Unmet Challenges in Patients with Crohn’s Disease
Abstract
:1. Introduction
2. Materials and Methods
- Accurate early disease diagnosis and phenotyping
- Variable disease course and response to treatment (including high treatment failure rates and lack of optimal disease monitoring, and decisions regarding the withdrawal of therapy)
- Standardized methods of surveillance
- Management of stricturing Crohn’s disease
- Management of cutaneous disease manifestations
3. Results
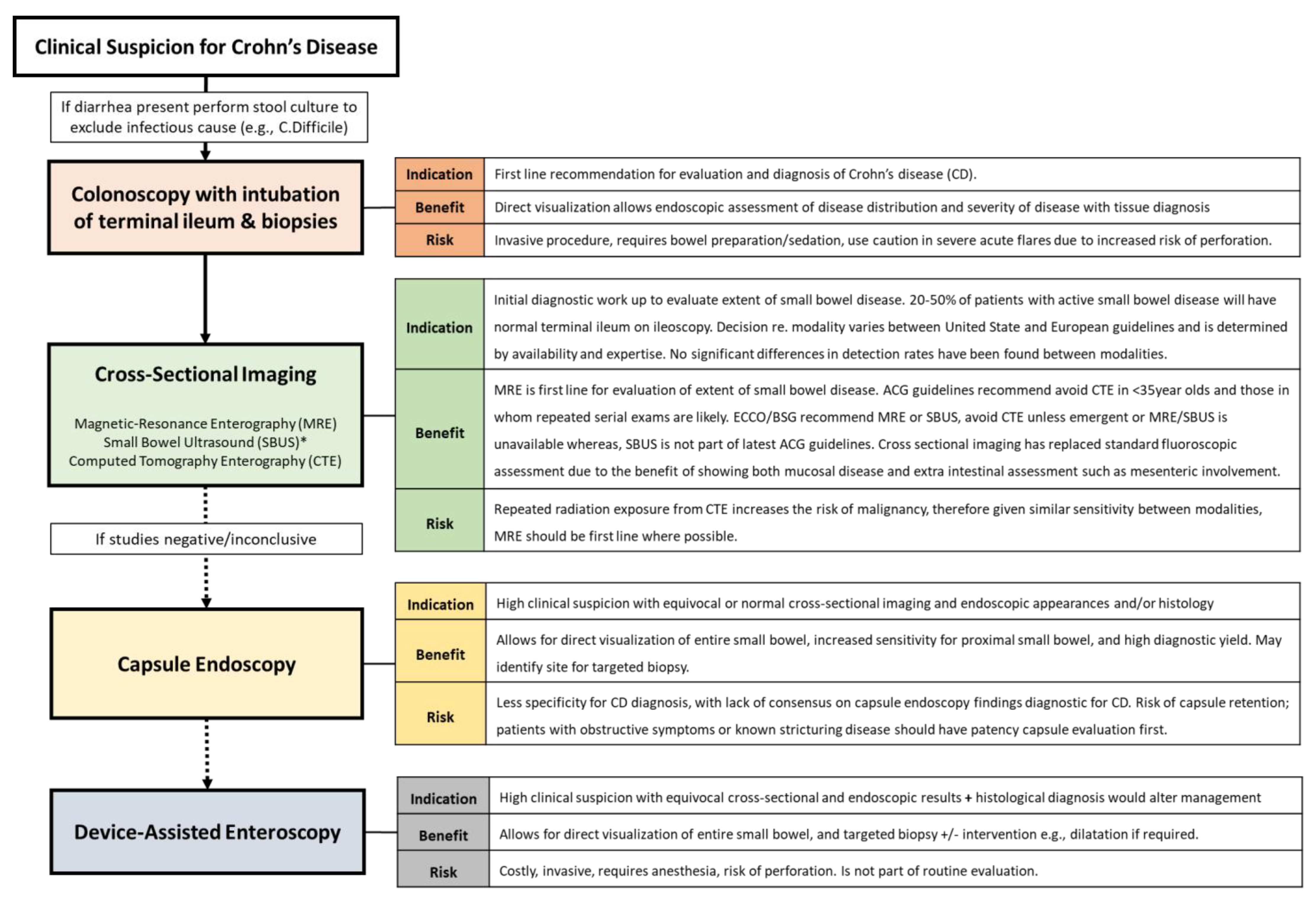
3.1. The Unmet Need: Accurate Early Disease Diagnosis and Phenotyping
3.1.1. What We Know
3.1.2. What Is Being Investigated
3.1.3. Remaining Gaps/Future Directions
3.2. Variable Disease Course and Response to Treatment (Including High Treatment Failure Rates and Lack of Optimal Disease Monitoring, and Decision Regarding Withdrawal of Therapy)
3.2.1. The Unmet Need: Variable Disease Course and Response to Treatment
What We Know
What Is Being Investigated
Remaining Gaps/Future Directions
3.2.2. The Unmet Need: High Treatment Failure Rates and Optimal Disease Monitoring
What We Know
What Is Being Investigated
| Current Biologics Recommended in Crohn’s Disease | |||
|---|---|---|---|
| Biologic Family Examples | Mechanism of Action | Secondary Failure Rates/ Complications | Guideline Recommendation * |
| Anti-TNFα -Infliximab -Adalimumab -Certolizumab -Golimumab | Non-specific inhibition of pro-inflammatory cytokines via TNF blocking. | 20–30% non-responders 30–40% initial responders, secondary failure. | Induction of remission and maintenance+/- thiopurine/methotrexate for maintenance. Patients should undergo latent/active tuberculosis testing prior to commencing. |
| Anti-IL12/23 -Ustekinumab | Targets p40 subunit on IL12/23 part of the T-helper cell pathway. | Similar failure rates to anti-TNFa (30–50%) Risk of pro-tumorigenic effect of blocking IL-12—new biologics targeting IL-23 alone are in development. | Induction of remission and maintenance or loss of response to anti-TNF therapy. |
| Anti-integrins -Natalizumab Anti-α4 -Vedolizumab Anti-α4β7 | Targets leukocyte trafficking via cell-adhesion molecule (CAM). Natalizumab acts via Vascular CAM-1 and Mucosal Addressin CAM-1 Vedolizumab is gut-specific via Mucosal Addressin CAM-1 only. | Risk of PML (progressive multi-focal leuko-encephalopathy) with Natalizumab if John Cunningham (JC) virus antibody positive. Vedolizumab is gut specific with no CNS uptake, no reported cases of PML. Failure rates in 34.3% monotherapy and 29.3% combination with thiopurine [33]. | Induction of remission and maintenance or loss of response to anti-TNF therapy. Natalizumab should not be used in combination with immuno-modulators. John Cunningham (JC) Virus serum antibody testing prior to commencing and every 6 months. AGA (2021) guidelines suggest against the use of Natalizumab [34]. Vedolizumab can be used in combination with immunomodulators. |
| New Drugs Recently Approved/Under Investigation for use in Crohn’s Disease | |||
| Drug Family Mechanism of Action | Drug Name | Completed Trials/Current Evidence | Active/Ongoing Trials. |
| Anti-IL23 Targets p19 subunit of IL-23. A promotor of Th17 cell immune response [31]. | Risankizumab Approved for use in Crohn’s disease (June 2022). | Phase 3 RCTs ADVANCE and MOTIVATE found Risankizumab was well tolerated and effective for the induction of remission (45% and 42%) vs. placebo in moderate to severe CD with loss of response to previous biologics [35]. Follow-up maintenance FORTIFY trial found greater clinical (52% vs. 41%) and endoscopic (47% vs. 22%) response vs. placebo [36]. | Phase 3 SEQUENCE trial comparing Risankizumab vs. Ustekinumab. NCT04524611 [37] |
| Mirikizumab | Phase 2 SERENITY trial, in patients with ileocolic or colonic CD, favorable endoscopic response (43.8% vs. 10.9%) and clinical remission (20.3% vs. 1.6%) in the highest dose treatment group vs. placebo. Maintenance remission at 52 weeks was 50–63.3% [38]. | Phase 3 trials VIVID-1 (NCT03926130) [39] and long term extension VIVID-2, (NCT04232553) [40]. | |
| Brazikumab (MEDI2070) | Phase 2 trial showed favorable safety profile in patients with moderate to severe Crohn’s who had failed anti-TNF. Clinical response at week 8 was found in 49.2% vs. 26.7% placebo with sustained response at week 24 [41]. IL-22 serum levels were found to be predictive of response to Brazikumab. Potential target for selecting appropriate patients for this treatment. | Phase 2b/3 INTREPID trial: Brazikumab vs. adalimumab vs. placebo NCT03759288 [42]. Phase 3 open label extension trial INTREPID-OLE studying long term safety NCT03961815 [43]. | |
| Guselkumab | Phase 2 GALAX-1 trial, showed efficacy in clinical response (60–64%) and remission (50–58%) in all doses of Guselkumab at 12 weeks. Results were similar to those treated with ustekinumab, although not a primary endpoint of the study [44]. | Phase 3 GRAVITI Guselkumab efficacy and safety NCT05197049 [45]. Phase 3 FUZION-CD Guselkumab in peri-anal CD NCT0534709 [46]. Phase 2 trial DUET CD Combination with Golimumab (anti-TNF) NCT05242471 [47]. | |
| Anti-TL1A Tumor necrosis factor like ligand 1A. Blocks TL1A- involved in T cell activation, Th2 pathological responses, and production of IL-5 and IL-3 in intestinal mucosa. | PRA023 | Phase 2a APOLLO-CD proof of concept trial reported 26% endoscopic response and 49% clinical remission at 12 weeks. Efficacy preserved in patients with previous biologic exposure [48]. | Placebo-controlled trials awaited to confirm findings. |
| TEV-48574 | Phase 2b RELIEVE UCCD efficacy and dose response for remission using TEV-48574 NCT05499130 [49]. Phase 2b RELIEVE UCCD LTE, long term extension of RELIEVE UCCD. Efficacy and dose response for maintenance regimens NCT05668013 [50]. | ||
| JAK-Inhibitors Inhibit JAK/TYK pathways, which mediate signal transduction downstream of many cytokines. | Filgotinib JAK1 inhibitor | Phase 2 FITZROY trial of 174 patients with ileal, ileocolonic or colonic CD. Induction of clinical remission was reported in 47% vs. 23% placebo following 10 weeks treatment [32]. Phase 2 DIVERGENCE-1 trial of filgotinib vs. placebo in Small Bowel Crohn’s disease showed no significant Difference in clinical remission, or MRI index of activity. | Phase 3 DIVERSITY NCT 02914561 [51] completed, awaiting publication of results. Phase 3 DIVERSITY LTE Long Term Extension NCT02914600 [52]. Long term efficacy and safety of filgotinib vs. placebo for remission induction and maintenance |
| Upadacitinib JAK-1 inhibitor. Approved May 2023 for moderate to severe CD with intolerance to or inadequate response from anti-TNF. | Phase 2 CELEST trial of efficacy and safety of multiple doses of Upadacitinib vs. placebo. Upadacitinib was superior in the induction of clinical and endoscopic remission as well as remission maintenance at 36 weeks compared to placebo [53]. Phase 3 U-EXCEL and U-EXCEED trials showed clinical remission in 39–50% vs. 21–29% in placebo, and endoscopic response 35–46% vs. 4–13% placebo. U-ENDURE maintenance trial reported clinical remission at 52 weeks in 37–47% patients vs. 15% placebo [54]. | Observational UPLIFT trial to assess speed of onset and durability of effectiveness of Upadacitinib NCT05930275 [55]. Not yet recruiting. | |
| Tofacitinib (-approved for use in UC) Non-selective JAK inhibitors. | Two Phase 2 randomized, double blinded, placebo-controlled trials involving 460 patients showed no benefit over placebo with Tofacitinib for induction or maintenance of remission in CD [56]. | No new/active trials at present. | |
| Deucravacitinib (BMS-986165) Tyrosine Kinase 2 (TYK2) inhibitor. | Phase 2 LATTICE-CD trials for long term efficacy and safety of Deucravacitinib. NCT03599622, NCT04877990 [57] | ||
| Sphingosine-1- Phosphate Receptor Modulators Inhibit leukocytes uptake in peripheral tissues. | Ozanimod S1P1, S1P5 selective. | Phase 2 STEPSTONE trial reported endoscopic response in 23.2% and clinical remission in 39.1% of patients [58]. | Phase 3 YELLOWSTONE trial program including 2 placebo-controlled induction trials (NCT03440372, NCT0344038) 1 maintenance (NCT03464097) and an open label extension study (NCT03467958) [59]. |
| Etrasimod S1PR1, S1PR4, S1PR5 selective. | Phase 2 CULTIVATE trial Sub-study A suggested endoscopic and clinical improvement following etrasimod, but small numbers and no placebo limit conclusions [60]. | Phase 2/3 CULTIVATE trial includes 5 sub-studies evaluating efficacy, safety, and tolerability of etrasimod. NCT04173273 [61] | |
Remaining Gaps/Future Direction
- Treatment Dilemma—Medical Treatment Versus Early Surgery
- Treatment Dilemma—When to Stop Biologic Agents
3.3. The Unmet Need: Standardized Methods of Surveillance
3.3.1. Surveillance for Recurrent Disease
What We know
What Is Being Investigated
Remaining Gaps/Future Directions
3.3.2. Surveillance for Cancer
What We Know
What Is Being Investigated
Remaining Gaps/Future Directions
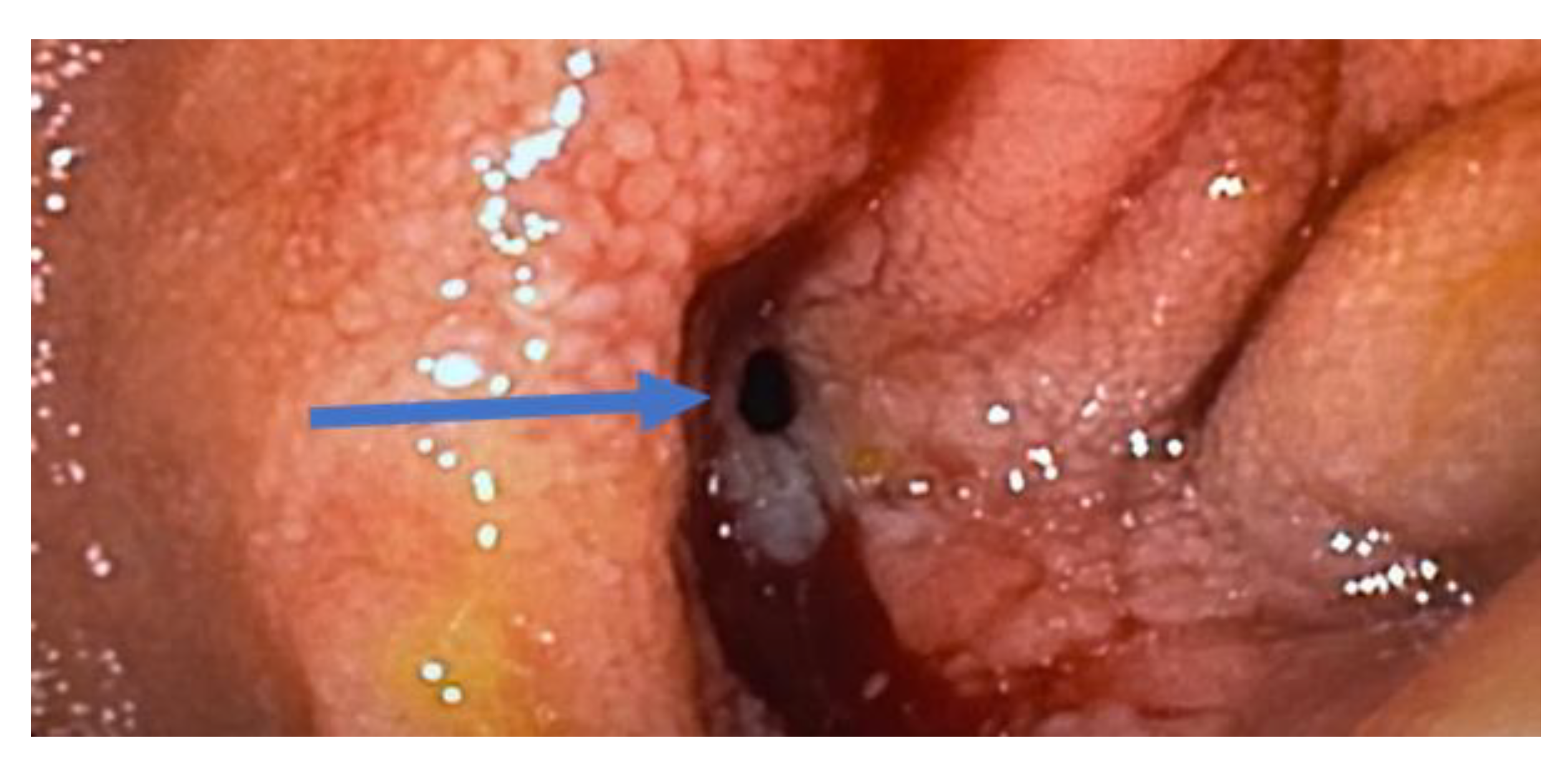
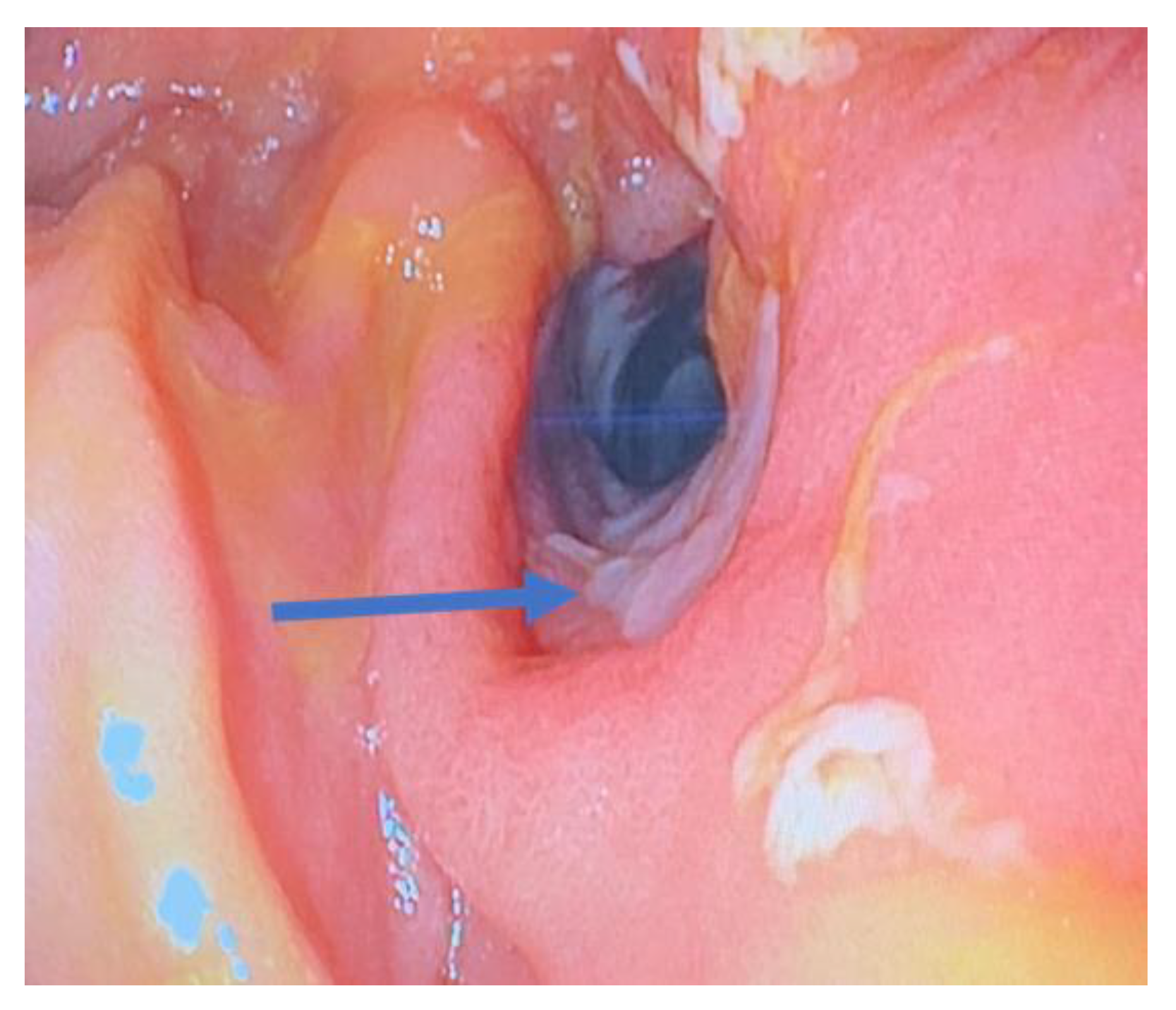
3.4. The Unmet Need: Management of Stricturing Crohn’s Disease
3.4.1. What We Know
3.4.2. What Is Being Investigated
- Medical therapy
- Endoscopic dilation
- Surgical therapy
3.4.3. Remaining Gaps/Future Directions
- Medical therapy
- Surgical therapy
3.5. The Unmet Need: Management of Cutaneous Crohn’s Disease
3.5.1. What We Know
3.5.2. What Is Being Investigated
3.5.3. Remaining Gaps/Future Direction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Sulz, M.C.; Burri, E.; Michetti, P.; Rogler, G.; Peyrin-Biroulet, L.; Seibold, F.; Swiss Ibdnet. Treatment Algorithms for Crohn’s Disease. Digestion 2020, 101 (Suppl. S1), 43–57. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Kopylov, U.; Chowers, Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Irving, P.; Burisch, J.; Driscoll, R.; Olsson, M.; Fullarton, J.R.; Rodgers-Gray, B.S.; Travis, S.P. IBD2020 global forum: Results of an international patient survey on quality of care. Intest. Res. 2018, 16, 537–545. [Google Scholar] [CrossRef]
- Perler, B.K.; Ungaro, R.; Baird, G.; Mallette, M.; Bright, R.; Shah, S.; Shapiro, J.; Sands, B.E. Presenting symptoms in inflammatory bowel disease: Descriptive analysis of a community-based inception cohort. BMC Gastroenterol. 2019, 19, 47. [Google Scholar] [CrossRef]
- Ajbar, A.; Cross, E.; Matoi, S.; Hay, C.A.; Baines, L.M.; Saunders, B.; Farmer, A.D.; Prior, J.A. Diagnostic Delay in Pediatric Inflammatory Bowel Disease: A Systematic Review. Dig. Dis. Sci. 2022, 67, 5444–5454. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, N.; Baillie, S.; Blackwell, J.; Bottle, A.; Petersen, I.; Creese, H.; Saxena, S.; Pollok, R.C. Systematic review with meta-analysis: Time to diagnosis and the impact of delayed diagnosis on clinical outcomes in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2023, 57, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.T.; Zhang, Y.; She, Y.; Goyal, H.; Wu, Z.Q.; Xu, H.G. Diagnostic Utility of Non-invasive Tests for Inflammatory Bowel Disease: An Umbrella Review. Front. Med. 2022, 9, 920732. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. S3), s1–s106. [Google Scholar] [CrossRef]
- Torres, J.; Petralia, F.; Sato, T.; Wang, P.; Telesco, S.E.; Choung, R.S.; Strauss, R.; Li, X.J.; Laird, R.M.; Gutierrez, R.L.; et al. Serum Biomarkers Identify Patients Who Will Develop Inflammatory Bowel Diseases Up to 5 Years Before Diagnosis. Gastroenterology 2020, 159, 96–104. [Google Scholar] [CrossRef]
- Noor, N.M.; Sousa, P.; Paul, S.; Roblin, X. Early Diagnosis, Early Stratification, and Early Intervention to Deliver Precision Medicine in IBD. Inflamm. Bowel Dis. 2022, 28, 1254–1264. [Google Scholar] [CrossRef]
- Steiner, C.A.; Berinstein, J.A.; Louissaint, J.; Higgins, P.D.R.; Spence, J.R.; Shannon, C.; Lu, C.; Stidham, R.W.; Fletcher, J.G.; Bruining, D.H.; et al. Biomarkers for the Prediction and Diagnosis of Fibrostenosing Crohn’s Disease: A Systematic Review. Clin. Gastroenterol. Hepatol. 2022, 20, 817–846.e10. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Seksik, P.; Cosnes, J. Complications and surgery in the inflammatory bowel diseases biological era. Curr. Opin. Gastroenterol. 2014, 30, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.A.; Horton, H.; Siegel, L.S.; Thompson, K.D.; Mackenzie, T.; Stewart, S.K.; Rice, P.W.; Stempak, J.M.; Dezfoli, S.; Haritunians, T.; et al. A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Aliment. Pharmacol. Ther. 2016, 43, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Kugathasan, S.; Denson, L.A.; Walters, T.D.; Kim, M.O.; Marigorta, U.M.; Schirmer, M.; Mondal, K.; Liu, C.; Griffiths, A.; Noe, J.D.; et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: A multicentre inception cohort study. Lancet 2017, 389, 1710–1718. [Google Scholar] [CrossRef]
- Biasci, D.; Lee, J.C.; Noor, N.M.; Pombal, D.R.; Hou, M.; Lewis, N.; Ahmad, T.; Hart, A.; Parkes, M.; McKinney, E.F.; et al. A blood-based prognostic biomarker in IBD. Gut 2019, 68, 1386–1395. [Google Scholar] [CrossRef]
- Yarur, A.J.; Strobel, S.G.; Deshpande, A.R.; Abreu, M.T. Predictors of aggressive inflammatory bowel disease. Gastroenterol. Hepatol. 2011, 7, 652–659. [Google Scholar]
- Ferrari, L.; Krane, M.K.; Fichera, A. Inflammatory bowel disease surgery in the biologic era. World J. Gastrointest. Surg. 2016, 8, 363–370. [Google Scholar] [CrossRef]
- Ghosh, S.; Panaccione, R. Anti-adhesion molecule therapy for inflammatory bowel disease. Therap. Adv. Gastroenterol. 2010, 3, 239–258. [Google Scholar] [CrossRef] [PubMed]
- de Buck van Overstraeten, A.; Wolthuis, A.; D’Hoore, A. Surgery for Crohn’s disease in the era of biologicals: A reduced need or delayed verdict? World J. Gastroenterol. 2012, 18, 3828–3832. [Google Scholar] [CrossRef]
- Tamilarasan, A.G.; Cunningham, G.; Irving, P.M.; Samaan, M.A. Recent advances in monoclonal antibody therapy in IBD: Practical issues. Frontline Gastroenterol. 2019, 10, 409–416. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Gecse, K.; Halfvarson, J.; Irving, P.M.; Jahnsen, J.; Peyrin-Biroulet, L.; Rogler, G.; Schreiber, S.; Danese, S. Clinical Practice of Adalimumab and Infliximab Biosimilar Treatment in Adult Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2021, 27, 106–122. [Google Scholar] [CrossRef]
- Danese, S.; Argollo, M.; Le Berre, C.; Peyrin-Biroulet, L. JAK selectivity for inflammatory bowel disease treatment: Does it clinically matter? Gut 2019, 68, 1893–1899. [Google Scholar] [CrossRef]
- Geremia, A.; Arancibia-Carcamo, C.V.; Fleming, M.P.; Rust, N.; Singh, B.; Mortensen, N.J.; Travis, S.P.; Powrie, F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011, 208, 1127–1133. [Google Scholar] [CrossRef]
- Park, S.C.; Jeen, Y.T. Anti-integrin therapy for inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 1868–1880. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Colombel, J.F.; Ungaro, R. Review article: Predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2018, 47, 896–905. [Google Scholar] [CrossRef]
- Ergen, E.N.; Yusuf, N. Inhibition of interleukin-12 and/or interleukin-23 for the treatment of psoriasis: What is the evidence for an effect on malignancy? Exp. Dermatol. 2018, 27, 737–747. [Google Scholar] [CrossRef]
- Parigi, T.L.; Iacucci, M.; Ghosh, S. Blockade of IL-23: What is in the Pipeline? J. Crohns Colitis 2022, 16 (Suppl. S2), ii64–ii72. [Google Scholar] [CrossRef]
- Vermeire, S.; Schreiber, S.; Petryka, R.; Kuehbacher, T.; Hebuterne, X.; Roblin, X.; Klopocka, M.; Goldis, A.; Wisniewska-Jarosinska, M.; Baranovsky, A.; et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): Results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017, 389, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Kirchgesner, J.; Desai, R.J.; Schneeweiss, M.C.; Beaugerie, L.; Schneeweiss, S.; Kim, S.C. Decreased risk of treatment failure with vedolizumab and thiopurines combined compared with vedolizumab monotherapy in Crohn’s disease. Gut 2022, 71, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Ho, E.Y.; Shmidt, E.; Singh, H.; Falck-Ytter, Y.; Sultan, S.; Terdiman, J.P. AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021, 160, 2496–2508. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.; Panaccione, R.; Baert, F.; Bossuyt, P.; Colombel, J.F.; Danese, S.; Dubinsky, M.; Feagan, B.G.; Hisamatsu, T.; Lim, A.; et al. Risankizumab as induction therapy for Crohn’s disease: Results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet 2022, 399, 2015–2030. [Google Scholar] [CrossRef]
- Ferrante, M.; Panaccione, R.; Baert, F.; Bossuyt, P.; Colombel, J.F.; Danese, S.; Dubinsky, M.; Feagan, B.G.; Hisamatsu, T.; Lim, A.; et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: Results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet 2022, 399, 2031–2046. [Google Scholar] [CrossRef]
- AbbVie. Study Comparing Intravenous (IV)/Subcutaneous (SC) Risankizumab to IV/SC Ustekinumab to Assess Change in Crohn’s Disease Activity Index (CDAI) in Adult Participants with Moderate to Severe Crohn’s Disease (CD) (SEQUENCE). 2022. Available online: https://clinicaltrials.gov/study/NCT04524611 (accessed on 5 August 2023).
- Sands, B.E.; Peyrin-Biroulet, L.; Kierkus, J.; Higgins, P.D.R.; Fischer, M.; Jairath, V.; Hirai, F.; D’Haens, G.; Belin, R.M.; Miller, D.; et al. Efficacy and Safety of Mirikizumab in a Randomized Phase 2 Study of Patients with Crohn’s Disease. Gastroenterology 2022, 162, 495–508. [Google Scholar] [CrossRef]
- Lilly, E. A Study of Mirikizumab (LY3074828) in Participants with Crohn’s Disease (VIVID-1). 2023. Available online: https://clinicaltrials.gov/study/NCT03926130 (accessed on 5 August 2023).
- Lilly, E. A Long-term Extension Study of Mirikizumab (LY3074828) in Participants with Crohn’s Disease (VIVID-2). 2023. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04232553 (accessed on 5 August 2023).
- Sands, B.E.; Chen, J.; Feagan, B.G.; Penney, M.; Rees, W.A.; Danese, S.; Higgins, P.D.R.; Newbold, P.; Faggioni, R.; Patra, K.; et al. Efficacy and Safety of MEDI2070, an Antibody Against Interleukin 23, in Patients with Moderate to Severe Crohn’s Disease: A Phase 2a Study. Gastroenterology 2017, 153, 77–86.e76. [Google Scholar] [CrossRef]
- AstraZeneca. An Active and Placebo-Controlled Study of Brazikumab in Participants with Moderately to Severely Active Crohn’s Disease (INTREPID). 2023. Available online: https://clinicaltrials.gov/study/NCT03759288 (accessed on 5 August 2023).
- AstraZeneca. Open-Label Extension Study of Brazikumab in Crohn’s Disease (INTREPID OLE). 2023. Available online: https://clinicaltrials.gov/study/NCT03961815 (accessed on 5 August 2023).
- Sandborn, W.J.; D’Haens, G.R.; Reinisch, W.; Panés, J.; Chan, D.; Gonzalez, S.; Weisel, K.; Germinaro, M.; Frustaci, M.E.; Yang, Z.; et al. Guselkumab for the Treatment of Crohn’s Disease: Induction Results From the Phase 2 GALAXI-1 Study. Gastroenterology 2022, 162, 1650–1664.e1658. [Google Scholar] [CrossRef]
- Janssen Research & Development, LLC. A Study of Guselkumab Subcutaneous Therapy in Participants with Moderately to Severely Active Crohn’s Disease (GRAVITI). 2022. Available online: https://clinicaltrials.gov/study/NCT05197049 (accessed on 5 August 2023).
- Janssen-Cilag-Ltd. A Study of Guselkumab in Participants with Fistulizing, Perianal Crohn’s Disease (FUZION CD). Available online: https://clinicaltrials.gov/study/NCT05347095 (accessed on 5 August 2023).
- Janssen Research & Development, LLC. A Study of Combination Therapy with Guselkumab and Golimumab in Participants with Moderately to Severely Active Crohn’s Disease (DUET-CD). Available online: https://clinicaltrials.gov/study/NCT05242471 (accessed on 5 August 2023).
- Feagan, B.G.; Sands, B.; Siegel, C.A.; Dubinsky, M.; Longman, R.; Sabinho, J.; Laurent, O.; Luo, A.; Lu, J.D.; Nguyen, D.; et al. DOP87 The Anti-TL1A Antibody PRA023 Demonstrated Proof-of-Concept in Crohn’s Disease: Phase 2a APOLLO-CD Study Results. J. Crohns Colitis 2023, 17 (Suppl. S1), i162–i164. [Google Scholar] [CrossRef]
- Teva Branded Pharmaceutical Products R&D, Inc. A Study to Test the Effect of TEV-48574 in Moderate to Severe Ulcerative Colitis or Crohn’s Disease (RELIEVE UCCD). 2023. Available online: https://clinicaltrials.gov/study/NCT05499130 (accessed on 5 August 2023).
- Teva Branded Pharmaceutical Products R&D, Inc. A Study to Evaluate the Long-Term Effect of TEV-48574 in Moderate to Severe Ulcerative Colitis or Crohn’s Disease. 2023. Available online: https://clinicaltrials.gov/study/NCT05668013 (accessed on 5 August 2023).
- Galapagos. Filgotinib in the Induction and Maintenance of Remission in Adults with Moderately to Severely Active Crohn’s Disease (DIVERSITY1). 2016. Available online: https://clinicaltrials.gov/study/NCT02914561 (accessed on 5 August 2023).
- Galapagos. Filgotinib in Long-Term Extension Study of Adults with Crohn’s Disease (DIVERSITYLTE). 2023. Available online: https://clinicaltrials.gov/study/NCT02914600 (accessed on 5 August 2023).
- Sandborn, W.J.; Feagan, B.G.; Loftus, E.V., Jr.; Peyrin-Biroulet, L.; Van Assche, G.; D’Haens, G.; Schreiber, S.; Colombel, J.F.; Lewis, J.D.; Ghosh, S.; et al. Efficacy and Safety of Upadacitinib in a Randomized Trial of Patients with Crohn’s Disease. Gastroenterology 2020, 158, 2123–2138.e8. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr.; Panés, J.; Lacerda, A.P.; Peyrin-Biroulet, L.; D’Haens, G.; Panaccione, R.; Reinisch, W.; Louis, E.; Chen, M.; Nakase, H.; et al. Upadacitinib Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2023, 388, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- AbbVie. Study to Assess Speed of Onset and Durability of Effectiveness of Upadacitinib in Adult Participants with Moderate to Severe Crohn’s Disease (CD) in Real World Clinical Practice. (UPlift). 2023. Available online: https://clinicaltrials.gov/study/NCT05930275 (accessed on 5 August 2023).
- Panés, J.; Sandborn, W.J.; Schreiber, S.; Sands, B.E.; Vermeire, S.; D’Haens, G.; Panaccione, R.; Higgins, P.D.R.; Colombel, J.F.; Feagan, B.G.; et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: Results of two phase IIb randomised placebo-controlled trials. Gut 2017, 66, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Squibb, B.-M. An Investigational Study of Experimental Medication BMS-986165 in Participants with Moderate to Severe Crohn’s Disease. Available online: https://clinicaltrials.gov/study/NCT03599622 (accessed on 31 July 2023).
- Feagan, B.G.; Sandborn, W.J.; Danese, S.; Wolf, D.C.; Liu, W.J.; Hua, S.Y.; Minton, N.; Olson, A.; D’Haens, G. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: A single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol. Hepatol. 2020, 5, 819–828. [Google Scholar] [CrossRef]
- Feagan, B.G.; Schreiber, S.; Afzali, A.; Rieder, F.; Hyams, J.; Kollengode, K.; Pearlman, J.; Son, V.; Marta, C.; Wolf, D.C.; et al. Ozanimod as a novel oral small molecule therapy for the treatment of Crohn’s disease: The YELLOWSTONE clinical trial program. Contemp. Clin. Trials 2022, 122, 106958. [Google Scholar] [CrossRef]
- D’Haens, G.; Dubinsky, M.C.; Peyrin-Biroulet, L.; Danese, S.; Sands, B.E.; Wolf, D.C.; Yarur, A.; Chiorean, M.; Dray, D.; Modesto, I.; et al. P632 Etrasimod induction therapy in moderately to severely active Crohn’s disease: Results from a phase 2, randomised, double-blind substudy. J. Crohns Colitis 2023, 17 (Suppl. S1), i764–i765. [Google Scholar] [CrossRef]
- Pfizer. A Study Evaluating the Efficacy and Safety of Oral Etrasimod in the Treatment of Adult Participants with Moderately to Severely Active Crohn’s Disease (CULTIVATE). 2023. Available online: https://clinicaltrials.gov/study/NCT04173273 (accessed on 5 August 2023).
- Cheng, F.; Huang, Z.; Wei, W.; Li, Z. Fecal microbiota transplantation for Crohn’s disease: A systematic review and meta-analysis. Tech. Coloproctol. 2021, 25, 495–504. [Google Scholar] [CrossRef]
- Yanai, H.; Levine, A.; Hirsch, A.; Boneh, R.S.; Kopylov, U.; Eran, H.B.; Cohen, N.A.; Ron, Y.; Goren, I.; Leibovitzh, H.; et al. The Crohn’s disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn’s disease (CDED-AD): An open-label, pilot, randomised trial. Lancet Gastroenterol. Hepatol. 2022, 7, 49–59. [Google Scholar] [CrossRef]
- Ponsioen, C.Y.; de Groof, E.J.; Eshuis, E.J.; Gardenbroek, T.J.; Bossuyt, P.M.M.; Hart, A.; Warusavitarne, J.; Buskens, C.J.; van Bodegraven, A.A.; Brink, M.A.; et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: A randomised controlled, open-label, multicentre trial. Lancet Gastroenterol. Hepatol. 2017, 2, 785–792. [Google Scholar] [CrossRef]
- Stevens, T.W.; Haasnoot, M.L.; D’Haens, G.R.; Buskens, C.J.; de Groof, E.J.; Eshuis, E.J.; Gardenbroek, T.J.; Mol, B.; Stokkers, P.C.F.; Bemelman, W.A.; et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: Retrospective long-term follow-up of the LIR!C trial. Lancet Gastroenterol Hepatol 2020, 5, 900–907. [Google Scholar] [CrossRef]
- Ledder, O. The Question That Doesn’t Seem to Go Away: Cancer Risk of Anti-TNF Therapy. Dig. Dis. Sci. 2022, 67, 6–7. [Google Scholar] [CrossRef]
- Minnis-Lyons, S.E.; Aiken, Z.; Chow, S.; Din, S. Managing IBD in patients with previous cancers. Frontline Gastroenterol. 2022, 13, e44–e50. [Google Scholar] [CrossRef]
- Sattler, L.; Hanauer, S.B.; Malter, L. Immunomodulatory Agents for Treatment of Patients with Inflammatory Bowel Disease (Review safety of anti-TNF, Anti-Integrin, Anti IL-12/23, JAK Inhibition, Sphingosine 1-Phosphate Receptor Modulator, Azathioprine/6-MP and Methotrexate). J. Crohn’s Colitis 2021, 15, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; D’Amico, F.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. TNF Inhibitors and Risk of Malignancy in Patients with Inflammatory Bowel Diseases: A Systematic Review. J. Crohn’s Colitis. 2021, 15, 840–859. [Google Scholar] [CrossRef]
- Poullenot, F.; Laharie, D. Management of Inflammatory Bowel Disease in Patients with Current or Past Malignancy. Cancers 2023, 15, 1083. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Panaccione, R.; Bossuyt, P.; Lukas, M.; Baert, F.; Vanasek, T.; Danalioglu, A.; Novacek, G.; Armuzzi, A.; Hebuterne, X.; et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet 2017, 390, 2779–2789. [Google Scholar] [CrossRef]
- Kishi, M.; Hirai, F.; Takatsu, N.; Hisabe, T.; Takada, Y.; Beppu, T.; Takeuchi, K.; Naganuma, M.; Ohtsuka, K.; Watanabe, K.; et al. A review on the current status and definitions of activity indices in inflammatory bowel disease: How to use indices for precise evaluation. J. Gastroenterol. 2022, 57, 246–266. [Google Scholar] [CrossRef]
- Enns, R.A.; Hookey, L.; Armstrong, D.; Bernstein, C.N.; Heitman, S.J.; Teshima, C.; Leontiadis, G.I.; Tse, F.; Sadowski, D. Clinical Practice Guidelines for the Use of Video Capsule Endoscopy. Gastroenterology 2017, 152, 497–514. [Google Scholar] [CrossRef]
- Benitez, J.M.; Meuwis, M.A.; Reenaers, C.; Van Kemseke, C.; Meunier, P.; Louis, E. Role of endoscopy, cross-sectional imaging and biomarkers in Crohn’s disease monitoring. Gut 2013, 62, 1806–1816. [Google Scholar] [CrossRef]
- Carvello, M.; D’Hoore, A.; Maroli, A.; Cuenca, C.; Vermeire, S.; Danese, S.; Bislenghi, G.; Spinelli, A. Postoperative Complications Are Associated with Early and Increased Rate of Disease Recurrence After Surgery for Crohn’s Disease. Dis. Colon Rectum 2022, 66, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhu, Y.; Xu, Y.; Cao, L.; Li, Y.; Gong, J.; Wang, Z.; Zhu, W. Endoscopic Evaluation at 1 Month After Ileocolic Resection for Crohn’s Disease Predicts Future Postoperative Recurrence and Is Safe. Dis. Colon Rectum 2022, 65, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Panes, J.; Jairath, V.; Levesque, B.G. Advances in Use of Endoscopy, Radiology, and Biomarkers to Monitor Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 362–373.e3. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Yang, S.K.; Park, S.H.; Lee, H.S.; Boo, S.J.; Park, J.H.; Na, S.Y.; Jung, K.W.; Kim, K.J.; Ye, B.D.; et al. Usefulness of C-reactive protein as a disease activity marker in Crohn’s disease according to the location of disease. Gut Liver 2015, 9, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Ordas, I.; Feagan, B.G.; Sandborn, W.J. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2012, 10, 1079–1087, quiz e85–86. [Google Scholar] [CrossRef]
- Papay, P.; Ignjatovic, A.; Karmiris, K.; Amarante, H.; Milheller, P.; Feagan, B.; D’Haens, G.; Marteau, P.; Reinisch, W.; Sturm, A.; et al. Optimising monitoring in the management of Crohn’s disease: A physician’s perspective. J. Crohn’s Colitis 2013, 7, 653–669. [Google Scholar] [CrossRef]
- Li, C.; Kuemmerle, J.F. Mechanisms that mediate the development of fibrosis in patients with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1250–1258. [Google Scholar] [CrossRef]
- Reese, G.E.; Purkayastha, S.; Tilney, H.S.; von Roon, A.; Yamamoto, T.; Tekkis, P.P. Strictureplasty vs resection in small bowel Crohn’s disease: An evaluation of short-term outcomes and recurrence. Color. Dis. 2007, 9, 686–694. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Brun, L.; Ballabeni, P.; Pittet, V.; Prinz Vavricka, B.M.; Zeitz, J.; Rogler, G.; Schoepfer, A.M. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am. J. Gastroenterol. 2011, 106, 110–119. [Google Scholar] [CrossRef]
- Antonelli, E.; Bassotti, G.; Tramontana, M.; Hansel, K.; Stingeni, L.; Ardizzone, S.; Genovese, G.; Marzano, A.V.; Maconi, G. Dermatological Manifestations in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 364. [Google Scholar] [CrossRef]
- Ungureanu, L.; Cosgarea, R.; Alexandru Badea, M.; Florentina Vasilovici, A.; Cosgarea, I.; Corina Senila, S. Cutaneous manifestations in inflammatory bowel disease (Review). Exp. Ther. Med. 2020, 20, 31–37. [Google Scholar] [CrossRef]
- Goyal, P.; Nijhawan, S.; Nijhawan, M.; Agrawal, S. Refractory cutaneous Crohn’s disease of the external genitalia in a female. Indian J. Sex Transm. Dis. AIDS 2020, 41, 110–113. [Google Scholar] [CrossRef]
- Marzano, A.V.; Borghi, A.; Stadnicki, A.; Crosti, C.; Cugno, M. Cutaneous manifestations in patients with inflammatory bowel diseases: Pathophysiology, clinical features, and therapy. Inflamm. Bowel Dis. 2014, 20, 213–227. [Google Scholar] [CrossRef]
- Patel, B.M.; Ramos Rivers, C.; Koutroumpakis, F.; Ahsan, M.; Dueker, J.; Hashash, J.; Johnston, E.; Barrie, A.; Harrison, J.; Schwartz, M.; et al. Ustekinumab-Induced Remission of Two Cases of Refractory Cutaneous Crohn’s Disease. Inflamm. Bowel Dis. 2021, 27, e124. [Google Scholar] [CrossRef]
- Wu, J.; Smogorzewski, J. Ustekinumab for the treatment of paradoxical skin reactions and cutaneous manifestations of inflammatory bowel diseases. Dermatol. Ther. 2021, 34, e14883. [Google Scholar] [CrossRef]
- Freeman, H.J. Natural history and clinical behavior of Crohn’s disease extending beyond two decades. J. Clin. Gastroenterol. 2003, 37, 216–219. [Google Scholar] [CrossRef]
- Schmoyer, C.J.; Saidman, J.; Bohl, J.L.; Bierly, C.L.; Kuemmerle, J.F.; Bickston, S.J. The Pathogenesis and Clinical Management of Stricturing Crohn Disease. Inflamm. Bowel Dis. 2021, 27, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lago, I.; Hoyo, J.D.; Perez-Girbes, A.; Garrido-Marin, A.; Casanova, M.J.; Chaparro, M.; Fernandez-Clotet, A.; Castro-Poceiro, J.; Garcia, M.J.; Sanchez, S.; et al. Early treatment with anti-tumor necrosis factor agents improves long-term effectiveness in symptomatic stricturing Crohn’s disease. United Eur. Gastroenterol. J. 2020, 8, 1056–1066. [Google Scholar] [CrossRef]
- Vuyyuru, S.K.; Kante, B.; Kumar, P.; Sahu, P.; Kedia, S.; Ranjan, M.K.; Sharma, R.; Panwar, R.; Makharia, G.; Ahuja, V. Real world analysis on the efficacy and safety of anti-tumor necrosis factor therapy in patients with stricturing Crohn’s disease. Sci. Rep. 2021, 11, 11704. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Carbonnel, F.; Laharie, D.; Stefanescu, C.; Hebuterne, X.; Abitbol, V.; Nachury, M.; Brixi, H.; Bourreille, A.; Picon, L.; et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: A multicentre, prospective, observational cohort (CREOLE) study. Gut 2018, 67, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Schulberg, J.D.; Wright, E.K.; Holt, B.A.; Hamilton, A.L.; Sutherland, T.R.; Ross, A.L.; Vogrin, S.; Miller, A.M.; Connell, W.C.; Lust, M.; et al. Intensive drug therapy versus standard drug therapy for symptomatic intestinal Crohn’s disease strictures (STRIDENT): An open-label, single-centre, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 318–331. [Google Scholar] [CrossRef]
- Paine, E.; Shen, B. Endoscopic therapy in inflammatory bowel diseases (with videos). Gastrointest. Endosc. 2013, 78, 819–835. [Google Scholar] [CrossRef]
- Bettenworth, D.; Gustavsson, A.; Atreja, A.; Lopez, R.; Tysk, C.; van Assche, G.; Rieder, F. A Pooled Analysis of Efficacy, Safety, and Long-term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Lourdusamy, V.; Njei, B.; Shen, B. Endoscopic balloon dilation in the management of strictures in Crohn’s disease: A systematic review and meta-analysis of non-randomized trials. Surg. Endosc. 2016, 30, 5434–5443. [Google Scholar] [CrossRef]
- Shen, B.; Kochhar, G.S.; Navaneethan, U.; Cross, R.K.; Farraye, F.A.; Iacucci, M.; Schwartz, D.A.; Gonzalez-Lama, Y.; Schairer, J.; Kiran, R.P.; et al. Endoscopic evaluation of surgically altered bowel in inflammatory bowel disease: A consensus guideline from the Global Interventional Inflammatory Bowel Disease Group. Lancet Gastroenterol. Hepatol. 2021, 6, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Reutemann, B.A.; Turkeltaub, J.A.; Al-Hawary, M.; Waljee, A.K.; Higgins, P.D.R.; Stidham, R.W. Endoscopic Balloon Dilation Size and Avoidance of Surgery in Stricturing Crohn’s Disease. Inflamm. Bowel Dis. 2017, 23, 1803–1809. [Google Scholar] [CrossRef]
- Lan, N.; Stocchi, L.; Ashburn, J.H.; Hull, T.L.; Steele, S.R.; Delaney, C.P.; Shen, B. Outcomes of Endoscopic Balloon Dilation vs Surgical Resection for Primary Ileocolic Strictures in Patients with Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Stidham, R.W.; Guentner, A.S.; Ruma, J.L.; Govani, S.M.; Waljee, A.K.; Higgins, P.D. Intestinal Dilation and Platelet:Albumin Ratio Are Predictors of Surgery in Stricturing Small Bowel Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 1112–1119.e2. [Google Scholar] [CrossRef]
- Kienle, P. Impact of Modern Drug Therapy on Surgery: Crohn’s Disease. Visc. Med. 2018, 34, 422–425. [Google Scholar] [CrossRef]
- Maggiori, L.; Panis, Y. Surgical management of IBD—From an open to a laparoscopic approach. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 297–306. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Geboes, K.; Vantrappen, G.; Beyls, J.; Kerremans, R.; Hiele, M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990, 99, 956–963. [Google Scholar] [CrossRef]
- Lian, L.; Stocchi, L.; Remzi, F.H.; Shen, B. Comparison of Endoscopic Dilation vs. Surgery for Anastomotic Stricture in Patients with Crohn’s Disease Following Ileocolonic Resection. Clin. Gastroenterol. Hepatol. 2017, 15, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Bobanga, I.D.; Bai, S.; Swanson, M.A.; Champagne, B.J.; Reynolds, H.J.; Delaney, C.P.; Barksdale, E.M., Jr.; Stein, S.L. Factors influencing disease recurrence after ileocolic resection in adult and pediatric onset Crohn’s disease. Am. J. Surg. 2014, 208, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Kavalukas, S.L.; Scheurlen, K.M.; Galandiuk, S. State-of-the-art surgery for Crohn’s disease: Part I-small intestine/ileal disease. Langenbecks Arch. Surg. 2022, 407, 885–895. [Google Scholar] [CrossRef] [PubMed]


| Cutaneous Manifestation Category | Definition |
|---|---|
| 1 | Disease-specific lesions. Same histopathological findings as Crohn’s.
|
| 2 | Mucocutaneous lesions associated with Crohn’s. |
| 3 | Reactive (inflammatory) lesions that share a pathogenetic mechanism with Crohn’s disease, but not the same histopathology. |
| 4 | Drug-related mucocutaneous lesions secondary to Crohn’s treatment. |
| 5 | Cutaneous lesions secondary to nutritional absorption. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheurlen, K.M.; Parks, M.A.; Macleod, A.; Galandiuk, S. Unmet Challenges in Patients with Crohn’s Disease. J. Clin. Med. 2023, 12, 5595. https://doi.org/10.3390/jcm12175595
Scheurlen KM, Parks MA, Macleod A, Galandiuk S. Unmet Challenges in Patients with Crohn’s Disease. Journal of Clinical Medicine. 2023; 12(17):5595. https://doi.org/10.3390/jcm12175595
Chicago/Turabian StyleScheurlen, Katharina M, Mary A Parks, Anne Macleod, and Susan Galandiuk. 2023. "Unmet Challenges in Patients with Crohn’s Disease" Journal of Clinical Medicine 12, no. 17: 5595. https://doi.org/10.3390/jcm12175595
APA StyleScheurlen, K. M., Parks, M. A., Macleod, A., & Galandiuk, S. (2023). Unmet Challenges in Patients with Crohn’s Disease. Journal of Clinical Medicine, 12(17), 5595. https://doi.org/10.3390/jcm12175595







