arranz, S.; Valderas-Martinez, P.; Portoles, O.; Corella, D. Differential Effects of Polyphenols and Alcohol of Red Wine on the Expression of Adhesion Molecules and Inflammatory Cytokines Related to Atherosclerosis: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2011, 95, 326–334. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Epidemiology 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French Paradox Revisited. Front. Pharmacol. 2012, 3, 141–159. [Google Scholar] [CrossRef]
- Kaur, G.; Rao, L.V.M.; Agrawal, A.; Pendurthi, U.R. Effect of wine phenolics on cytokine-induced C-reactive protein expression. J. Thromb. Haemost. 2007, 5, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, I.; Spagnuolo, C.; Russo, G.; Russo, M.; Cervellera, C.; Moccia, S. The Pro-Oxidant Activity of Red Wine Polyphenols Induces an Adaptive Antioxidant Response in Human Erythrocytes. Antioxidants 2021, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Shafreen, R.M.B.; Lakshmi, S.A.; Pandian, S.K.; Kim, Y.-M.; Deutsch, J.; Katrich, E.; Gorinstein, S. In Vitro and In Silico Interaction Studies with Red Wine Polyphenols against Different Proteins from Human Serum. Molecules 2021, 26, 6686. [Google Scholar] [CrossRef] [PubMed]
- Snopec, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of red wine consumption to human health protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef]
- Cavallini, G.; Straniero, S.; Donati, A.; Bergamini, E. Resveratrol requires red wine polyphenols for optimum antioxidant activity. J. Nutr. Health Aging 2016, 20, 540–545. [Google Scholar] [CrossRef]
- Montorsi, P.; Montorsi, F.; Schulman, C.C. Is erectile dysfunction the “tip of the iceberg” of a systemic vascular disorder? Eur. Urol. 2003, 44, 352–354. [Google Scholar] [CrossRef]
- Torres, A.; Cachofeiro, V.; Millán, J.; Lahera, V.; Nieto, M.; Martin, R.; Bello, E.; Alvarez-Sala, L.; Nieto, M. Red wine intake but not other alcoholic beverages increases total antioxidant capacity and improves pro-inflammatory profile after an oral fat diet in healthy volunteers. Rev. Clínica Española 2015, 215, 486–494. [Google Scholar] [CrossRef]
- Nova, E.; San Mauro-Martín, I.; Díaz-Prieto, L.E.; Marcos, A. Wine and beer within a moderate alcohol intake is associated with higher levels of HDL-c and adiponectin. Nutr. Res. 2019, 63, 42–50. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Minno, G.D.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef]
- Muñoz-Bernal, Ó.A.; Coria-Oliveros, A.J.; de la Rosa, L.A.; Rodrigo-García, J.; Del Rocío Martínez-Ruiz, N.; Sayago-Ayerdi, S.G.; Alvarez-Parrilla, E. Cardioprotective effect of red wine and grape pomace. Food Res. Int. 2021, 140, 110069–110076. [Google Scholar] [CrossRef]
- De Paula, G.C.; de Oliveira, J.; Engel, D.F.; Lopes, S.C.; Moreira, E.L.G.; Figueiredo, C.P.; Prediger, R.D.; Fabro de Bem, A. Red wine consumption mitigates the cognitive impairments in low-density lipoprotein receptor knockout (LDLr-/-) mice. Nutr. Neurosci. 2021, 24, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, Y. Absorption and metabolism of red wine polyphenols and their potential health benefits in cardiovascular function. Am. J. Clin. Nutr. 2012, 95, 1496–1497, author reply 1497–1498. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of Wine, Alcohol and Polyphenols on Cardiovascular Disease Risk Factors: Evidences from Human Studies. Alcohol Alcohol. 2013, 48, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Duluc, L.; Jacques, C.; Soleti, R.; Iacobazzi, F.; Simard, G.; Andriantsitohaina, R. Modulation of mitochondrial capacity and angiogenesis by red wine polyphenols via estrogen receptor, NADPH oxidase and nitric oxide synthase pathways. Int. J. Biochem. Cell Biol. 2013, 45, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Pace-Asciak, C.R. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen. Pharmacol. 1996, 27, 363–366. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, L.; Wu, R.X.; Yue, S.Q.; Pei, J.M. The vasorelaxing effect of resveratrol on abdominal aorta from rats and its underlying mechanisms. Vascul. Pharmacol. 2013, 58, 64–70. [Google Scholar] [CrossRef]
- Boydens, C.; Pauwels, B.; Decaluwé, K.; Brouckaert, P.; Van de Voorde, J. Relaxant and Antioxidant Capacity of the Red Wine Polyphenols, Resveratrol and Quercetin, on Isolated Mice Corpora Cavernosa. J. Sex. Med. 2014, 12, 303–312. [Google Scholar] [CrossRef]
- Neves, D.R.G.L.M.; Tomada, I.M.A.S.C.M.; Assunção, M.M.B.; Marques, F.A.P.; Almeida, H.M.N.; Andrade, J.P.A.V. Effects of Chronic Red Wine Consumption on the Expression of Vascular Endothelial Growth Factor, Angiopoietin 1, Angiopoietin 2, and Its Receptors in Rat Erectile Tissue. J. Food Sci. 2010, 75, 79–86. [Google Scholar] [CrossRef]
- Yetik-Anacak, G.; Dereli, M.V.; Sevin, G.; Ozzayım, O.; Erac, Y.; Ahmed, A. Resveratrol Stimulates Hydrogen Sulfide (H2S) Formation to Relax Murine Corpus Cavernosum. J. Sex. Med. 2015, 12, 2004–2012. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I.; Calhau, C. Modulation of aromatase activity by diet polyphenolic compounds. J. Agric. Food Chem. 2006, 54, 3535–3540. [Google Scholar] [CrossRef]
- Jenkinson, C.; Petroczi, A.; Naughton, D.P. Red wine and component flavonoids inhibit UGT2B17 in vitro. Nutr. J. 2012, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Radisavljevic, Z.M.; Siroky, M.B.; Azadzoi, K.M. Dietary antioxidants improve arteriogenic erectile dysfunction. Int. J. Androl. 2010, 34, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Gholami, S.S.; Rogers, R.; Chang, J.; Ho, H.C.; Grazziottin, T.; Lin, C.S.; Lue, T.F. The effect of vascular endothelial growth factor and adeno-associated virus mediated brain derived neurotrophic factor on neurogenic and vasculogenic erectile dysfunction induced by hyperlipidemia. J. Urol. 2003, 169, 1577–1581. [Google Scholar] [CrossRef]
- Kwangsung, P.; Kyu, Y.A.; Min-Kyung, K.; Song, E.L.; Taek, W.K.; Soo-Bang, R. Intracavernosal Injection of Vascular Endothelial Growth Factor Improves Erectile Function in Aged Rats. Eur. Urol. 2004, 46, 403–407. [Google Scholar] [CrossRef]
- Isaac, U.E.; Obeten, K.E.; Igiri, A.O. Effects of regular administration of red wine on testicular profile and body weight in adult male experimental model. J. Clin. Med. Kaz. 2021, 18, 75–80. [Google Scholar] [CrossRef]
- Kaya, C.; Uslu, Z.; Karaman, I. Is endothelial function impaired in erectile dysfunction patients? Int. J. Impot. Res. 2006, 18, 55–60. [Google Scholar] [CrossRef]
- Mondaini, N.; Cai, T.; Gontero, P.; Gavazzi, A.; Lombardi, G.; Boddi, V.; Bartoletti, R. Regular Moderate Intake of Red Wine Is Linked to a Better Women’s Sexual Health. J. Sex. Med. 2009, 6, 2772–2777. [Google Scholar] [CrossRef]
- Chew, K.K.; Bremner, A.; Stuckey, B.; Earle, C.; Jamrozik, K. Alcohol consumption and male erectile dysfunction: An unfounded reputation for risk? J. Sex. Med. 2009, 6, 1386–1394. [Google Scholar] [CrossRef]
- Anil, K.B.; Shalini, M.; Sanjai, R.J.; Prasannakumar, D.R. Sexual dysfunction in women with alcohol dependence syndrome: A study from India. Asian J. Psychiatr. 2017, 28, 9–14. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Arranz, S.; Valderas-Martínez, P.; Casas, R.; Sacanella, E.; Llorach, R.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; et al. Dealcoholized Red Wine Decreases Systolic and Diastolic Blood Pressure and Increases Plasma Nitric Oxide. Circ. Res. 2012, 111, 1065–1068. [Google Scholar] [CrossRef]
- Golan, R.; Gepner, Y.; Shai, I. Wine and Health—New Evidence. Eur. J. Clin. Nutr. 2018, 72, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Corder, R.; Mullen, W.; Khan, N.Q.; Marks, S.C.; Wood, E.G.; Carrier, M.J.; Crozier, A. Red wine procyanidins and vascular health. Nature 2006, 444, 566. [Google Scholar] [CrossRef]
- Bartoletti, R.; Mondaini, N.; Cai, T. Red Wine, Sex, and a Genius. Eur. Urol. 2008, 53, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Yuyun, M.F.; Ng, L.L.; Ng, G.A. Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy? Microvasc. Res. 2018, 119, 7–12. [Google Scholar] [CrossRef]
- Corder, R. Endothelin and Its Inhibitors. In Handbook of Experimental Pharmacology; Warner, T.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 35–67. [Google Scholar]
- Burnett, A.L. The Role of Nitric Oxide in Erectile Dysfunction: Implications for Medical Therapy. J. Clin. Hypertens. 2006, 8, 53–62. [Google Scholar] [CrossRef]
- Little, P.J.; Askew, C.D.; Xu, S.; Kamato, D. Endothelial Dysfunction and Cardiovascular Disease: History and Analysis of the Clinical Utility of the Relationship. Biomedicines 2021, 9, 699. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Sheen, J.-M.; Hu, W.L.; Hung, Y.-C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxid. Med. Cell Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef]
- Feinman, L.; Lieber, C.S. Ethanol and lipid metabolism. Am. J. Clin. Nutr. 1999, 70, 791–792. [Google Scholar] [CrossRef]
- Li, J.M.; Mukamal, K.J. An update on alcohol and atherosclerosis. Curr. Opin. Lipidol. 2004, 15, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Saremi, A.; Rohit, A. The Cardiovascular Implications of Alcohol and Red Wine. Am. J. Ther. 2008, 15, 265–277. [Google Scholar] [CrossRef]
- Wollin, S.D.; Jones, P.J.H. Alcohol, Red Wine and Cardiovascular Disease. J. Nutr. 2001, 131, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Esumi, H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim. Pol. 2003, 50, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen sulfide as a neuromodulator. Mol. Neurobiol. 2002, 26, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Pauls, R.N. Anatomy of the clitoris and the female sexual response. Clin. Anat. 2015, 28, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Mollaioli, D.; Ciocca, G.; Limoncin, E.; Di Sante, S.; Gravina, G.L.; Carosa, E.; Lenzi, A.; Jannini, E.A.F. Lifestyles and sexuality in men and women: The gender perspective in sexual medicine. Reprod. Biol. Endocrinol. 2020, 18, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Peugh, J.; Belenko, S. Alcohol, drugs, and sexual function: A review. J. Psychoact. Drugs 2001, 33, 223–332. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; La Vignera, S. Chronic consumption of alcohol and sperm parameters: Ourexperience and the main evidences. Andrologia 2015, 47, 368–379. [Google Scholar] [CrossRef]
- Leiblum, S.R.; Rosen, R.C. Alcohol and human sexual response. In Alcoholism and Sexual Dysfunction: Issues in Clinical Management; Powell, D.J., Ed.; Haworth Press: Binghampton, NY, USA, 1984. [Google Scholar]
- Sam, F.E.; Ma, T.-Z.; Salifu, R.; Wang, J.; Jiang, Y.-M.; Zhang, B.; Han, S.-Y. Techniques for Dealcoholization of Wines: Their Impact on Wine Phenolic Composition, Volatile Composition, and Sensory Characteristics. Foods 2021, 10, 2498. [Google Scholar] [CrossRef]
- Shirai, M.; Miyoshi, Y.; Ogasa, T.; Miyoshi, M.; Ishikawa, K.; Hiramatsu, I.; Uesaka, Y.; Nozaki, T.; Koyama, T.; Tsujimura, A. Oral Testofen, L-Citrulline, Resveratrol, and Caffeine Supplement Drink Improves Sexual Function in Men with Phosphodiesterase 5 Inhibitors: Randomized, Double-Blind, Placebo-Controlled Crossover Pilot Study. World J. Mens Health 2021, 39, 733–739. [Google Scholar] [CrossRef]
- Fusco, G.M.; Cirillo, L.; Abate, M.; Morra, S.; Morgera, V.; Barone, B.; Crocetto, F.; Cacace, G.; Di Bello, F.; Spirito, L.; et al. Male infertility, what Mobile Health Applications “know”: Quality analysis and adherence to European Association of Urology Guidelines. Arch. Ital. Urol. Androl. 2022, 94, 470–475. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).