Abstract
The post-transplant evolution of antihuman leukocyte antigen donor-specific antibodies (anti-HLA DSAs) includes three clinical patterns: resolved preformed DSAs, persistent preformed DSAs, and de novo DSAs. The aim of this retrospective study was to analyze the impact of resolved preformed, persistent preformed, and de novo anti-HLA-A, -B, and -DR DSAs in kidney transplant recipients on long-term renal allograft outcomes. This is a post hoc analysis of the study conducted in our transplant center. One hundred eight kidney transplant recipients were included in the study. Patients were followed for a minimum of 24 months after allograft biopsy, which was performed 3 to 24 months after kidney transplantation. The identification of persistent preformed DSAs at the time of biopsy was the most significant predictor of the combined endpoint of the study (>30% decline in estimated glomerular filtration rate or death-censored graft loss; HR = 5.96, 95% CI 2.041–17.431, p = 0.0011), followed by the occurrence of de novo DSAs (HR = 4.48, 95% CI 1.483–13.520, p = 0.0079). No increased risk was observed in patients with resolved preformed DSAs (HR = 1.10, 95% CI 0.139–8.676, p = 0.9305). Patients with resolved preformed DSAs have similar graft prognoses as patients without DSAs, therefore, the persistence of preformed DSAs and development of de novo DSAs are associated with inferior long-term allograft outcomes.
1. Introduction
The presence of donor-specific antibodies (DSAs) directed against human leukocyte antigens (HLA) is a risk factor for antibody-mediated rejection (ABMR) and allograft loss in kidney transplant (KTx) recipients [1]. Over the last decade, the identification of anti-HLA DSAs and their characteristics has improved due to the use of solid-phase assay technology, particularly single antigen bead (SAB) testing [2]. In addition, the destructive effect of anti-HLA DSAs is determined by antibody characteristics including class, specificity, mean fluorescent intensity (MFI), complement-biding ability, and IgG subclass [3,4].
DSAs may occur before transplantation (preformed) or arise de novo after transplantation. Alloimmunization before KTx can be caused by pregnancy, blood transfusion, or prior transplant [5,6]. The development of de novo DSAs is associated with several clinical events such as pregnancy, blood transfusion, minimization of immunosuppression, nonadherence, and implementation of a homograft [7,8,9]. Regarding the post-transplant evolution of DSAs, there are three identified clinical patterns: persistent preformed DSAs, resolved preformed DSAs, and de novo DSAs [10]. Patients with persistent preformed DSAs are at a higher risk of ABMR or kidney allograft loss compared to patients with resolved or no preformed DSAs. Factors associated with the persistence of DSAs after transplantation are class II, high MFI, and complement-binding ability [10,11]. However, independently of the evolution status after KTx, preformed DSAs (even with low MFI values) are an important risk factor for decreased graft survival [12]. Patients with identified circulating de novo DSAs display a significantly increased risk of chronic ABMR and renal allograft loss compared to patients with preexisting DSAs [13,14]. Regardless of the preformed/de novo status, the presence of DSAs corresponds with a higher incidence of diagnosis of subclinical ABMR [15,16]. DSA positivity after KTx is associated with increased ABMR-related gene expression, even in biopsy samples with no molecular or histologic rejection [17].
In solid-organ transplantation, the monitoring of anti-HLA DSAs is currently under investigation. Recently, clinical recommendations for the post-transplant assessment of anti-HLA DSAs were published [18]. Nevertheless, there are some deficits in the existing knowledge that should be addressed. There is a need for a better understanding of the clinical role of persistent preformed anti-HLA DSAs and to investigate factors that lead to the clearance of preformed anti-HLA DSAs after transplantation [11]. As the evolution of DSAs after KTx may modify the outcomes, therapeutic interventions could be implemented [18].
The aim of this retrospective study was to analyze the impact of resolved preformed, persistent preformed, and de novo anti-HLA-A, -B, and -DR DSAs in kidney transplant recipients with long-term renal allograft outcomes.
2. Materials and Methods
2.1. Study Design and Data Collection
This is a post hoc analysis of the study conducted in our transplant center [19]. All 108 consecutive kidney transplant recipients from brain-dead deceased donors from the mentioned study were included in this retrospective study. All patients underwent an ultrasound-guided renal allograft biopsy between 2018 and 2020, 3 to 24 months after KTx, and were followed for a minimum of 24 months after the biopsy (except in cases of death or graft failure).
Patients were divided post hoc into four groups according to their DSA status at the time of biopsy: no anti-HLA DSAs, resolved preformed anti-HLA DSAs, persistent preformed anti-HLA DSAs, and de novo anti-HLA DSAs.
All transplantations were performed with negative complement-dependent cytotoxicity crossmatch. Donor–recipient pairs were ABO blood group compatible. All patients were of white ethnicity. None of the patients received a desensitization protocol before KTx. The decision of whether and what induction therapy (basiliximab or anti-thymocyte globulin (ATG)) to administer was made individually for each patient after the assessment of their immunological risk. Mostly, patients with a high immunological risk received ATG while patients with an intermediate immunological risk received basiliximab. In all patients, the maintenance immunosuppressive regimen consisted of the standard of care agents: a calcineurin inhibitor (tacrolimus or cyclosporine) with mycophenolate mofetil and prednisone. Only two patients were treated with cyclosporine due to tacrolimus intolerance. Biopsies were assessed using Banff classification criteria [20].
At the time of biopsy, serum samples were obtained from all the patients and stored for further analysis. Sera were tested for the presence of anti-HLA DSAs and their characteristics including class, specificity, MFI, C1q-binding capacity, and IgG subclass according to the protocol described previously [19]. Clinical and laboratory data including the DSA status of the patients before KTx were retrospectively extracted from the medical records. All patients were tested for anti-HLA DSAs by SAB assay before transplantation. Antibodies with MFI values above 500 were determined as positive. The identification of donor HLA antibody specificity was limited to HLA-A, HLA-B, and HLA-DR. Donor typing was only available for HLA-A, HLA-B, and HLA-DR; the cross-reactive epitope groups were not considered. The resolved preformed anti-HLA DSAs were defined as antibodies detected before KTx but not detected at the time of the kidney transplant biopsy procedure.
The primary outcome of the study was defined as a permanent 30% decline (two consecutive laboratory assessments ≥ 3 months apart) in the estimated glomerular filtration rate (eGFR) compared to the baseline at the time of biopsy or death-censored graft loss (need for dialysis or retransplantation). The Chronic Kidney Disease Epidemiology Collaboration 2009 (CKD-EPI) creatinine equation was used to determine the eGFR.
Approval of the study was obtained from the Medical University of Warsaw medical ethics committee (Warsaw, Poland). The study was performed according to the principles expressed in the Declaration of Helsinki. All patients provided informed written consent.
2.2. Statistical Analysis
Statistical analysis was conducted using R software. The Shapiro–Wilk test was used to assess the normality of distribution. Continuous variables were presented as the means with standard deviation (SD) or medians with quartiles 1 and 3 (Q1–Q3) as appropriate. Categorical variables are presented as frequencies and percentages. Differences between continuous variables were assessed using the t-test, Mann–Whitney U test, and one-way analysis of variance (F-test or Kruskal–Wallis test), depending on the number and distribution of the compared variables. The Chi-square test was used to compare categorical variables. The Yates continuity correction was used when the frequency of events was low (<5). Logistic regression was performed to identify variables associated with DSA’s status. Event-free survival was assessed using the Kaplan–Meier method and log-rank test. Univariate and multivariate Cox proportional hazards models were used to quantify the hazard ratios for the study outcome. A backward stepwise elimination method was applied. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Demographic and Clinical Characteristics
A total of 108 consecutive patients who received kidney transplants from brain-dead deceased donors and underwent renal allograft biopsy 3 to 24 months post-KTx were included in the study. Based on the DSA status at the time of biopsy, four groups were identified: no anti-HLA DSAs (N = 80), resolved preformed anti-HLA DSAs (N = 9), persistent preformed anti-HLA DSAs (N = 9), and de novo anti-HLA DSAs (N = 10). The demographic and clinical characteristics of the groups are provided in Table 1. The groups did not differ in age, sex, body mass index, type of renal replacement therapy, cause of end-stage renal disease, age and sex of the donor, duration of cold ischemia, or number of HLA mismatches. Moreover, no statistical difference was found in terms of clinical characteristics such as the use of tacrolimus versus cyclosporine for maintenance immunosuppression, eGFR and proteinuria at biopsy, protocol biopsy, time from KTx to biopsy procedure, and C4d deposition in biopsy specimens. There was a statistical difference regarding the history of previous renal transplantation, induction of immunosuppression, panel-reactive antibodies, and diagnosis of ABMR at biopsy. The prior KTxs had 2.5% of patients without DSAs, 22.2% of patients with resolved preformed DSAs, 77.8% of patients with persistent preformed DSAs, and 80% of patients with de novo DSAs. The transplant procedure was performed without the induction of immunosuppression in 85% of patients without DSA. In patients with preformed DSAs (resolved and persistent), the induction protocol with anti-thymocyte globulin was the most popular. Half of the patients with de novo DSAs received basiliximab as an induction of immunosuppression. The panel-reactive antibody >5% was the most common in patients with persistent preformed anti-HLA DSAs (55.6%). ABMR was diagnosed in 2.5% of patients without DSAs and in 22.2% and 20% of KTx recipients with preformed and de novo DSAs, respectively.

Table 1.
Characteristics of the groups.
3.2. Characteristics of Anti-HLA DSAs before and after Transplantation
Table 2 depicts the characteristics of anti-HLA DSAs before transplantation. No significant difference was shown between the number, HLA class specificity, and MFI of resolved and persistent anti-HLA DSAs. Nevertheless, the median MFI level of resolved preformed DSAs was lower compared to the median MFI level of persistent preformed DSAs (983 vs. 1905, not statistically significant).

Table 2.
Characteristics of anti-HLA DSAs before transplantation.
Table 3 shows the characteristics of anti-HLA DSAs at the time of biopsy. There was no significant difference between the persistent preformed and de novo anti-HLA DSAs regarding the number, HLA class specificity, MFI, C1q-binding capacity, and IgG subclasses. However, the median MFI level of persistent preformed anti-HLA DSAs was higher in comparison with de novo anti-HLA DSAs (3843 vs. 1693, not statistically significant).

Table 3.
Characteristics of anti-HLA DSAs after transplantation.
3.3. Clinical Outcomes
All patients were followed for a minimum of 24 months after the biopsy procedure. The clinical outcomes are shown in Table 4. The combined endpoint of the study (>30% decline in eGFR or death-censored graft loss) was reached by 66.7% of patients with persistent preformed DSAs, 50% of patients with de novo DSAs, 11.1% of patients with resolved preformed DSAs, and 10% of patients without DSAs (p < 0.0001). Two patients (22.2%) with persistent preformed DSAs experienced graft loss. As many as 42.9% of patients with persistent preformed DSAs had proteinuria at the end of follow-up, ≥50 mg/dL (p = 0.0001). No statistical difference between the groups was identified regarding the time of follow-up after biopsy, median proteinuria at the end of follow-up, time from biopsy to the primary outcome, and mortality.

Table 4.
Clinical outcomes.
3.4. Survival Analysis
The event-free survival according to DSA status is shown in Figure 1 and the univariate Cox analysis is demonstrated in Table 5. Compared to patients without anti-HLA DSAs, the persistence of preformed anti-HLA DSAs after KTx and development of de novo anti-HLA DSAs at the time of biopsy were significantly associated with inferior survival (p < 0.0001 and p = 0.0090, respectively). However, there was no statistical difference regarding the combined endpoint survival between patients with resolved preformed anti-HLA DSAs after transplantation and patients without anti-HLA DSAs (p = 0.918).
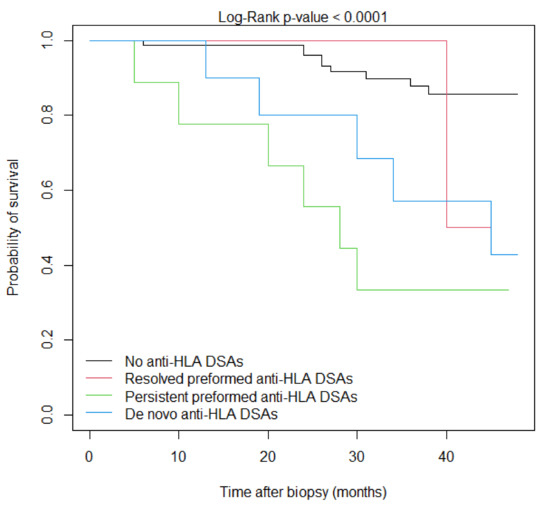
Figure 1.
Event-free Kaplan–Meier survival curves according to anti-HLA DSA status at the time of biopsy.

Table 5.
Univariate Cox proportional hazards model for the combined endpoint according to the evolution of preformed anti-HLA DSAs and development of de novo anti-HLA DSAs at the time of biopsy.
The multivariate Cox regression model for the risk of the combined endpoint is depicted in Table 6. In this model, the identification of persistent preformed anti-HLA DSAs at the time of biopsy is the most significant predictor of inferior graft outcomes (HR = 5.96, p = 0.0011), followed by the occurrence of de novo anti-HLA DSAs (HR = 4.48, p = 0.0079), proteinuria at biopsy ≥ 50 mg/dL (HR = 1.02, p = 0.0428), and increased donor’s age, with borderline statistical significance (HR = 1.03, p = 0.0530).

Table 6.
Multivariate Cox proportional hazards model for the combined endpoint. To build the model, a univariate analysis was undertaken, and significant factors were entered into the multivariate model. A backward stepwise elimination method was used.
3.5. Factors Associated with DSA Status
Multivariate logistic regression analysis revealed several clinical, independent variables significant with DSA status (Table 7). The use of anti-thymocyte globulin therapy was a risk factor for resolved preformed anti-HLA DSAs. The persistence of preformed anti-HLA DSAs was associated with previous transplantation and the increased age of the donor. Moreover, a prior transplant was identified to be a risk factor for the development of de novo anti-HLA DSAs.

Table 7.
Factors associated with DSA status.
4. Discussion
In the study, the clinical impact of the evolution of anti-HLA DSAs in kidney transplant recipients was assessed. In recent years, transplant matching has evolved due to the development of new technologies. Moreover, the characteristics of donors and recipients have changed, which alters KTx outcomes [21]. Currently, in most transplant centers, histocompatibility testing for solid organ transplantation includes ABO blood group compatibility, HLA donor–recipient matching, and crossmatching testing [22]. Anti-HLA antibody screening is crucial for assessing the immunological risk in kidney transplant recipients. However, the interpretation of DSAs should be conducted with great care; it demands an understanding of the complexity of antibodies and the technical aspects of detection assays [23].
In the cohort, 50% of patients with preformed anti-HLA DSAs cleared their antibodies after transplantation. None of the patients received desensitization treatment. This is directly in line with previous findings by Sanev et al. In their large study, which included 924 kidney transplant recipients, they also demonstrated that the persistence of DSAs is associated with higher MFI values and antibodies directed against HLA class II [10]. However, this is not shown in our analysis, probably due to the smaller number of patients.
Our results present that KTx recipients with resolved preformed anti-HLA DSAs have similar renal allograft outcomes regarding the eGFR and graft survival compared to patients without DSAs. Overall, these findings are in accordance with the results reported by other researchers [10,24,25]. The mechanism of clearance of DSAs after transplant without desensitization therapy is not fully explained. It could be hypothesized that an immunosuppressive regimen used after transplantation could decrease the production of weak antibodies [10]. In our study, the clearance of DSAs was associated with the use of ATG. It could be speculated that some preformed DSAs could be clinically irrelevant or in some way may be influenced by ATG. Another circumstance that potentially drives DSA disappearance is the development of graft accommodation, but this process is still under investigation [26].
The detection of persistent preformed anti-HLA DSAs at the time of biopsy and the identification of de novo anti-HLA DSAs are the main independent predictors of worse graft outcomes, defined as a 30% sustained decline of the GFR or graft failure. No significant difference between the characteristics of these antibodies was found. This might imply that current, circulating DSAs are more essential in the prediction of the renal allograft compared to resolved DSAs. A similar conclusion was reached in studies involving not only adult renal transplant recipients but also patients after other solid organ transplantation such as heart, lung, or intestine [27,28,29,30]. A history of previous transplantation is independently associated with the persistence of preformed DSAs. This is consistent with what has been found by Caillard et al. [24]. In addition, the persistence of DSAs was associated with increased donor age. It is difficult to explain such results, but it should be mentioned that the donor’s age was also determined to be a risk factor for overall graft failure [31]. In our model, prior transplantation is also associated with the development of de novo DSAs. Similar findings were reported before in the liver transplant recipients cohort [32]. These results could suggest that in the case of kidney retransplantation, immunological status and histocompatibility in an individual patient need to be carefully assessed.
Our findings highlight the clinical impact of the clearance of anti-HLA DSAs after renal transplantation. This is particularly important within the context of emerging new desensitization therapies [33,34]. However, factors leading to the clearance or persistence of preformed anti-HLA DSAs should be further investigated. In clinical practice, in kidney transplant recipients, therapeutic decisions should be based on the detected, circulating anti-HLA DSAs rather than considering pre-transplant antibodies. Nevertheless, there is a fundamental need for more studies assessing post-transplant DSA positivity without allograft dysfunction with reference to the utility of kidney allograft biopsies or potential noninvasive biomarkers such as donor-derived cell-free DNA [18].
One of the limitations of the present study is the post hoc, retrospective nature of the analysis. In addition, a relatively modest number of patients had been identified with DSAs in this single-center investigation. The characteristics of pre-transplant antibodies such as C1q-binding capacity and IgG subclasses were not assessed. Donor typing did not include HLA-C, -DP, and -DQ, therefore, antibodies against these antigens were not analyzed and the ABMR risk could not be assessed precisely. The cross-reactive epitope groups were not considered. Patients were tested for post-transplant DSAs only at the time of kidney allograft biopsy, which was performed 3 to 24 months after the transplant. Follow-up biopsies are not available. The use of different agents in the induction of immunosuppressive therapy was inevitable.
To conclude, kidney transplant recipients with resolved preformed anti-HLA DSAs have similar graft prognoses as patients without DSAs. The persistence of preformed anti-HLA DSAs after kidney transplantation and the occurrence of de novo anti-HLA DSAs are independent predictors for inferior long-term allograft outcomes. Candidates for kidney retransplantation should undergo a cautious immunological risk assessment. These findings are valuable in light of the development of new desensitization protocols. Although transplant immunology evolves rapidly, there are many gaps in the current knowledge and further studies are needed to answer clinical questions.
Author Contributions
Conceptualization, M.G. and M.D.; methodology, M.G. and M.D.; validation, K.C. and K.Z.; formal analysis, M.G.; investigation, M.G., K.C. and K.Z.; resources, M.G., K.C., K.Z. and M.D.; data curation, M.G., K.C. and K.Z.; writing—original graft preparation, M.G.; writing—review and editing, M.D.; visualization, M.G.; supervision, M.D.; project administration, M.G.; funding acquisition, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed from budget funds for science in the years 2018–2021 as a research project under the “Diamond Grant” program (no. DI2017 002147) supported by the Ministry of Science and Higher Education of Poland.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Medical University of Warsaw (KB/160/2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Konvalinka, A.; Tinckam, K. Utility of HLA Antibody Testing in Kidney Transplantation. J. Am. Soc. Nephrol. 2015, 26, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, H.C.; Gebel, H.M.; Bray, R.A. Understanding solid-phase HLA antibody assays and the value of MFI. Hum. Immunol. 2017, 78, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Viglietti, D.; Loupy, A.; Vernerey, D.; Bentlejewski, C.; Gosset, C.; Aubert, O.; Duong van Huyen, J.P.; Jouven, X.; Legendre, C.; Glotz, D.; et al. Value of Donor-Specific Anti-HLA Antibody Monitoring and Characterization for Risk Stratification of Kidney Allograft Loss. J. Am. Soc. Nephrol. 2017, 28, 702–715. [Google Scholar] [CrossRef]
- Muñoz-Herrera, C.M.; Gutiérrez-Bautista, J.F.; López-Nevot, M.Á. Complement Binding Anti-HLA Antibodies and the Survival of Kidney Transplantation. J. Clin. Med. 2023, 12, 2335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Donor-Specific Antibodies in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2018, 13, 182–192. [Google Scholar] [CrossRef]
- Oweira, H.; Ramouz, A.; Ghamarnejad, O.; Khajeh, E.; Ali-Hasan-Al-Saegh, S.; Nikbakhsh, R.; Reißfelder, C.; Rahbari, N.; Mehrabi, A.; Sadeghi, M. Risk Factors of Rejection in Renal Transplant Recipients: A Narrative Review. J. Clin. Med. 2022, 11, 1392. [Google Scholar] [CrossRef]
- Sharma, A.; Cherukuri, A.; Mehta, R.B.; Sood, P.; Hariharan, S. High Calcineurin Inhibitor Intrapatient Variability Is Associated with Renal Allograft Inflammation, Chronicity, and Graft Loss. Transplant. Direct 2019, 5, e424. [Google Scholar] [CrossRef]
- Coti, I.; Wenda, S.; Andreeva, A.; Kocher, A.; Laufer, G.; Fischer, G.; Andreas, M. Donor-specific HLA antibodies after fresh decellularized vs cryopreserved native allograft implantation. Hla 2020, 96, 580–588. [Google Scholar] [CrossRef]
- Snanoudj, R.; Kamar, N.; Cassuto, E.; Caillard, S.; Metzger, M.; Merville, P.; Thierry, A.; Jollet, I.; Grimbert, P.; Anglicheau, D.; et al. Epitope load identifies kidney transplant recipients at risk of allosensitization following minimization of immunosuppression. Kidney Int. 2019, 95, 1471–1485. [Google Scholar] [CrossRef]
- Senev, A.; Lerut, E.; Van Sandt, V.; Coemans, M.; Callemeyn, J.; Sprangers, B.; Kuypers, D.; Emonds, M.P.; Naesens, M. Specificity, strength, and evolution of pretransplant donor-specific HLA antibodies determine outcome after kidney transplantation. Am. J. Transplant. 2019, 19, 3100–3113. [Google Scholar] [CrossRef]
- Redondo-Pachón, D.; Pérez-Sáez, M.J.; Mir, M.; Gimeno, J.; Llinás, L.; García, C.; Hernández, J.J.; Yélamos, J.; Pascual, J.; Crespo, M. Impact of persistent and cleared preformed HLA DSA on kidney transplant outcomes. Hum. Immunol. 2018, 79, 424–431. [Google Scholar] [CrossRef]
- Ziemann, M.; Altermann, W.; Angert, K.; Arns, W.; Bachmann, A.; Bakchoul, T.; Banas, B.; von Borstel, A.; Budde, K.; Ditt, V.; et al. Preformed Donor-Specific HLA Antibodies in Living and Deceased Donor Transplantation: A Multicenter Study. Clin. J. Am. Soc. Nephrol. 2019, 14, 1056–1066. [Google Scholar] [CrossRef]
- Aubert, O.; Loupy, A.; Hidalgo, L.; Duong van Huyen, J.-P.; Higgins, S.; Viglietti, D.; Jouven, X.; Glotz, D.; Legendre, C.; Lefaucheur, C.; et al. Antibody-Mediated Rejection Due to Preexisting versus De Novo Donor-Specific Antibodies in Kidney Allograft Recipients. J. Am. Soc. Nephrol. 2017, 28, 1912–1923. [Google Scholar] [CrossRef]
- Haas, M.; Mirocha, J.; Reinsmoen, N.L.; Vo, A.A.; Choi, J.; Kahwaji, J.M.; Peng, A.; Villicana, R.; Jordan, S.C. Differences in pathologic features and graft outcomes in antibody-mediated rejection of renal allografts due to persistent/recurrent versus de novo donor-specific antibodies. Kidney Int. 2017, 91, 729–737. [Google Scholar] [CrossRef]
- Loupy, A.; Suberbielle-Boissel, C.; Hill, G.S.; Lefaucheur, C.; Anglicheau, D.; Zuber, J.; Martinez, F.; Thervet, E.; Méjean, A.; Charron, D.; et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am. J. Transplant. 2009, 9, 2561–2570. [Google Scholar] [CrossRef]
- Yamamoto, T.; Watarai, Y.; Takeda, A.; Tsujita, M.; Hiramitsu, T.; Goto, N.; Narumi, S.; Katayama, A.; Morozumi, K.; Uchida, K.; et al. De Novo Anti-HLA DSA Characteristics and Subclinical Antibody-Mediated Kidney Allograft Injury. Transplantation 2016, 100, 2194–2202. [Google Scholar] [CrossRef]
- Madill-Thomsen, K.S.; Böhmig, G.A.; Bromberg, J.; Einecke, G.; Eskandary, F.; Gupta, G.; Hidalgo, L.G.; Myslak, M.; Viklicky, O.; Perkowska-Ptasinska, A.; et al. Donor-Specific Antibody Is Associated with Increased Expression of Rejection Transcripts in Renal Transplant Biopsies Classified as No Rejection. J. Am. Soc. Nephrol. 2021, 32, 2743–2758. [Google Scholar] [CrossRef]
- Lefaucheur, C.; Louis, K.; Morris, A.B.; Taupin, J.L.; Nickerson, P.; Tambur, A.R.; Gebel, H.M.; Reed, E.F. Clinical recommendations for posttransplant assessment of anti-HLA (Human Leukocyte Antigen) donor-specific antibodies: A Sensitization in Transplantation: Assessment of Risk consensus document. Am. J. Transplant. 2023, 23, 115–132. [Google Scholar] [CrossRef]
- Gniewkiewicz, M.; Czerwinska, K.; Zielniok, K.; Durlik, M. Association of Circulating Anti-HLA Donor-Specific Antibodies and Their Characteristics, including C1q-Binding Capacity, in Kidney Transplant Recipients with Long-Term Renal Graft Outcomes. J. Clin. Med. 2023, 12, 1312. [Google Scholar] [CrossRef]
- Haas, M.; Loupy, A.; Lefaucheur, C.; Roufosse, C.; Glotz, D.; Seron, D.; Nankivell, B.J.; Halloran, P.F.; Colvin, R.B.; Akalin, E.; et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am. J. Transplant. 2018, 18, 293–307. [Google Scholar] [CrossRef]
- Bestard, O.; Thaunat, O.; Bellini, M.I.; Böhmig, G.A.; Budde, K.; Claas, F.; Couzi, L.; Furian, L.; Heemann, U.; Mamode, N.; et al. Alloimmune Risk Stratification for Kidney Transplant Rejection. Transplant. Int. 2022, 35, 10138. [Google Scholar] [CrossRef] [PubMed]
- Markkinen, S.; Helanterä, I.; Lauronen, J.; Lempinen, M.; Partanen, J.; Hyvärinen, K. Mismatches in Gene Deletions and Kidney-related Proteins as Candidates for Histocompatibility Factors in Kidney Transplantation. Kidney Int. Rep. 2022, 7, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Schinstock, C.A.; Gandhi, M.J.; Stegall, M.D. Interpreting Anti-HLA Antibody Testing Data: A Practical Guide for Physicians. Transplantation 2016, 100, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Caillard, S.; Becmeur, C.; Gautier-Vargas, G.; Olagne, J.; Muller, C.; Cognard, N.; Perrin, P.; Braun, L.; Heibel, F.; Lefebre, F.; et al. Pre-existing donor-specific antibodies are detrimental to kidney allograft only when persistent after transplantation. Transplant. Int. 2017, 30, 29–40. [Google Scholar] [CrossRef]
- Kimball, P.M.; Baker, M.A.; Wagner, M.B.; King, A. Surveillance of alloantibodies after transplantation identifies the risk of chronic rejection. Kidney Int. 2011, 79, 1131–1137. [Google Scholar] [CrossRef]
- Kenta, I.; Takaaki, K. Molecular Mechanisms of Antibody-Mediated Rejection and Accommodation in Organ Transplantation. Nephron 2020, 144 (Suppl. 1), 2–6. [Google Scholar] [CrossRef]
- Irving, C.A.; Carter, V.; Gennery, A.R.; Parry, G.; Griselli, M.; Hasan, A.; Kirk, C.R. Effect of persistent versus transient donor-specific HLA antibodies on graft outcomes in pediatric cardiac transplantation. J. Heart Lung Transplant. 2015, 34, 1310–1317. [Google Scholar] [CrossRef]
- Le Pavec, J.; Suberbielle, C.; Lamrani, L.; Feuillet, S.; Savale, L.; Dorfmüller, P.; Stephan, F.; Mussot, S.; Mercier, O.; Fadel, E. De-novo donor-specific anti-HLA antibodies 30 days after lung transplantation are associated with a worse outcome. J. Heart Lung Transplant. 2016, 35, 1067–1077. [Google Scholar] [CrossRef]
- Abu-Elmagd, K.M.; Wu, G.; Costa, G.; Lunz, J.; Martin, L.; Koritsky, D.A.; Murase, N.; Irish, W.; Zeevi, A. Preformed and de novo donor specific antibodies in visceral transplantation: Long-term outcome with special reference to the liver. Am. J. Transplant. 2012, 12, 3047–3060. [Google Scholar] [CrossRef]
- Fujiyama, N.; Satoh, S.; Saito, M.; Numakura, K.; Inoue, T.; Yamamoto, R.; Saito, T.; Kanda, S.; Narita, S.; Mitobe, Y.; et al. Impact of persistent preformed and de novo donor-specific antibodies detected at 1 year after kidney transplantation on long-term graft survival in Japan: A retrospective study. Clin. Exp. Nephrol. 2019, 23, 1398–1406. [Google Scholar] [CrossRef]
- Betjes, M.G.H.; Sablik, K.S.; Otten, H.G.; Roelen, D.L.; Claas, F.H.; de Weerd, A. Pretransplant Donor-Specific Anti-HLA Antibodies and the Risk for Rejection-Related Graft Failure of Kidney Allografts. J. Transplant. 2020, 2020, 5694670. [Google Scholar] [CrossRef]
- Del Bello, A.; Congy-Jolivet, N.; Muscari, F.; Lavayssière, L.; Esposito, L.; Cardeau-Desangles, I.; Guitard, J.; Dörr, G.; Suc, B.; Duffas, J.P.; et al. Prevalence, Incidence and Risk Factors for Donor-Specific Anti-HLA Antibodies in Maintenance Liver Transplant Patients. Am. J. Transplant. 2014, 14, 867–875. [Google Scholar] [CrossRef]
- Grimaldi, V.; Pagano, M.; Moccia, G.; Maiello, C.; De Rosa, P.; Napoli, C. Novel insights in the clinical management of hyperimmune patients before and after transplantation. Curr. Res. Immunol. 2023, 4, 100056. [Google Scholar] [CrossRef]
- Leal, R.; Pardinhas, C.; Martinho, A.; Sá, H.O.; Figueiredo, A.; Alves, R. Strategies to Overcome HLA Sensitization and Improve Access to Retransplantation after Kidney Graft Loss. J. Clin. Med. 2022, 11, 5753. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).