Treatment and Prevention of Postoperative Leakage after Gastrectomy for Gastric Cancer
Abstract
:1. Introduction
2. Causes of Postoperative Leakage
3. Clinical Manifestation and Diagnosis of Postoperative Leakage
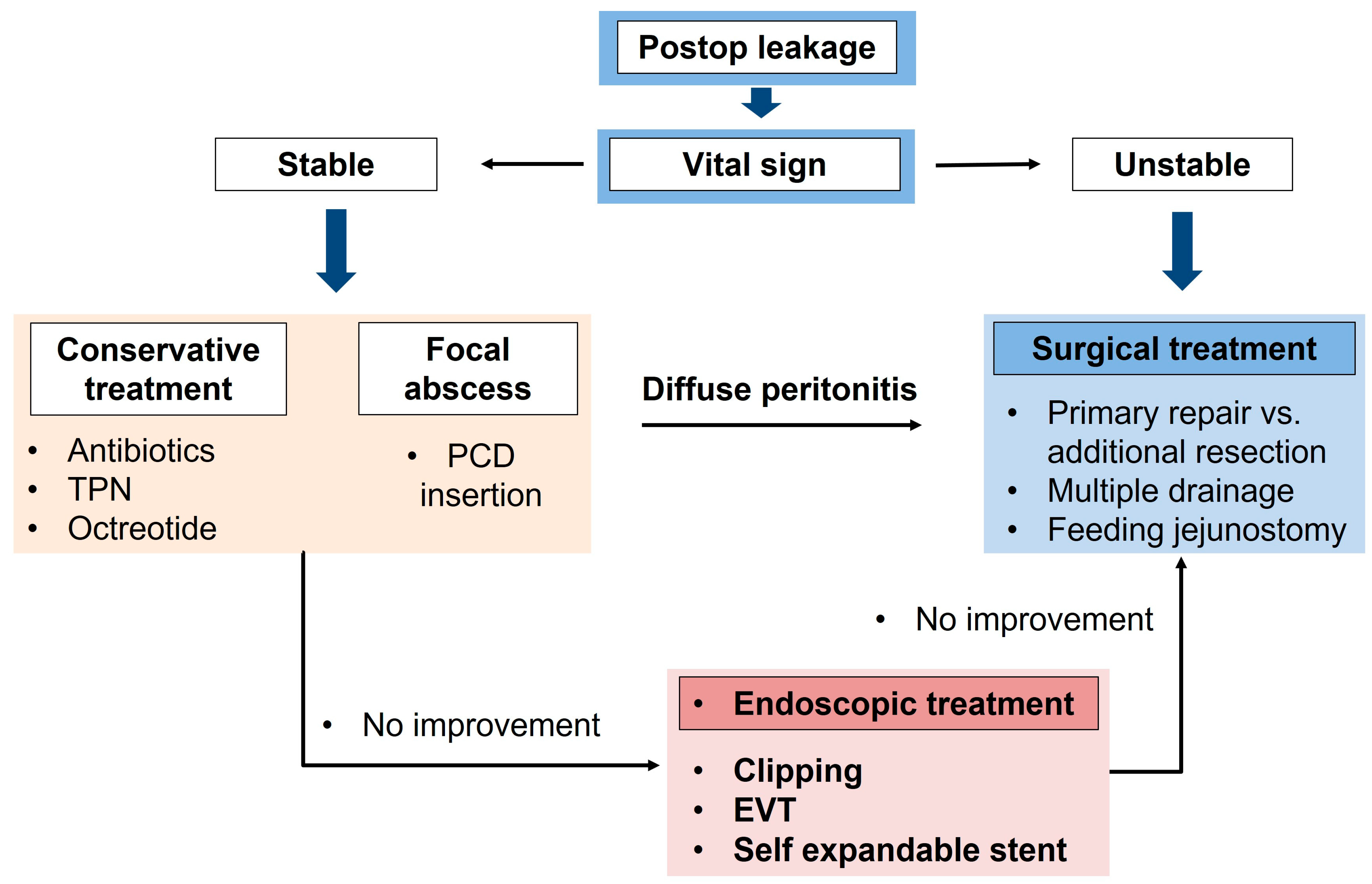
4. Basic Treatment Strategy for Postoperative Leakage
- Broad-spectrum antibiotic infusions should be initially conducted;
- Intestinal intraluminal stenting is one option for invasive management [28,30]. If nonoperative interventions do not induce successful results for leakage management, the patient has an unstable hemodynamic status, or peritonitis occurs in the whole abdomen due to leakage, the patient should undergo an emergency operation;
5. Endoscopic Treatment of Postoperative Leakage
- Sealant and fibrin glue can be sprayed or injected using an endoscope after the formation of the fistula tract, rather than immediately after the leakage occurs. According to Rbabgo et al., a closure rate of approximately 86.6% without complications was reported [59].
- Endosuturing also showed its utility in managing chronic fistulae. Similar instruments such as over-the-scope clips (OTSC) demonstrated positive effects on gastrocutaneous fistulae and gastric leaks in several studies [58,59,60,61]. According to a systematic review, a 100% success rate was reported when applying leakage within one week, but <60% over time [62,63].
- Endoscopic stenting is a viable treatment option for leaks at esophagojejunostomy sites following gastrectomy or prosthetic gastrectomy [64,65]. Puli et al. [66] conducted a meta-analysis on self-expandable metallic stents (SEMS) and self-expanding plastic stents (SEPS) and reported a leak closure rate of approximately 87.8% when the stents were removed 4 to 8 weeks after insertion. It should be noted that partially covered SEMS carry a risk of tissue in-growth complicating the removal process [67].
- EVAC (endoscopic vacuum-assisted closure) was initially introduced for anastomotic leaks after rectal surgery and was also applied after upper gastrointestinal (UGI) surgery [68]. Recent studies showed its effectiveness, particularly in esophageal cancer, and ongoing attempts are being made to explore its use after gastric cancer surgery [69,70].
6. Factors Associated with Postoperative Leakage
- Location of leakage site: Anastomotic leakage vs. duodenal stump leakage
- 2.
- Existence of surgical drainage or percutaneous drainage
- 3.
- Remaining omentum
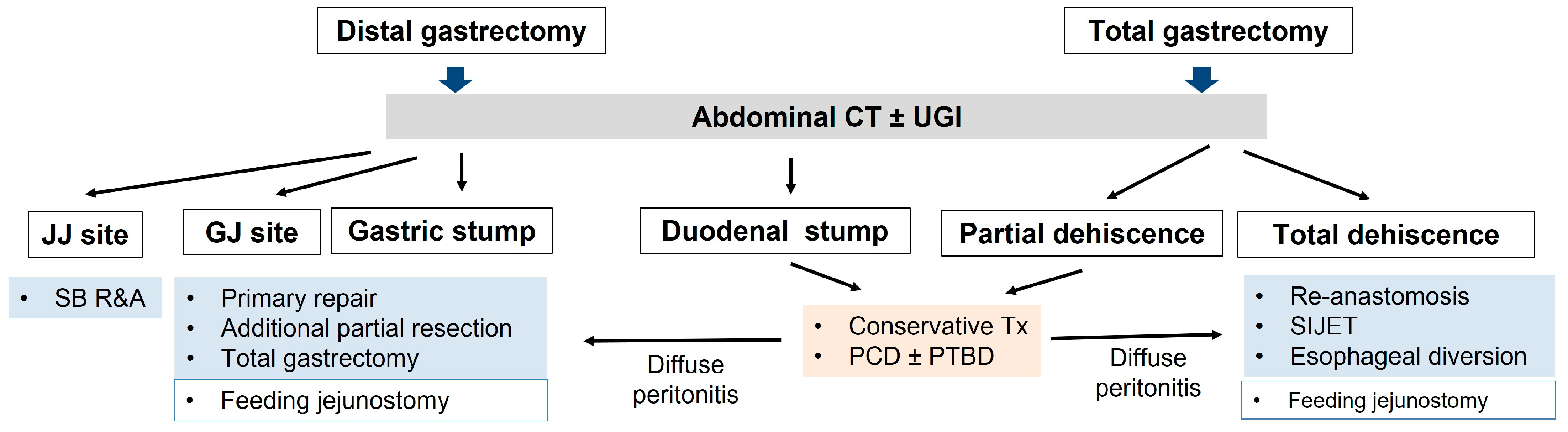
7. Surgical Treatment Strategy in Patients with Postoperative Leakage
- Basic surgical treatment strategy
- ①
- ②
- ③
- ④
- Insertion of multiple surgical drains in the dependent position during reoperation: when performing the reoperation, sufficient drainage of discharge is important even in cases with the recurrence of leakage.
- ①
- Diffuse peritonitis signs with the abrupt onset of peritonitis symptoms
- Bursting of the anastomotic site;
- Expectation of persistent peritonitis due to food content spillage.
- ②
- Gastrojejunostomy site leakage is more likely to require reoperation than duodenal stump leakage
- ③
- Total dehiscence is more likely to need reoperation than partial dehiscence at the esophagojejunostomy anastomotic site
- 3.
- Surgical treatment after distal gastrectomy
- ①
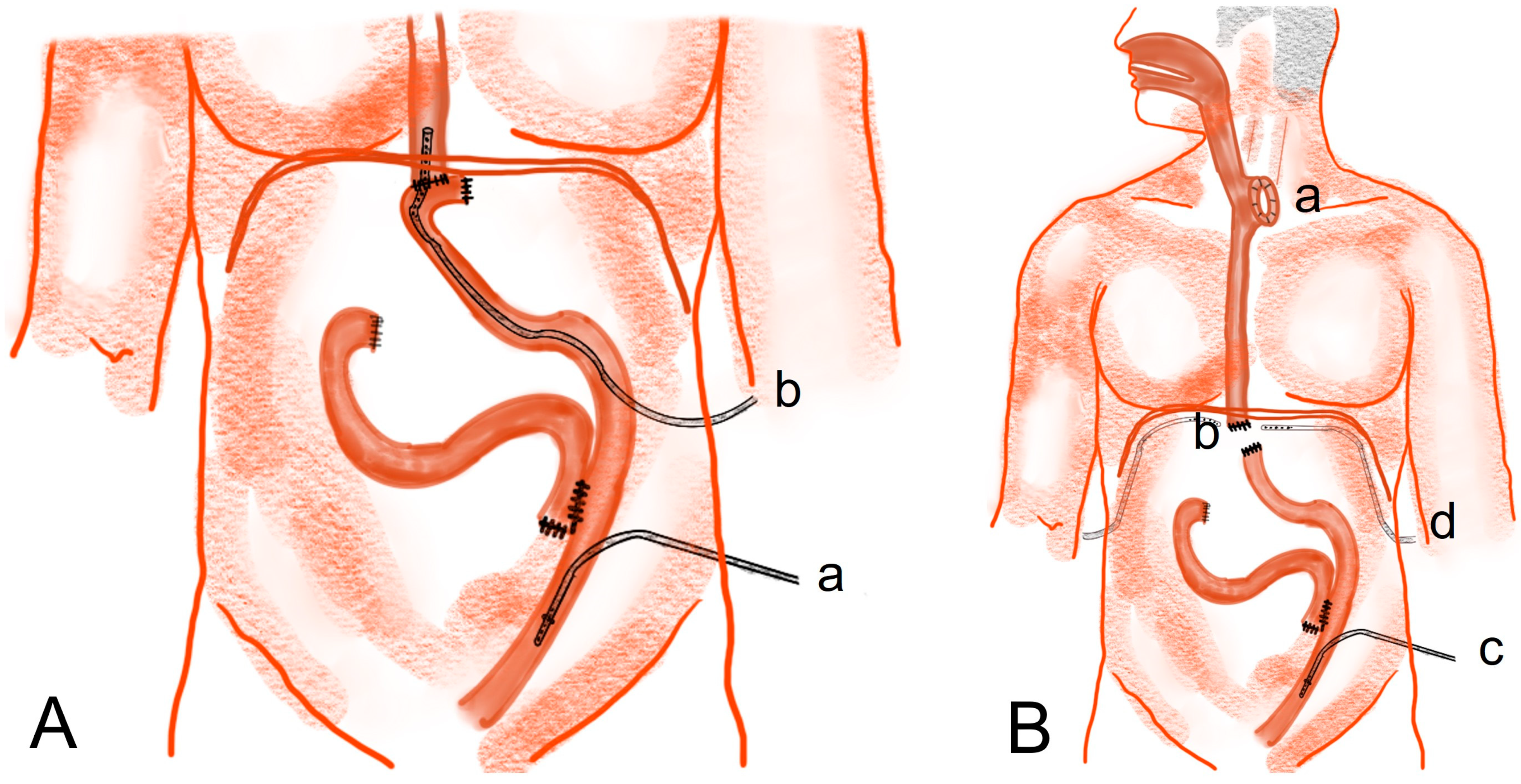
- Rupturing of the stapler common entry hole at the gastrojejunostomy site (Figure 3A(b)): In general, when there is rupturing around the gastrojejunostomy site, the edema or inflammation in the surrounding tissues is severe, and so, proper debridement should be performed before closure. If the inflammation is severe, a new gastrojejunostomy is recommended after resection of the previous anastomosis. If the remnant stomach is too small, total gastrectomy should be performed if necessary.
- ②
- Leakage at the gastric stump site (Figure 3A(c)): In most cases, ischemic changes due to a decrease in blood supply are likely to be the cause of gastric stump leakage. Therefore, it is recommended to perform reresection of the gastric stump at the remnant stomach.
- ③
- Leakage at the jejunojejunostomy site (Figure 3A(d)): In most cases, inflammation occurred, so it is necessary to perform resection and jejunojejunostomy reanastomosis rather than primary repair, which can cause leakage again after reoperation. Additional procedures recommended during reoperation are feeding jejunostomy and the insertion of multiple surgical drains.
- 4.
- Surgical treatment for complete dehiscence of the esophagojejunostomy site after total gastrectomy
- ①
- Re-anastomosis with feeding jejunostomy
- ②
- Insertion of a continuous suction isoperistaltic jejuno-esophagostomy tube (SIJET)
- ③
- Esophageal diversion and clamping of the distal esophagus with feeding jejunostomy (esophageal exclusion)
8. Basic Strategy for the Prevention of Postoperative Leakage
- Preservation of sufficient blood circulation: Resection of the remnant stomach with proper blood supply is needed. In cases of total gastrectomy, the preservation of mesentery vessels around the Roux limb is essential (Figure 7A).
- Reduction in tension at the anastomotic site: If the length of the Roux limp is increased by cutting the mesentery vessel, the tension at the anastomotic site can be reduced (Figure 7B).
- Stapling failure: When dissecting a stomach or jejunum with a stapler, it is important to choose a stapler with an appropriate size and height. When dissecting very thick tissues or organs, the largest stapler should be selected [34].
- Failure of suturing: When suturing using threads at the common channel of the stapler entry site, breakdown of the suture material may occur. In the case of barbed suture threads, the thickness and strength are reduced compared to those of general threads, so it is better to select a thread that is thicker than the general thread. The author of this paper commonly uses a barbed thread to close the stapler common entry hole at the gastrojejunostomy site. Previously, the author performed single-layer suturing using 3-0 barbed thread, but the author recently implemented double-layer suturing using 2-0 barbed thread.
- Prevention of gastric stasis: If gastric stasis occurs after distal gastrectomy, an increase in pressure around the anastomosis can affect the occurrence of leakage at the anastomotic site. Therefore, an anastomosis method that does not cause gastric stasis should be selected. In addition, the diet protocol changes as the patient are taught to eat a small amount or dominant liquid diet immediately after gastrectomy.
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gotoda, T.; Yanagisawa, A.; Sasako, M.; Ono, H.; Nakanishi, Y.; Shimoda, T.; Kato, Y. Incidence of lymph node metastasis from early gastric cancer: Estimation with a large number of cases at two large centers. Gastric Cancer 2000, 3, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Kim, I.H.; Kang, S.J.; Choi, M.; Kim, B.H.; Eom, B.W.; Kim, B.J.; Min, B.H.; Choi, C.I.; Shin, C.M.; et al. Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach. J. Gastric Cancer 2023, 23, 3–106. [Google Scholar] [CrossRef]
- Hahn, K.Y.; Park, C.H.; Lee, Y.K.; Chung, H.; Park, J.C.; Shin, S.K.; Lee, Y.C.; Kim, H.I.; Cheong, J.H.; Hyung, W.J.; et al. Comparative study between endoscopic submucosal dissection and surgery in patients with early gastric cancer. Surg. Endosc. 2018, 32, 73–86. [Google Scholar] [CrossRef]
- Kim, D.Y.; Hong, S.J.; Cho, G.S.; Jeong, G.A.; Kim, H.K.; Han, J.P.; Lee, Y.N.; Ko, B.M.; Lee, M.S. Long-term efficacy of endoscopic submucosal dissection compared with surgery for early gastric cancer: A retrospective cohort study. Gut Liver 2014, 8, 519–525. [Google Scholar] [CrossRef]
- Choi, K.S.; Jung, H.Y.; Choi, K.D.; Lee, G.H.; Song, H.J.; Kim, D.H.; Lee, J.H.; Kim, M.Y.; Kim, B.S.; Oh, S.T.; et al. EMR versus gastrectomy for intramucosal gastric cancer: Comparison of long-term outcomes. Gastrointest. Endosc. 2011, 73, 942–948. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, Y.A.; Kim, C.G.; Ryu, K.W.; Kim, Y.W.; Sim, J.A.; Yun, Y.H.; Choi, I.J. Serial intermediate-term quality of life comparison after endoscopic submucosal dissection versus surgery in early gastric cancer patients. Surg. Endosc. 2018, 32, 2114–2122. [Google Scholar] [CrossRef]
- Eom, S.S.; Choi, W.; Eom, B.W.; Park, S.H.; Kim, S.J.; Kim, Y.I.; Yoon, H.M.; Lee, J.Y.; Kim, C.G.; Kim, H.K.; et al. A Comprehensive and Comparative Review of Global Gastric Cancer Treatment Guidelines. J. Gastric Cancer 2022, 22, 3–23. [Google Scholar] [CrossRef]
- Association, J.G.C. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.H.; Zhang, X.T.; Li, Y.F.; Tang, L.; Qu, X.J.; Ying, J.E.; Zhang, J.; Sun, L.Y.; Lin, R.B.; Qiu, H.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021, 41, 747–795. [Google Scholar] [CrossRef]
- Papenfuss, W.A.; Kukar, M.; Oxenberg, J.; Attwood, K.; Nurkin, S.; Malhotra, U.; Wilkinson, N.W. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann. Surg. Oncol. 2014, 21, 3008–3014. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.; Ashley, M. Postgastrectomy Complications; David, I., Soybel, M., Eds.; UpToDate: Waltham, MA, USA, 2022. [Google Scholar]
- Gouzi, J.L.; Huguier, M.; Fagniez, P.L.; Launois, B.; Flamant, Y.; Lacaine, F.; Paquet, J.C.; Hay, J.M. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann. Surg. 1989, 209, 162–166. [Google Scholar] [CrossRef]
- Seufert, R.M.; Schmidt-Matthiesen, A.; Beyer, A. Total gastrectomy and oesophagojejunostomy—A prospective randomized trial of hand-sutured versus mechanically stapled anastomoses. Br. J. Surg. 1990, 77, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Stillman, M.D.; Yoon, S.S. Open and minimally invasive gastrectomy in Eastern and Western patient populations: A review of the literature and reasons for differences in outcomes. J. Surg. Oncol. 2022, 126, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Association, I.C.o.t.K.G.C. Korean Gastric Cancer Association-Led Nationwide Survey on Surgically Treated Gastric Cancers in 2019. J. Gastric Cancer 2021, 21, 221–235. [Google Scholar] [CrossRef]
- Costantino, C.L.; Mullen, J.T. Minimally Invasive Gastric Cancer Surgery. Surg. Oncol. Clin. N. Am. 2019, 28, 201–213. [Google Scholar] [CrossRef]
- Kostakis, I.D.; Alexandrou, A.; Armeni, E.; Damaskos, C.; Kouraklis, G.; Diamantis, T.; Tsigris, C. Comparison Between Minimally Invasive and Open Gastrectomy for Gastric Cancer in Europe: A Systematic Review and Meta-analysis. Scand. J. Surg. 2017, 106, 3–20. [Google Scholar] [CrossRef]
- Shibao, K.; Honda, S.; Adachi, Y.; Kohi, S.; Kudou, Y.; Matayoshi, N.; Sato, N.; Hirata, K. An advanced bipolar device helps reduce the rate of postoperative pancreatic fistula in laparoscopic gastrectomy for gastric cancer patients: A propensity score-matched analysis. Langenbecks Arch. Surg. 2022, 407, 3479–3486. [Google Scholar] [CrossRef]
- Hong, J.; Wang, Y.P.; Wang, J.; Hua, L.C.; Hao, H.K. The safety and feasibility of intra-corporeal gastroduodenostomy using a self-pulling and latter transected method (Delta SPLT) in totally laparoscopic distal gastrectomy. Jl. Surg. Oncol. 2021, 123 (Suppl. 1), S25–S29. [Google Scholar] [CrossRef]
- Washio, M.; Yamashita, K.; Niihara, M.; Hosoda, K.; Hiki, N. Postoperative pancreatic fistula after gastrectomy for gastric cancer. Ann. Gastroenterol. Surg. 2020, 4, 618–627. [Google Scholar] [CrossRef]
- Wang, S.Y.; Hong, J.; Hao, H.K. A comparative study of delta-shaped and conventional Billroth I anastomosis after laparoscopic distal gastrectomy for gastric cancer. Surg. Endosc. 2017, 31, 3191–3202. [Google Scholar] [CrossRef] [PubMed]
- Umemura, A.; Koeda, K.; Sasaki, A.; Fujiwara, H.; Kimura, Y.; Iwaya, T.; Akiyama, Y.; Wakabayashi, G. Totally laparoscopic total gastrectomy for gastric cancer: Literature review and comparison of the procedure of esophagojejunostomy. Asian J. Surg. 2015, 38, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Sasako, M.; Katai, H.; Sano, T.; Maruyama, K. Decreasing complication rates with stapled esophagojejunostomy following a learning curve. Gastric Cancer 2000, 3, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schardey, J.; von Ahnen, T.; Schardey, E.; Kappenberger, A.; Zimmermann, P.; Kühn, F.; Andrassy, J.; Werner, J.; Arbogast, H.; Wirth, U. Antibiotic Bowel Decontamination in Gastrointestinal Surgery—A Single-Center 20 Years’ Experience. Front. Surg. 2022, 9, 874223. [Google Scholar] [CrossRef] [PubMed]
- Roos, D.; Dijksman, L.M.; Tijssen, J.G.; Gouma, D.J.; Gerhards, M.F.; Oudemans-van Straaten, H.M. Systematic review of perioperative selective decontamination of the digestive tract in elective gastrointestinal surgery. Br. J. Surg. 2013, 100, 1579–1588. [Google Scholar] [CrossRef]
- Schardey, H.M.; Joosten, U.; Finke, U.; Staubach, K.H.; Schauer, R.; Heiss, A.; Kooistra, A.; Rau, H.G.; Nibler, R.; Lüdeling, S.; et al. The prevention of anastomotic leakage after total gastrectomy with local decontamination. A prospective, randomized, double-blind, placebo-controlled multicenter trial. Ann. Surg. 1997, 225, 172–180. [Google Scholar] [CrossRef]
- Kähler, G. Anastomotic Leakage after Upper Gastrointestinal Surgery: Endoscopic Treatment. Visc. Med. 2017, 33, 202–206. [Google Scholar] [CrossRef]
- Virgilio, E.; Ceci, D.; Cavallini, M. Surgical Endoscopic Vacuum-assisted Closure Therapy (EVAC) in Treating Anastomotic Leakages After Major Resective Surgery of Esophageal and Gastric Cancer. Anticancer Res. 2018, 38, 5581–5587. [Google Scholar] [CrossRef]
- Liesenfeld, L.F.; Schmidt, T.; Zhang-Hagenlocher, C.; Sauer, P.; Diener, M.K.; Müller-Stich, B.P.; Hackert, T.; Büchler, M.W.; Schaible, A. Self-Expanding Metal Stents for Anastomotic Leaks After Upper Gastrointestinal Cancer Surgery. J. Surg. Res. 2021, 267, 516–526. [Google Scholar] [CrossRef]
- Turrentine, F.E.; Denlinger, C.E.; Simpson, V.B.; Garwood, R.A.; Guerlain, S.; Agrawal, A.; Friel, C.M.; LaPar, D.J.; Stukenborg, G.J.; Jones, R.S. Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J. Am. Coll. Surg. 2015, 220, 195–206. [Google Scholar] [CrossRef]
- Roh, C.K.; Choi, S.; Seo, W.J.; Cho, M.; Kim, H.I.; Lee, S.K.; Lim, J.S.; Hyung, W.J. Incidence and treatment outcomes of leakage after gastrectomy for gastric cancer: Experience of 14,075 patients from a large volume centre. Eur. J. Surg. Oncol. 2021, 47, 2304–2312. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, Y.; Fukagawa, T.; Morita, S.; Ohashi, M.; Saka, M.; Katai, H. Identification of risk factors for esophagojejunal anastomotic leakage after gastric surgery. World J. Surg. 2012, 36, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Migita, K.; Takayama, T.; Matsumoto, S.; Wakatsuki, K.; Enomoto, K.; Tanaka, T.; Ito, M.; Nakajima, Y. Risk factors for esophagojejunal anastomotic leakage after elective gastrectomy for gastric cancer. J. Gastrointest. Surg. 2012, 16, 1659–1665. [Google Scholar] [CrossRef]
- Hummel, R.; Bausch, D. Anastomotic Leakage after Upper Gastrointestinal Surgery: Surgical Treatment. Visc. Med. 2017, 33, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, M.; Otsuki, S.; Fujimori, Y.; Sato, Y.; Nakagawa, M.; Kojima, K. Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. World J. Gastroenterol. 2015, 21, 9656–9665. [Google Scholar] [CrossRef]
- Schietroma, M.; Cecilia, E.M.; Carlei, F.; Sista, F.; De Santis, G.; Piccione, F.; Amicucci, G. Prevention of anastomotic leakage after total gastrectomy with perioperative supplemental oxygen administration: A prospective randomized, double-blind, controlled, single-center trial. Ann. Surg. Oncol. 2013, 20, 1584–1590. [Google Scholar] [CrossRef]
- Aurello, P.; Magistri, P.; D’Angelo, F.; Valabrega, S.; Sirimarco, D.; Tierno, S.M.; Nava, A.K.; Ramacciato, G. Treatment of esophagojejunal anastomosis leakage: A systematic review from the last two decades. Am. Surg. 2015, 81, 450–453. [Google Scholar] [CrossRef]
- Bracale, U.; Peltrini, R.; De Luca, M.; Ilardi, M.; Di Nuzzo, M.M.; Sartori, A.; Sodo, M.; Danzi, M.; Corcione, F.; De Werra, C. Predictive Factors for Anastomotic Leakage after Laparoscopic and Open Total Gastrectomy: A Systematic Review. J. Clin. Med. 2022, 11, 5022. [Google Scholar] [CrossRef]
- Woodfield, C.A.; Levine, M.S. The postoperative stomach. Eur. J. Radiol. 2005, 53, 341–352. [Google Scholar] [CrossRef]
- Kim, A.; Ko, C.S.; Kim, B.S.; Kim, H.S. Intracorporeal bi-directional pouch jejunojejunostomy following Roux-en-Y anastomosis: A simple reconstruction technique using an endoscopic linear stapler. Wideochirurgia I Inne Tech. Maloinwazyjne = Videosurgery Other Miniinvasive Tech. 2021, 16, 704–709. [Google Scholar] [CrossRef]
- Upponi, S.; Ganeshan, A.; D’Costa, H.; Betts, M.; Maynard, N.; Bungay, H.; Slater, A. Radiological detection of post-oesophagectomy anastomotic leak—A comparison between multidetector CT and fluoroscopy. Br. J. Radiol. 2008, 81, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, J.H.; Shin, C.I.; Kim, S.H.; Han, J.K.; Choi, B.I. CT findings suggesting anastomotic leak and predicting the recovery period following gastric surgery. Eur. Radiol. 2015, 25, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Lamb, P.J.; Griffin, S.M.; Chandrashekar, M.V.; Richardson, D.L.; Karat, D.; Hayes, N. Prospective study of routine contrast radiology after total gastrectomy. Br. J. Surg. 2004, 91, 1015–1019. [Google Scholar] [CrossRef]
- Tonouchi, H.; Mohri, Y.; Tanaka, K.; Ohi, M.; Kobayashi, M.; Yamakado, K.; Kusunoki, M. Diagnostic sensitivity of contrast swallow for leakage after gastric resection. World J. Surg. 2007, 31, 128–131. [Google Scholar] [CrossRef]
- Hogan, B.A.; Winter, D.C.; Broe, D.; Broe, P.; Lee, M.J. Prospective trial comparing contrast swallow, computed tomography and endoscopy to identify anastomotic leak following oesophagogastric surgery. Surg. Endosc. 2008, 22, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.; Krukowski, Z.H.; Al-Khairy, G.; Russell, E.M.; Park, K.G. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br. J. Surg. 2001, 88, 1157–1168. [Google Scholar] [CrossRef]
- Totten, H.P.; Jensen, A.L. Complications following subtotal gastrectomy. Calif. Med. 1956, 84, 162–167. [Google Scholar] [PubMed]
- Wu, M.H.; Lin, M.T.; Chen, W.J. Effect of perioperative parenteral nutritional support for gastric cancer patients undergoing gastrectomy. Hepatogastroenterology 2008, 55, 799–802. [Google Scholar]
- Riboli, E.B.; Bertoglio, S.; Arnulfo, G.; Terrizzi, A. Treatment of esophageal anastomotic leakages after cancer resection. The role of total parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 1986, 10, 82–85. [Google Scholar] [CrossRef]
- Shan, Y.S.; Sy, E.D.; Lin, P.W. Role of somatostatin in the prevention of pancreatic stump-related morbidity following elective pancreaticoduodenectomy in high-risk patients and elimination of surgeon-related factors: Prospective, randomized, controlled trial. World J. Surg. 2003, 27, 709–714. [Google Scholar] [CrossRef]
- Gouillat, C.; Chipponi, J.; Baulieux, J.; Partensky, C.; Saric, J.; Gayet, B. Randomized controlled multicentre trial of somatostatin infusion after pancreaticoduodenectomy. Br. J. Surg. 2001, 88, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Montorsi, M.; Zago, M.; Mosca, F.; Capussotti, L.; Zotti, E.; Ribotta, G.; Fegiz, G.; Fissi, S.; Roviaro, G.; Peracchia, A.; et al. Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resections: A prospective, controlled, randomized clinical trial. Surgery 1995, 117, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Chen, J.H.; Zhang, X.H.; Xu, J.B.; He, Y.L.; Cai, S.R.; Han, F.H.; Chen, C.Q. Effect of somatostatin in advanced gastric cancer after D2 radical gastrectomy. World J. Gastroenterol. 2014, 20, 14927–14933. [Google Scholar] [CrossRef] [PubMed]
- Tsou, C.C.; Lo, S.S.; Fang, W.L.; Wu, C.W.; Chen, J.H.; Hsieh, M.C.; Shen, K.H. Risk factors and management of anastomotic leakage after radical gastrectomy for gastric cancer. Hepatogastroenterology 2011, 58, 218–223. [Google Scholar]
- Lo, C.H.; Chen, J.H.; Wu, C.W.; Lo, S.S.; Hsieh, M.C.; Lui, W.Y. Risk factors and management of intra-abdominal infection after extended radical gastrectomy. Am. J. Surg. 2008, 196, 741–745. [Google Scholar] [CrossRef]
- Schots, J.P.M.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P. Abdominal Drainage and Amylase Measurement for Detection of Leakage After Gastrectomy for Gastric Cancer. J. Gastrointest. Surg. 2018, 22, 1163–1170. [Google Scholar] [CrossRef]
- El-Sourani, N.; Miftode, S.; Bockhorn, M.; Arlt, A.; Meinhardt, C. Endoscopic Management of Anastomotic Leakage after Esophageal Surgery: Ten Year Analysis in a Tertiary University Center. Clin. Endosc. 2022, 55, 58–66. [Google Scholar] [CrossRef]
- Rábago, L.R.; Ventosa, N.; Castro, J.L.; Marco, J.; Herrera, N.; Gea, F. Endoscopic treatment of postoperative fistulas resistant to conservative management using biological fibrin glue. Endoscopy 2002, 34, 632–638. [Google Scholar] [CrossRef]
- von Renteln, D.; Denzer, U.W.; Schachschal, G.; Anders, M.; Groth, S.; Rösch, T. Endoscopic closure of GI fistulae by using an over-the-scope clip (with videos). Gastrointest. Endosc. 2010, 72, 1289–1296. [Google Scholar] [CrossRef]
- Kouklakis, G.; Zezos, P.; Liratzopoulos, N.; Gatopoulou, A.; Oikonomou, A.; Pitiakoudis, M.; Efremidou, E.; Simopoulos, C. Endoscopic treatment of a gastrocutaneous fistula using the over-the-scope-clip system: A case report. Diagn. Ther. Endosc. 2011, 2011, 384143. [Google Scholar] [CrossRef] [Green Version]
- Albert, J.G.; Friedrich-Rust, M.; Woeste, G.; Strey, C.; Bechstein, W.O.; Zeuzem, S.; Sarrazin, C. Benefit of a clipping device in use in intestinal bleeding and intestinal leakage. Gastrointest. Endosc. 2011, 74, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Kirschniak, A.; Subotova, N.; Zieker, D.; Königsrainer, A.; Kratt, T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg. Endosc. 2011, 25, 2901–2905. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Thompson, C.C. Endoscopic therapy for postoperative leaks and fistulae. Gastrointest. Endosc. Clin. N. Am. 2013, 23, 123–136. [Google Scholar] [CrossRef]
- van Boeckel, P.G.; Sijbring, A.; Vleggaar, F.P.; Siersema, P.D. Systematic review: Temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment. Pharmacol. Ther. 2011, 33, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Puli, S.R.; Spofford, I.S.; Thompson, C.C. Use of self-expandable stents in the treatment of bariatric surgery leaks: A systematic review and meta-analysis. Gastrointest. Endosc. 2012, 75, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Eisendrath, P.; Cremer, M.; Himpens, J.; Cadière, G.B.; Le Moine, O.; Devière, J. Endotherapy including temporary stenting of fistulas of the upper gastrointestinal tract after laparoscopic bariatric surgery. Endoscopy 2007, 39, 625–630. [Google Scholar] [CrossRef]
- Wedemeyer, J.; Schneider, A.; Manns, M.P.; Jackobs, S. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest. Endosc. 2008, 67, 708–711. [Google Scholar] [CrossRef]
- Hwang, J.J.; Jeong, Y.S.; Park, Y.S.; Yoon, H.; Shin, C.M.; Kim, N.; Lee, D.H. Comparison of Endoscopic Vacuum Therapy and Endoscopic Stent Implantation With Self-Expandable Metal Stent in Treating Postsurgical Gastroesophageal Leakage. Medicine 2016, 95, e3416. [Google Scholar] [CrossRef]
- Laukoetter, M.G.; Mennigen, R.; Neumann, P.A.; Dhayat, S.; Horst, G.; Palmes, D.; Senninger, N.; Vowinkel, T. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): A prospective cohort study. Surg. Endosc. 2017, 31, 2687–2696. [Google Scholar] [CrossRef]
- Aurello, P.; Sirimarco, D.; Magistri, P.; Petrucciani, N.; Berardi, G.; Amato, S.; Gasparrini, M.; D’Angelo, F.; Nigri, G.; Ramacciato, G. Management of duodenal stump fistula after gastrectomy for gastric cancer: Systematic review. World J. Gastroenterol. 2015, 21, 7571–7576. [Google Scholar] [CrossRef]
- Zizzo, M.; Ugoletti, L.; Manzini, L.; Castro Ruiz, C.; Nita, G.E.; Zanelli, M.; De Marco, L.; Besutti, G.; Scalzone, R.; Sassatelli, R.; et al. Management of duodenal stump fistula after gastrectomy for malignant disease: A systematic review of the literature. BMC Surg. 2019, 19, 55. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Park, J.H.; Kim, T.H.; Jung, E.J.; Jeong, C.Y.; Ju, Y.T.; Kim, J.Y.; Park, T.J.; Lee, Y.J.; Jeong, S.H. Risk Factors for Reoperation Following Radical Gastrectomy in Gastric Cancer Patients. Am. Surg. 2021, 23, 31348211050842. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Kang, J.H.; Jung, M.R.; Ryu, S.Y.; Jeong, O. Abdominal Drainage in the Prevention and Management of Major Intra-Abdominal Complications after Total Gastrectomy for Gastric Carcinoma. J. Gastric Cancer 2020, 20, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, N.; Matsubara, T.; Hayashi, H.; Takai, K.; Fujii, Y.; Tajima, Y. Significance of prophylactic intra-abdominal drain placement after laparoscopic distal gastrectomy for gastric cancer. World J. Surg. Oncol. 2015, 13, 181. [Google Scholar] [CrossRef] [Green Version]
- Mengardo, V.; Weindelmayer, J.; Veltri, A.; Giacopuzzi, S.; Torroni, L.; de Manzoni, G. Current practice on the use of prophylactic drain after gastrectomy in Italy: The Abdominal Drain in Gastrectomy (ADiGe) survey. Updat. Surg. 2022, 74, 1839–1849. [Google Scholar] [CrossRef]
- Petrowsky, H.; Demartines, N.; Rousson, V.; Clavien, P.A. Evidence-based value of prophylactic drainage in gastrointestinal surgery: A systematic review and meta-analyses. Ann. Surg. 2004, 240, 1074–1084. [Google Scholar] [CrossRef]
- Kakeji, Y.; Ishikawa, T.; Suzuki, S.; Akazawa, K.; Irino, T.; Miyashiro, I.; Ono, H.; Suzuki, H.; Tanabe, S.; Kadowaki, S.; et al. A retrospective 5-year survival analysis of surgically resected gastric cancer cases from the Japanese Gastric Cancer Association nationwide registry (2001–2013). Gastric Cancer 2022, 25, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Makuuchi, R.; Irino, T.; Tanizawa, Y.; Bando, E.; Kawamura, T.; Terashima, M. Esophagojejunal anastomotic leakage following gastrectomy for gastric cancer. Surg. Today 2019, 49, 187–196. [Google Scholar] [CrossRef]
- Gong, W.; Li, J. Combat with esophagojejunal anastomotic leakage after total gastrectomy for gastric cancer: A critical review of the literature. Int. J. Surg. 2017, 47, 18–24. [Google Scholar] [CrossRef]
- Lee, H.J.; Hyung, W.J.; Yang, H.K.; Han, S.U.; Park, Y.K.; An, J.Y.; Kim, W.; Kim, H.I.; Kim, H.H.; Ryu, S.W.; et al. Short-term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy With D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann. Surg. 2019, 270, 983–991. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, C.; Sun, Y.; Su, X.; Cao, H.; Hu, J.; Xue, Y.; Suo, J.; Tao, K.; He, X.; et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 1350–1357. [Google Scholar] [CrossRef]
- Sun, Z.; Shenoi, M.M.; Nussbaum, D.P.; Keenan, J.E.; Gulack, B.C.; Tyler, D.S.; Speicher, P.J.; Blazer, D.G., 3rd. Feeding jejunostomy tube placement during resection of gastric cancers. J. Surg. Res. 2016, 200, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blakely, A.M.; Ajmal, S.; Sargent, R.E.; Ng, T.T.; Miner, T.J. Critical analysis of feeding jejunostomy following resection of upper gastrointestinal malignancies. World J. Gastrointest. Surg. 2017, 9, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.C.; Kwon, S.H. Endoscopic Vacuum-Assisted Closure (E-VAC) Treatment in a Patient with Delayed Anastomotic Perforation following a Perforated Gastric Conduit Repair after an Ivor-Lewis Esophagectomy. Ann. Thorac. Cardiovasc. Surg. Off. J. Assoc. Thorac. Cardiovasc. Surg. Asia 2016, 22, 363–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bludau, M.; Hölscher, A.H.; Herbold, T.; Leers, J.M.; Gutschow, C.; Fuchs, H.; Schröder, W. Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC). Surg. Endosc. 2014, 28, 896–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smallwood, N.R.; Fleshman, J.W.; Leeds, S.G.; Burdick, J.S. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg. Endosc. 2016, 30, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Bludau, M.; Fuchs, H.F.; Herbold, T.; Maus, M.K.H.; Alakus, H.; Popp, F.; Leers, J.M.; Bruns, C.J.; Hölscher, A.H.; Schröder, W.; et al. Results of endoscopic vacuum-assisted closure device for treatment of upper GI leaks. Surg. Endosc. 2018, 32, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Suter, K.; Bui, H.T.; Chan, S.T. Management of Severe Sleeve Gastrectomy Leaks by a Roux-en-Y Gastrojejunostomy and Suction Isoperistaltic Jejuno-gastroesophagostomy Tube (SIJGET): A Novel Approach. Obes. Surg. 2022, 32, 2816–2819. [Google Scholar] [CrossRef] [PubMed]
- Persson, S.; Rouvelas, I.; Irino, T.; Lundell, L. Outcomes following the main treatment options in patients with a leaking esophagus: A systematic literature review. Dis. Esophagus 2017, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.N.; Bui, H.T.; Abdulrasool, H.; Chan, S.T.F. Continuous Suction Isoperistaltic Gastroesophagostomy for Esophageal Perforation. Ann. Thorac. Surg. 2019, 107, e293–e295. [Google Scholar] [CrossRef]
- Raymond, D.P.; Watson, T.J. Esophageal diversion. Oper. Tech. Thorac. Cardiovasc. Surg. 2008, 13, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Allum, W.H.; Bonavina, L.; Cassivi, S.D.; Cuesta, M.A.; Dong, Z.M.; Felix, V.N.; Figueredo, E.; Gatenby, P.A.; Haverkamp, L.; Ibraev, M.A.; et al. Surgical treatments for esophageal cancers. Ann. N. Y. Acad. Sci. 2014, 1325, 242–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urschel, H.C., Jr.; Razzuk, M.A.; Wood, R.E.; Galbraith, N.; Pockey, M.; Paulson, D.L. Improved management of esophageal perforation: Exclusion and diversion in continuity. Ann. Surg. 1974, 179, 587–591. [Google Scholar] [CrossRef]
- Mariette, C.; De Botton, M.L.; Piessen, G. Surgery in esophageal and gastric cancer patients: What is the role for nutrition support in your daily practice? Ann. Surg. Oncol. 2012, 19, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jeong, S.H.; Lee, Y.J.; Kim, T.H.; Kim, J.M.; Kim, D.H.; Kwag, S.J.; Kim, J.Y.; Park, T.; Jeong, C.Y.; et al. Safety and efficacy of post-anastomotic intraoperative endoscopy to avoid early anastomotic complications during gastrectomy for gastric cancer. Surg. Endosc. 2020, 34, 5312–5319. [Google Scholar] [CrossRef]
- Kanaji, S.; Ohyama, M.; Yasuda, T.; Sendo, H.; Suzuki, S.; Kawasaki, K.; Tanaka, K.; Fujino, Y.; Tominaga, M.; Kakeji, Y. Can the intraoperative leak test prevent postoperative leakage of esophagojejunal anastomosis after total gastrectomy? Surg. Today 2016, 46, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Yanaga, K.; Kashiwagi, H.; Hanyuu, N.; Iwabuchi, S. Significance of intraoperative endoscopy in total gastrectomy for gastric cancer. Surg. Endosc. 2010, 24, 2633–2636. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-H.; Lee, J.-K.; Seo, K.W.; Min, J.-S. Treatment and Prevention of Postoperative Leakage after Gastrectomy for Gastric Cancer. J. Clin. Med. 2023, 12, 3880. https://doi.org/10.3390/jcm12123880
Jeong S-H, Lee J-K, Seo KW, Min J-S. Treatment and Prevention of Postoperative Leakage after Gastrectomy for Gastric Cancer. Journal of Clinical Medicine. 2023; 12(12):3880. https://doi.org/10.3390/jcm12123880
Chicago/Turabian StyleJeong, Sang-Ho, Jin-Kwon Lee, Kyung Won Seo, and Jae-Seok Min. 2023. "Treatment and Prevention of Postoperative Leakage after Gastrectomy for Gastric Cancer" Journal of Clinical Medicine 12, no. 12: 3880. https://doi.org/10.3390/jcm12123880
APA StyleJeong, S.-H., Lee, J.-K., Seo, K. W., & Min, J.-S. (2023). Treatment and Prevention of Postoperative Leakage after Gastrectomy for Gastric Cancer. Journal of Clinical Medicine, 12(12), 3880. https://doi.org/10.3390/jcm12123880






