Prognostic Significance of Chromogranin A Expression in the Initial and Second Biopsies in Metastatic Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinicopathological Data
2.2. Biopsy and Histopathologic Review
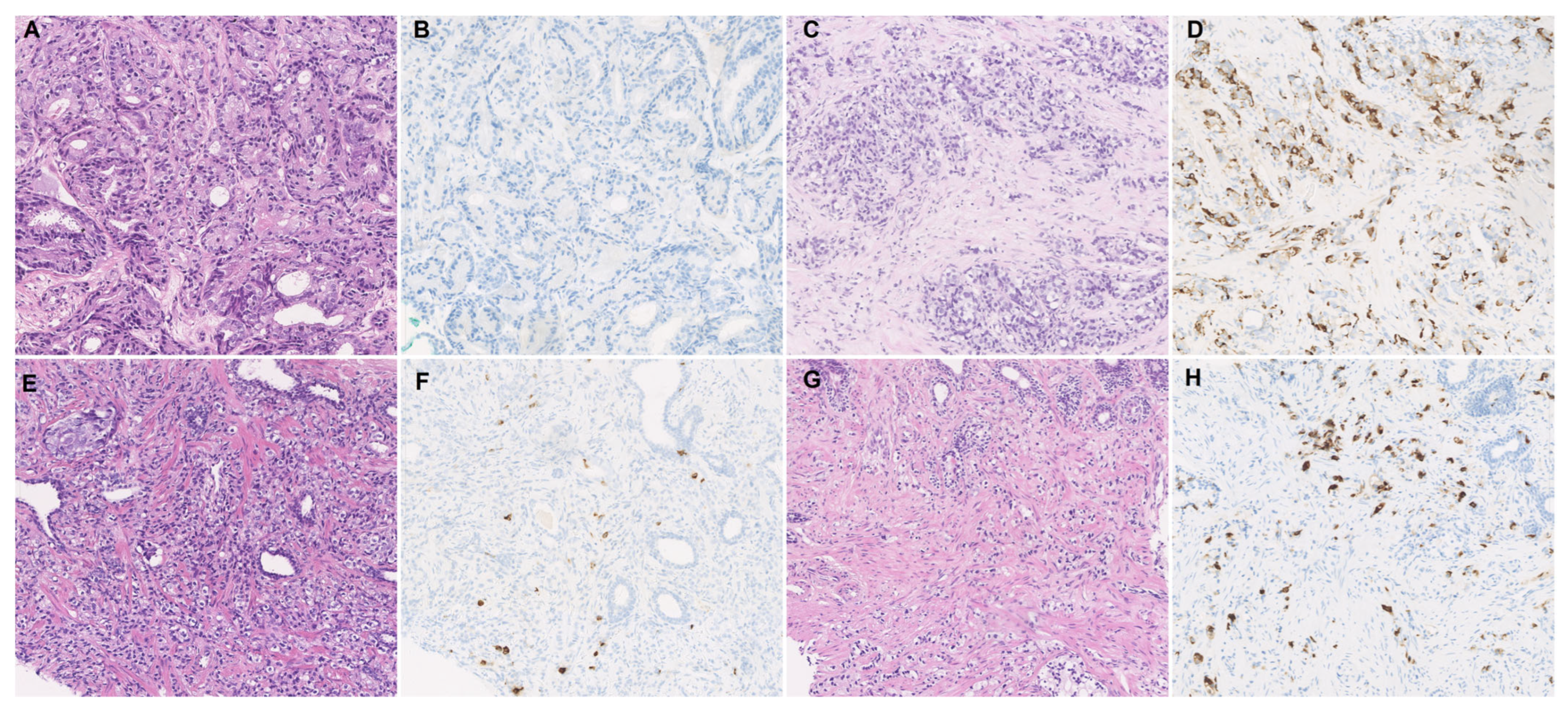
2.3. Immunohistochemistry
2.4. Statistical Analyses
3. Results
3.1. The Clinical Characteristics of the Patient Cohort
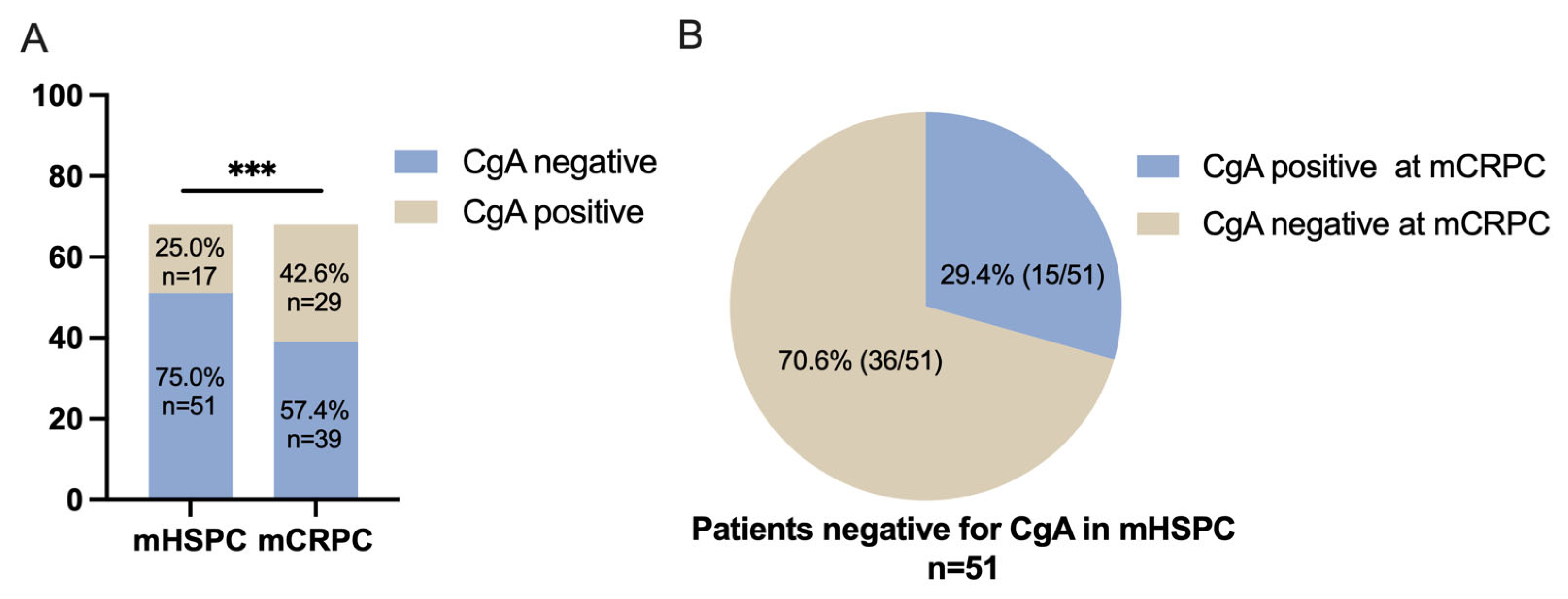
3.2. The Tendency of CgA Expression When Developing from mHSPC to mCRPC
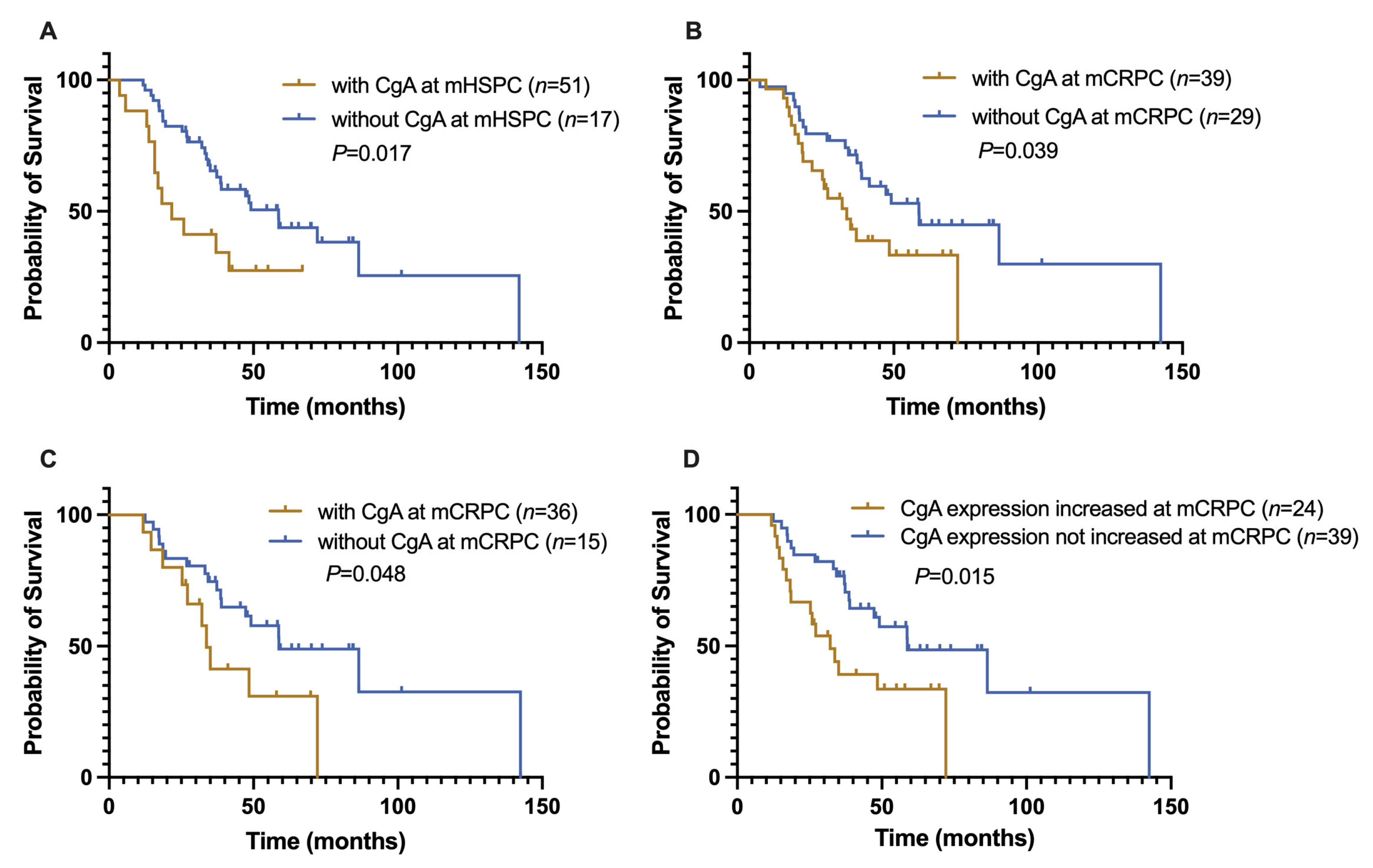
3.3. Analysis of the Relationship of Clinicopathological Variables with Survival
3.4. Prognostic Significance of the Change in CgA Expression Status of Advanced PCa
3.5. Hazard Ratio Assessment of Risk Factors of Advanced PCa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhu, Y.; Mo, M.; Wei, Y.; Wu, J.; Pan, J.; Freedland, S.J.; Zheng, Y.; Ye, D. Epidemiology and Genomics of Prostate Cancer in Asian Men. Nat. Rev. Urol. 2021, 18, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging Mechanisms of Resistance to Androgen Receptor Inhibitors in Prostate Cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Jee, B.; Chung, J.H.; Song, W.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; et al. Repression of Slc22a3 by the Ar-V7/Yap1/Taz Axis in Enzalutamide-Resistant Castration-Resistant Prostate Cancer. FEBS J. 2022, 290, 1645–1662. [Google Scholar] [CrossRef] [PubMed]
- Bluemn, E.G.; Coleman, I.M.; Lucas, J.M.; Coleman, R.T.; Hernandez-Lopez, S.; Tharakan, R.; Bianchi-Frias, D.; Dumpit, R.F.; Kaipainen, A.; Corella, A.N.; et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through Fgf Signaling. Cancer Cell 2017, 32, 474–489.e6. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Lobo, A.; Cheng, L. The 2022 Revision of the World Health Organization Classification of Tumors of the Urinary System and Male Genital Organs: Advances and Challenges. Hum. Pathol. 2022, 82, 469–482. [Google Scholar] [CrossRef]
- Wang, H.T.; Yao, Y.H.; Li, B.G.; Tang, Y.; Chang, J.W.; Zhang, J. Neuroendocrine Prostate Cancer (Nepc) Progressing from Conventional Prostatic Adenocarcinoma: Factors Associated with Time to Development of Nepc and Survival from Nepc Diagnosis-a Systematic Review and Pooled Analysis. J. Clin. Oncol. 2014, 32, 3383–3390. [Google Scholar] [CrossRef]
- Mitsui, Y.; Arichi, N.; Hiraki, M.; Harada, Y.; Yasumoto, H.; Shiina, H. Tissue Chromogranin a Expression during Prostate Cancer Progression: Prediction of Chemosensitivity. Urol. J. 2015, 12, 2165–2172. [Google Scholar]
- Heck, M.M.; Thaler, M.A.; Schmid, S.C.; Seitz, A.K.; Tauber, R.; Kubler, H.; Maurer, T.; Thalgott, M.; Hatzichristodoulou, G.; Hoppner, M.; et al. Chromogranin a and Neurone-Specific Enolase Serum Levels as Predictors of Treatment Outcome in Patients with Metastatic Castration-Resistant Prostate Cancer Undergoing Abiraterone Therapy. BJU Int. 2017, 119, 30–37. [Google Scholar] [CrossRef]
- Sainio, M.; Visakorpi, T.; Tolonen, T.; Ilvesaro, J.; Bova, G.S. Expression of Neuroendocrine Differentiation Markers in Lethal Metastatic Castration-Resistant Prostate Cancer. Pathol. Res. Pract. 2018, 214, 848–856. [Google Scholar] [CrossRef]
- Conteduca, V.; Oromendia, C.; Eng, K.W.; Bareja, R.; Sigouros, M.; Molina, A.; Faltas, B.M.; Sboner, A.; Mosquera, J.M.; Elemento, O.; et al. Clinical Features of Neuroendocrine Prostate Cancer. Eur. J. Cancer 2019, 121, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. Eau-Estro-Siog Guidelines on Prostate Cancer. Part Ii: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Mankuzhy, N.P.; Almahariq, M.F.; Ye, H.; Amin, M.; Stone, B.; Krauss, D.J. Investigation of the Prognostic Significance of Neuroendocrine Differentiation in Gleason Score 7 to 10 Prostate Adenocarcinoma in Patients with Distant Metastasis after Definitive Radiotherapy. Am. J. Clin. Pathol 2021, 155, 879–886. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Albisinni, S.; Presicce, F.; Lombardo, R.; Cancrini, F.; Tubaro, A. Serum Levels of Chromogranin a Are Not Predictive of High-Grade, Poorly Differentiated Prostate Cancer: Results from an Italian Biopsy Cohort. Urol. Oncol. 2014, 32, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Bollito, E.; Cracco, C.M.; Volante, M.; Ciccone, G.; Porpiglia, F.; Papotti, M.; Scarpa, R.M.; Dogliotti, L. The Prognostic Role of Immunohistochemical Chromogranin a Expression in Prostate Cancer Patients Is Significantly Modified by Androgen-Deprivation Therapy. Prostate 2010, 70, 718–726. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Urinary and Male Genital Tumours, 5th ed.; IARC: Lyon, France, 2022.
- Parimi, V.; Goyal, R.; Poropatich, K.; Yang, X.J. Neuroendocrine Differentiation of Prostate Cancer: A Review. Am. J. Clin. Exp. Urol. 2014, 2, 273–285. [Google Scholar]
- Wang, W.; Epstein, J.I. Small Cell Carcinoma of the Prostate. A Morphologic and Immunohistochemical Study of 95 Cases. Am. J. Surg. Pathol. 2008, 32, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Cindolo, L.; Cantile, M.; Franco, R.; Chiodini, P.; Schiavo, G.; Forte, I.; Zlobec, I.; Salzano, L.; Botti, G.; Gidaro, S.; et al. Parallel Determination of Neurod1, Chromogranin-a, Ki67 and Androgen Receptor Expression in Surgically Treated Prostate Cancers. Int. Braz. J. Urol. 2011, 37, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Krauss, D.J.; Amin, M.; Stone, B.; Ye, H.; Hayek, S.; Cotant, M.; Hafron, J.; Brabbins, D.S. Chromogranin a Staining as a Prognostic Variable in Newly Diagnosed Gleason Score 7-10 Prostate Cancer Treated with Definitive Radiotherapy. Prostate 2014, 74, 520–527. [Google Scholar] [CrossRef]
- Kokubo, H.; Yamada, Y.; Nishio, Y.; Fukatsu, H.; Honda, N.; Nakagawa, A.; Saga, S.; Tsuzuki, T.; Hara, K. Immunohistochemical Study of Chromogranin a in Stage D2 Prostate Cancer. Urology 2005, 66, 135–140. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Wang, J.; Zhu, Z.; Luo, L.; Li, E.; Tang, F.; Zhao, Z. Serum Neuroendocrine Markers Predict Therapy Outcome of Patients with Metastatic Castration-Resistant Prostate Cancer: A Meta-Analysis. Urol. Int. 2019, 102, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Giridhar, K.V.; Sanhueza, C.; Hillman, D.W.; Alkhateeb, H.; Carlson, R.; Tan, W.; Costello, B.A.; Quevedo, F.; Pagliaro, L.; Kohli, M. Serum Chromogranin-a-Based Prognosis in Metastatic Castration-Resistant Prostate Cancer. Prostate Cancer Prostatic Dis. 2018, 21, 431–437. [Google Scholar] [CrossRef]
- Niedworok, C.; Tschirdewahn, S.; Reis, H.; Lehmann, N.; Szucs, M.; Nyirady, P.; Romics, I.; Rubben, H.; Szarvas, T. Serum Chromogranin a as a Complementary Marker for the Prediction of Prostate Cancer-Specific Survival. Pathol. Oncol. Res. 2017, 23, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.O.; Ather, M.H. Chromogranin A—Serum Marker for Prostate Cancer. J. Pak. Med. Assoc. 2011, 61, 108–111. [Google Scholar]
- Fan, L.; Wang, Y.; Chi, C.; Pan, J.; Xun, S.; Xin, Z.; Hu, J.; Zhou, L.; Dong, B.; Xue, W. Chromogranin a and Neurone-Specific Enolase Variations During the First 3 Months of Abiraterone Therapy Predict Outcomes in Patients with Metastatic Castration-Resistant Prostate Cancer. BJU Int. 2017, 120, 226–232. [Google Scholar] [CrossRef]
- Grimaldi, F.; Valotto, C.; Barbina, G.; Visentini, D.; Trianni, A.; Cerruto, M.A.; Zattoni, F. The Possible Role of Chromogranin a as a Prognostic Factor in Organ-Confined Prostate Cancer. Int. J. Biol. Markers 2006, 21, 229–234. [Google Scholar] [CrossRef]
- Conteduca, V.; Burgio, S.L.; Menna, C.; Carretta, E.; Rossi, L.; Bianchi, E.; Masini, C.; Amadori, D.; De Giorgi, U. Chromogranin a Is a Potential Prognostic Marker in Prostate Cancer Patients Treated with Enzalutamide. Prostate 2014, 74, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Bech, P.R.; Martin, N.M.; Ramachandran, R.; Bloom, S.R. The Biochemical Utility of Chromogranin a, Chromogranin B and Cocaine- and Amphetamine-Regulated Transcript for Neuroendocrine Neoplasia. Ann. Clin. Biochem. 2014, 51 Pt 1, 8–21. [Google Scholar] [CrossRef]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.; Varambally, S.; et al. Divergent Clonal Evolution of Castration-Resistant Neuroendocrine Prostate Cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef]
- Su, R.; Chen, L.; Jiang, Z.; Yu, M.; Zhang, W.; Ma, Z.; Ji, Y.; Shen, K.; Xin, Z.; Qi, J.; et al. Comprehensive Analysis of Androgen Receptor Status in Prostate Cancer with Neuroendocrine Differentiation. Front. Oncol. 2022, 12, 955166. [Google Scholar] [CrossRef]
- Aggarwal, R.; Huang, J.; Alumkal, J.J.; Zhang, L.; Feng, F.Y.; Thomas, G.V.; Weinstein, A.S.; Friedl, V.; Zhang, C.; Witte, O.N.; et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-Institutional Prospective Study. J. Clin. Oncol. 2018, 36, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Amin, M.B.; Beltran, H.; Lotan, T.L.; Mosquera, J.M.; Reuter, V.E.; Robinson, B.D.; Troncoso, P.; Rubin, M.A. Proposed Morphologic Classification of Prostate Cancer with Neuroendocrine Differentiation. Am. J. Surg. Pathol. 2014, 38, 756–767. [Google Scholar] [CrossRef]
- Abida, W.; Cyrta, J.; Heller, G.; Prandi, D.; Armenia, J.; Coleman, I.; Cieslik, M.; Benelli, M.; Robinson, D.; Van Allen, E.M.; et al. Genomic Correlates of Clinical Outcome in Advanced Prostate Cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 11428–11436. [Google Scholar] [CrossRef]
- Nava Rodrigues, D.; Casiraghi, N.; Romanel, A.; Crespo, M.; Miranda, S.; Rescigno, P.; Figueiredo, I.; Riisnaes, R.; Carreira, S.; Sumanasuriya, S.; et al. Rb1 Heterogeneity in Advanced Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Liu, M.; Han, D.; Li, M.; Toure, A.A.; Wang, Z.; Besschetnova, A.; Patalano, S.; Macoska, J.A.; Gao, S.; et al. Rb1 Loss in Castration-Resistant Prostate Cancer Confers Vulnerability to Lsd1 Inhibition. Oncogene 2022, 41, 852–864. [Google Scholar] [CrossRef]
- Ku, S.Y.; Rosario, S.; Wang, Y.; Mu, P.; Seshadri, M.; Goodrich, Z.W.; Goodrich, M.M.; Labbe, D.P.; Gomez, E.C.; Wang, J.; et al. Rb1 and Trp53 Cooperate to Suppress Prostate Cancer Lineage Plasticity, Metastasis, and Antiandrogen Resistance. Science 2017, 355, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, M.D.; Corella, A.; Coleman, I.; De Sarkar, N.; Kaipainen, A.; Ha, G.; Gulati, R.; Ang, L.; Chatterjee, P.; Lucas, J.; et al. Combined Tp53 and Rb1 Loss Promotes Prostate Cancer Resistance to a Spectrum of Therapeutics and Confers Vulnerability to Replication Stress. Cell Rep. 2020, 31, 107669. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Spring, D.J.; DePinho, R.A. Genetics and Biology of Prostate Cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef]
- Steffan, J.J.; Koul, S.; Meacham, R.B.; Koul, H.K. The Transcription Factor Spdef Suppresses Prostate Tumor Metastasis. J. Biol. Chem. 2012, 287, 29968–29978. [Google Scholar] [CrossRef]
- Dardenne, E.; Beltran, H.; Benelli, M.; Gayvert, K.; Berger, A.; Puca, L.; Cyrta, J.; Sboner, A.; Noorzad, Z.; MacDonald, T.; et al. N-Myc Induces an Ezh2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell 2016, 30, 563–577. [Google Scholar] [CrossRef]
- Kim, E.; Kim, M.; Woo, D.H.; Shin, Y.; Shin, J.; Chang, N.; Oh, Y.T.; Kim, H.; Rheey, J.; Nakano, I.; et al. Phosphorylation of Ezh2 Activates Stat3 Signaling Via Stat3 Methylation and Promotes Tumorigenicity of Glioblastoma Stem-Like Cells. Cancer Cell 2013, 23, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, K.; Yeh, S.; Sun, Y.; Liang, L.; Xiao, Y.; Xu, W.; Niu, Y.; Cheng, L.; Maity, S.N.; et al. Lncrna-P21 Alters the Antiandrogen Enzalutamide-Induced Prostate Cancer Neuroendocrine Differentiation Via Modulating the Ezh2/Stat3 Signaling. Nat. Commun. 2019, 10, 2571. [Google Scholar] [CrossRef]
- Shan, J.; Al-Muftah, M.A.; Al-Kowari, M.K.; Abuaqel, S.W.J.; Al-Rumaihi, K.; Al-Bozom, I.; Li, P.; Chouchane, L. Targeting Wnt/Ezh2/Microrna-708 Signaling Pathway Inhibits Neuroendocrine Differentiation in Prostate Cancer. Cell Death Discov. 2019, 5, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, D.; Zhou, T.; Song, H.; Hulsurkar, M.; Su, N.; Liu, Y.; Wang, Z.; Shao, L.; Ittmann, M.; et al. Androgen Deprivation Promotes Neuroendocrine Differentiation and Angiogenesis through Creb-Ezh2-Tsp1 Pathway in Prostate Cancers. Nat. Commun. 2018, 9, 4080. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Initial Biopsy | Repeated Biopsy | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | With CgA Expression | Without CgA Expression | Total | With CgA Expression | Without CgA Expression | |||
| (n = 68) | (n = 17) | (n = 51) | p | (n = 68) | (n = 29) | (n = 39) | p | |
| Median age (range) | 68 (51–84) | 67 (51–83) | 69 (52–84) | 0.228 | 70 (54–86) | 67 (54–85) | 70 (54–86) | 0.136 |
| ISUP/WHO2016 grade group | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Group 3 | 4 (5.9) | 2 (11.8) | 2 (3.9) | 0.400 | 2 (2.9) | 1 (3.4) | 1 (2.6) | 0.977 |
| Group 4 | 17 (25) | 3 (17.6) | 14 (27.5) | 3 (4.4) | 1 (3.4) | 2 (5.1) | ||
| Group 5 | 47 (69.1) | 12 (70.6) | 35 (68.6) | 41 (60.3) | 18 (62.1) | 23 (59.0) | ||
| Not grouped | 22 (32.4) | 9 (31.1) | 13 (33.3) | |||||
| Gleason score | ||||||||
| <9 | 21 (30.9) | 5 (29.4) | 16 (31.4) | 0.880 | 5 (7.4) | 2 (6.9) | 3 (7.7) | 0.967 |
| ≥9 | 47 (69.1) | 12 (70.6) | 35 (68.6) | 41 (60.3) | 18 (62.1) | 23 (59.0) | ||
| Not grouped | 22 (32.4) | 9 (31) | 13 (33.3) | |||||
| Castration | ||||||||
| Surgery | 22 (32.4) | 8 (47.1) | 14 (27.5) | 0.134 | 22 (32.4) | 10 (34.5) | 12 (30.8) | 0.746 |
| Drugs | 46 (67.6) | 9 (52.9) | 37 (72.5) | 46 (67.6) | 19 (65.5) | 27 (69.2) | ||
| Metastasis | ||||||||
| Visceral metastasis | 2 (2.9) | 1 (5.9) | 1 (2.0) | 0.440 | 2 (2.9) | 1 (3.4) | 1 (2.6) | 1.000 |
| Bone metastasis | 66 (97.1) | 16 (94.1) | 50 (98.0) | 66 (97.1) | 28 (96.6) | 38 (97.4) | ||
| PSA (ng/mL) | ||||||||
| ≥100 | 43 (63.2) | 10 (58.8) | 33 (64.7) | 0.663 | 43 (63.2) | 15 (51.7) | 28 (71.8) | 0.090 |
| <100 | 25 (36.8) | 7 (41.2) | 18 (35.3) | 25 (36.8) | 14 (48.3) | 11 (28.2) | ||
| Variates | OS1st (Months) | OS2nd (Months) | ||
|---|---|---|---|---|
| Median (95% CI) | p | Median (95% CI) | p | |
| Residual tumor of mCRPC (%) | ||||
| ≥40% vs. <40% | 47.8 (25.5–70.0) vs. 58.7 (43.5–73.8) | 0.487 | 23.6 (11.4–35.8) vs. 36.5 (25.3–47.7) | 0.100 |
| Age at first diagnosis (y) | ||||
| ≥68 vs. <68 | 49.1 (35.6–62.6) vs. 41.5 (15.1–67.9) | 0.415 | 25.9 (11.3–40.4) vs. 29.5 (13.3–45.6) | 0.822 |
| Age at mCRPC status (y) | ||||
| ≥70 vs. <70 | 50.0 (35.5–64.5) vs. 41.5 (22.4–60.6) | 0.129 | 25.9 (10.8–41.0) vs. 29.5 (19.3–39.6) | 0.824 |
| GS at mHSPC status | ||||
| ≥9 vs. <9 | 41.3 (26.1–56.5) vs. 86.5 (35.1–137.8) | 0.015 | 22.2 (12.6–31.8) vs. 44.0 | 0.004 |
| GS at mCRPC status | ||||
| ≥9 vs. <9 | 49.1 (25.3–73.0) vs. 58.7 (0–120.9) | 0.625 | 32.3 (25.9–38.7) vs. 46.4 (27.4–65.3) | 0.415 |
| ISUP/WHO 2016 grade group | ||||
| Group 3 vs. group 4 vs. group 5 | 67.3 (38.2–96.4) vs. 64.7 (49.1–80.4) vs. 51.8 (32.9–70.8) | 0.128 | 50.9 (28.8–72.9) vs. 42.2 (32.2–52.1) vs. 25.8 (20.2–31.3) | 0.074 |
| CgA status of mHSPC | ||||
| Positive vs. negative | 21.7 (9.7–33.7) vs. 58.7 (44.2–73.1) | 0.017 | 12.1 (7.4–16.8) vs. 33.3 (23.4–43.3) | 0.103 |
| CgA ≥ 10% vs. CgA < 10% | 15.8 (4.9–26.8) vs. 48.4 (25.6–71.3) | <0.001 | 10.9 (0.7–21.0) vs. 31.9 (21.7–42.1) | 0.001 |
| CgA status of mCRPC | ||||
| Positive vs. negative | 35.0 (11.5–58.5) vs. 58.7 (39.6–77.8) | 0.025 | 14.7 (8.5–20.8) vs. 37.3 (23.4–51.2) | 0.023 |
| CgA ≥ 10% vs. CgA < 10% | 20.5 (14.0–27.0) vs. 51.1 (39.8–62.4) | 0.007 | 12.1 (9.7–14.5) vs. 31.9 (21.2–42.6) | 0.001 |
| Castration | ||||
| Surgical vs. medical | 47.8 (30.0–66.0) vs. 49.1 (35.4–62.8) | 0.743 | 25.2 (7.5–43.0) vs. 27.0 (16.6–37.3) | 0.459 |
| Metastasis at mCRPC | ||||
| Visceral metastasis vs. bone metastasis only | 59.9 (14.9–104.9) vs. 48.4 (30.0–66.9) | 0.843 | 49.2 (22.2–76.2) vs. 25.2 (12.9–37.6) | 0.600 |
| Serum PSA at mCRPC (ng/mL) | ||||
| ≥100 vs. <100 | 41.5 (25.4–57.6) vs. 58.8 (35.0–82.6) | 0.332 | 31.3 (7.9–54.8) vs. 25.2 (15.8–34.7) | 0.697 |
| Variables | Data Type | OS1st (Months) | OS2nd (Months) | ||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Age at mHSPC status | Continuous | 0.99 (0.96–1.03) | 0.588 | 1.00 (0.97–1.04) | 0.754 |
| Age at mCRPC status | Continuous | 0.97 (0.94–1.00) | 0.058 | 1.00 (0.96–1.03) | 0.833 |
| Tumor% at mCRPC status | Continuous | 1.00 (0.99–1.01) | 0.773 | 1.00 (1.00–1.01) | 0.381 |
| Gleason score at mHSPC status | ≥9 vs. <9 | 2.048 (1.02–4.48) | 0.055 | 2.26 (1.13–4.97) | 0.015 |
| CgA status of mHSPC | ≥1% vs. <1% | 2.28 (1.14–4.58) | 0.020 | 1.75 (0.89–3.48) | 0.108 |
| Proportion of CgA at mHSPC status | ≥10% vs. <10% | 4.29 (1.89–9.73) | <0.001 | 2.81 (1.26–6.26) | 0.012 |
| CgA status of mCRPC | ≥1% vs. <1% | 1.77 (1.07–2.95) | 0.028 | 1.78 (1.08–2.95) | 0.025 |
| Proportion of CgA at mCRPC status | ≥10% vs. <10% | 2.27 (1.24–4.16) | 0.008 | 2.84 (1.53–5.29) | 0.001 |
| Castration method | Surgery vs. medicine | 1.09 (0.65–1.82) | 0.744 | 1.21 (0.73–2.01) | 0.460 |
| Metastasis of mCRPC | Viscera vs. bone | 1.11 (0.40–3.08) | 0.843 | 0.76 (0.27–2.12) | 0.601 |
| Serum PSA level of mCRPC (ng/mL) | ≥100 vs. <100 | 1.32 (0.75–2.31) | 0.334 | 1.15 (0.64–1.93) | 0.698 |
| Variates | Data Type | OS1st (Months) | OS2nd (Months) | ||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| For mHSPC | |||||
| Age at mHSPC status | Continuous | 1.00 (0.96–1.04) | 0.804 | 1.00 (0.96–1.04) | 0.994 |
| GS at first biopsy | ≥9 vs. <9 | 1.97 (0.98–4.33) | 0.070 | 2.19 (1.09–4.82) | 0.037 |
| CgA expression | ≥1% vs. <1% | 2.16 (1.04–4.26) | 0.031 | 1.67 (0.81–3.23) | 0.145 |
| For mCRPC | |||||
| Age at mCRPC status | Continuous | 1.05 (0.97–1.17) | 0.265 | 1.03 (0.95–1.12) | 0.533 |
| GS at second biopsy | ≥9 vs. <9 | 7.37 (1.84–54.79) | 0.020 | 4.92 (1.33–31.52) | 0.054 |
| CgA expression | ≥10% vs. <10% | 20.19 (3.04–329.9) | 0.008 | 5.17 (1.10–33.13) | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Tang, Y.; Wei, Y.; Qian, J.; Kang, Y.; Wang, D.; Xu, M.; Nie, L.; Chen, X.; Chen, N.; et al. Prognostic Significance of Chromogranin A Expression in the Initial and Second Biopsies in Metastatic Prostate Cancer. J. Clin. Med. 2023, 12, 3362. https://doi.org/10.3390/jcm12103362
Huang Z, Tang Y, Wei Y, Qian J, Kang Y, Wang D, Xu M, Nie L, Chen X, Chen N, et al. Prognostic Significance of Chromogranin A Expression in the Initial and Second Biopsies in Metastatic Prostate Cancer. Journal of Clinical Medicine. 2023; 12(10):3362. https://doi.org/10.3390/jcm12103362
Chicago/Turabian StyleHuang, Zhuo, Ying Tang, Yuyan Wei, Jingyu Qian, Yifan Kang, Duohao Wang, Miao Xu, Ling Nie, Xueqin Chen, Ni Chen, and et al. 2023. "Prognostic Significance of Chromogranin A Expression in the Initial and Second Biopsies in Metastatic Prostate Cancer" Journal of Clinical Medicine 12, no. 10: 3362. https://doi.org/10.3390/jcm12103362
APA StyleHuang, Z., Tang, Y., Wei, Y., Qian, J., Kang, Y., Wang, D., Xu, M., Nie, L., Chen, X., Chen, N., & Zhou, Q. (2023). Prognostic Significance of Chromogranin A Expression in the Initial and Second Biopsies in Metastatic Prostate Cancer. Journal of Clinical Medicine, 12(10), 3362. https://doi.org/10.3390/jcm12103362






