Sarcopenia Is a Prognostic Factor in Patients Undergoing Percutaneous Endoscopic Gastrostomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Diagnosis of Sarcopenia
2.3. Outcomes
2.4. Statistical Analysis
3. Results
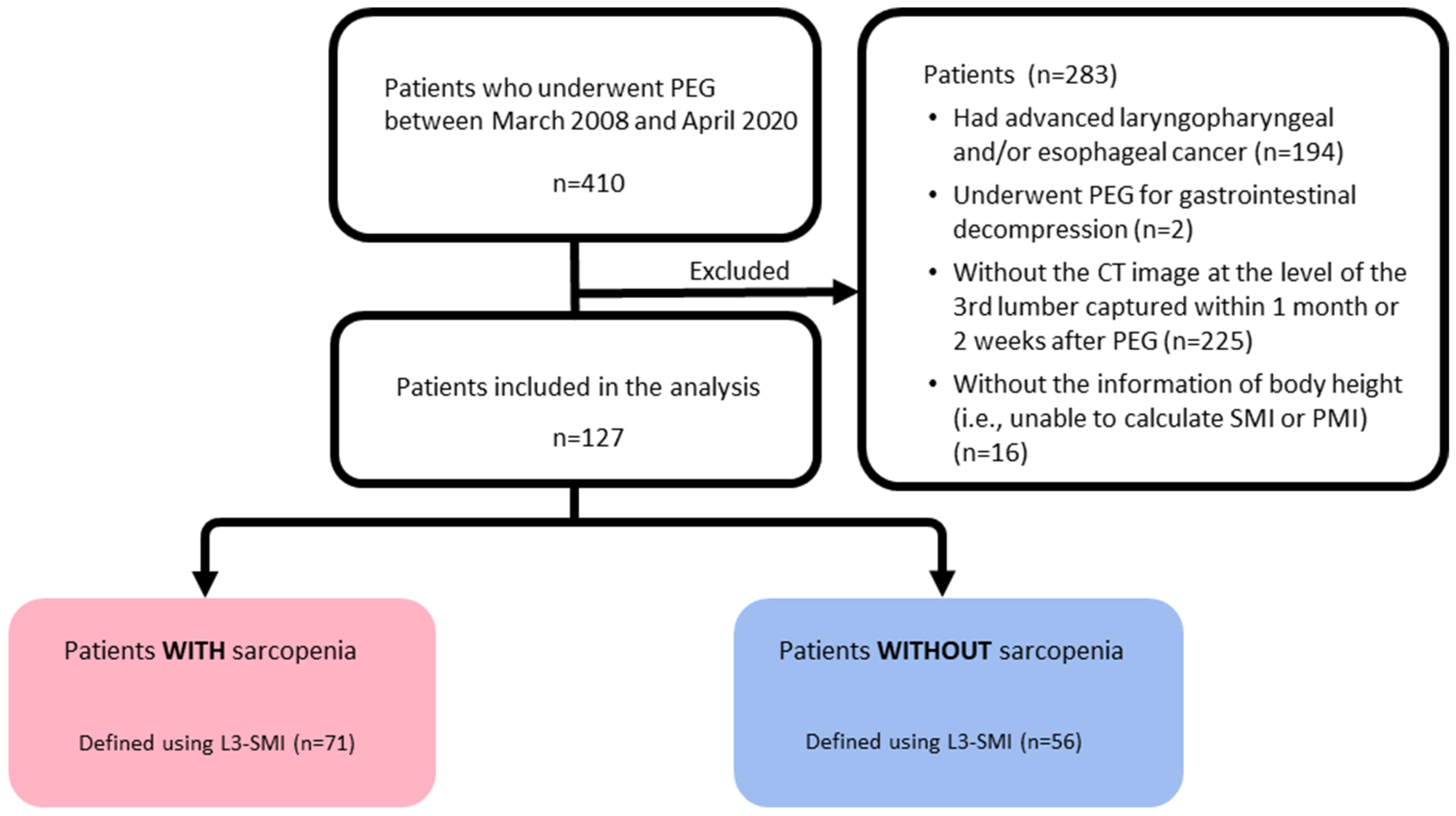
3.1. Patients Enrollments
3.2. Patients Characteristics
3.3. Overall Follow-Up Period and Events
3.4. Survival Rates and Log-Rank Analyses at 90 and 180 Days and One Year
3.5. Univariate and Multivariate Cox Proportional Hazard Analyses
3.6. Covariate-Balancing Propensity Score Matching Analyses
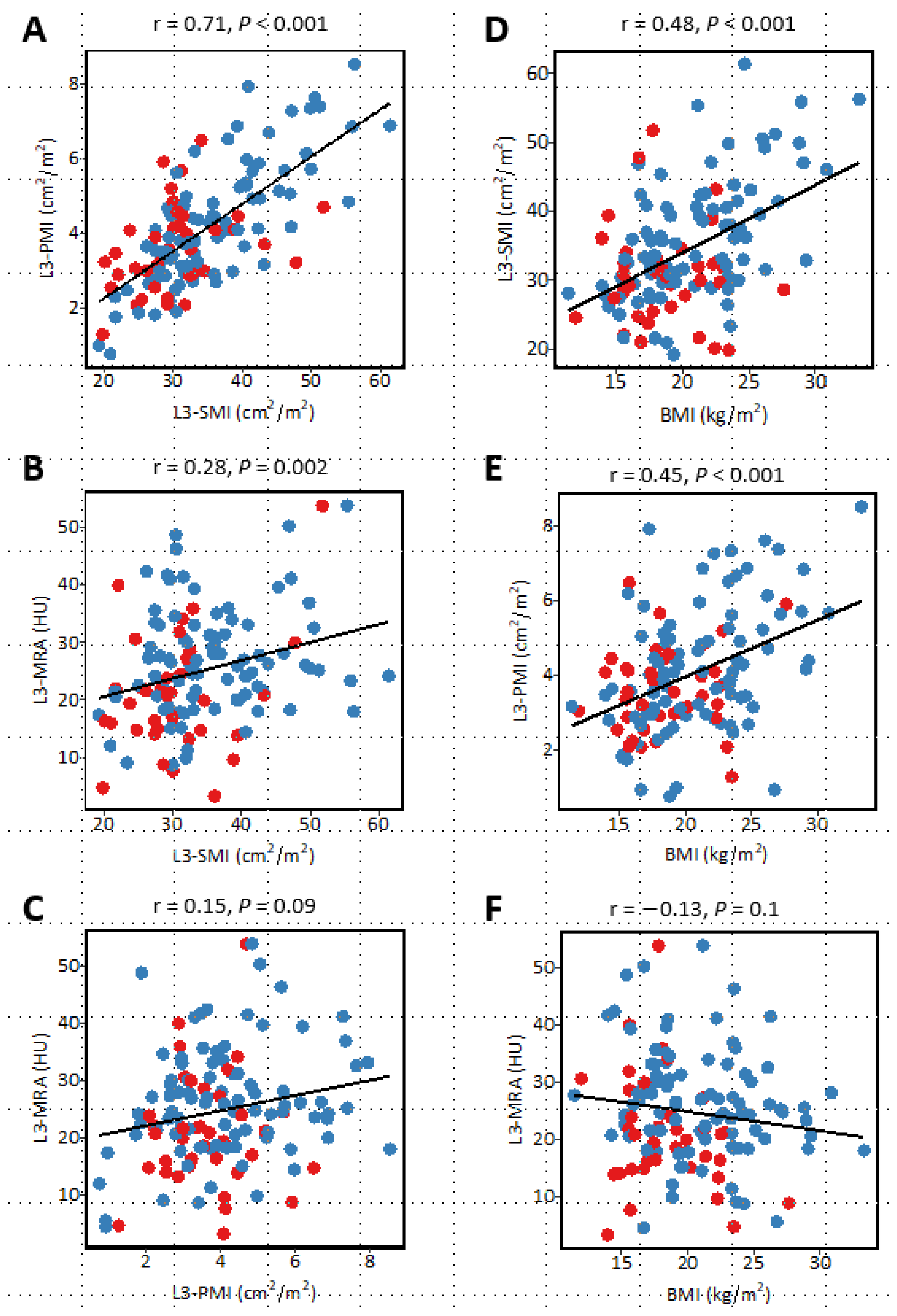
3.7. Correlation between Sarcopenia-Related Indices and Relationship with Body Mass Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauderer, M.W.; Ponsky, J.L.; Izant, R.J. Gastrostomy without laparotomy: A percutaneous endoscopic technique. Nutrition 1998, 14, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Rustom, I.K.; Jebreel, A.; Tayyab, M.; England, R.J.A.; Stafford, N.D. Percutaneous endoscopic, radiological and surgical gastrostomy tubes: A comparison study in head and neck cancer patients. J. Laryngol. Otol. 2006, 120, 463–466. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tamez, S.; Murakami, A.; Taira, A.; Mizuhara, A.; Horiuchi, A.; Mihara, C.; Ako, E.; Muramatsu, H.; Okano, H.; et al. Survival of geriatric patients after percutaneous endoscopic gastrostomy in Japan. World J. Gastroenterol. 2010, 16, 5084–5091. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, J.; Lagergren, P.; Martin, L.; Mattsson, F.; Lagergren, J. Albumin and C-reactive protein levels predict short-term mortality after percutaneous endoscopic gastrostomy in a prospective cohort study. Gastrointest. Endosc. 2011, 73, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T.; Kubo, T.; Ryan, S.; Tomizawa, M.; Yoshida, S.-I.; Takagi, K.; Furui, K.; Gotoh, T. Long-term outcome after placement of a percutaneous endoscopic gastrostomy tube. Geriatr. Gerontol. Int. 2008, 8, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef]
- Kim, G.; Kang, S.H.; Kim, M.Y.; Baik, S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186990. [Google Scholar] [CrossRef]
- Zhang, X.M.; Chen, D.; Xie, X.H.; Zhang, J.E.; Zeng, Y.; Cheng, A.S. Sarcopenia as a predictor of mortality among the critically ill in an intensive care unit: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 339. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, C.B.; Loveday, B.P.; Shrikhande, S.V.; Windsor, J.A.; Pandanaboyana, S. Impact of preoperative sarcopenia on postoperative outcomes following pancreatic resection: A systematic review and meta-analysis. Pancreatology 2018, 18, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chi, J.; Liu, Y.; Fan, L.; Hu, K. Impact of preoperative sarcopenia on postoperative complications and prognosis of gastric cancer resection: A meta-analysis of cohort studies. Arch. Gerontol. Geriatr. 2022, 98, 104534. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-C.; Wu, C.-H.; Tien, Y.-W.; Lu, T.-P.; Wang, Y.-H.; Chen, B.-B. Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur. Radiol. 2021, 31, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Iritani, S.; Imai, K.; Takai, K.; Hanai, T.; Ideta, T.; Miyazaki, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M.; Moriwaki, H. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J. Gastroenterol. 2015, 50, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef]
- Mccusker, A.; Khan, M.; Kulvatunyou, N.; Zeeshan, M.; Sakran, J.V.; Hayek, H.; O’Keeffe, T.; Hamidi, M.; Tang, A.; Joseph, B. Sarcopenia defined by a computed tomography estimate of the psoas muscle area does not predict frailty in geriatric trauma patients. Am. J. Surg. 2019, 218, 261–265. [Google Scholar] [CrossRef]
- Dello, S.A.W.G.; Lodewick, T.M.; van Dam, R.M.; Reisinger, K.W.; Broek, M.A.J.V.D.; von Meyenfeldt, M.F.; Bemelmans, M.H.A.; Damink, S.W.M.O.; Dejong, C.H.C. Sarcopenia negatively affects preoperative total functional liver volume in patients undergoing liver resection. HPB 2013, 15, 165–169. [Google Scholar] [CrossRef]
- Tegels, J.J.; van Vugt, J.L.; Reisinger, K.W.; Hulsewé, K.W.; Hoofwijk, A.G.; Derikx, J.P.; Stoot, J.H. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J. Surg. Oncol. 2015, 112, 403–407. [Google Scholar] [CrossRef]
- Reisinger, K.W.; van Vugt, J.L.A.; Tegels, J.J.W.; Snijders, C.; Hulsewé, K.W.E.; Hoofwijk, A.G.M.; Stoot, J.H.; Von Meyenfeldt, M.F.; Beets, G.L.; Derikx, J.P.M.; et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann. Surg. 2015, 261, 345–352. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016, 32, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Flores, A.O.; Álvarez-Villaseñor, A.D.S.; Fuentes-Orozco, C.; Ramírez-Campos, K.M.; Ramírez-Arce, A.D.R.; Macías-Amezcua, M.D.; Chavez-Tostado, M.; Hernández-Machuca, J.S.; González-Ojeda, A. Long-term outcome after percutaneous endoscopic gastrostomy in geriatric Mexican patients. Geriatr. Gerontol. Int. 2015, 15, 19–26. [Google Scholar] [CrossRef]
- Okumura, S.; Kaido, T.; Hamaguchi, Y.; Fujimoto, Y.; Masui, T.; Mizumoto, M.; Hammad, A.; Mori, A.; Takaori, K.; Uemoto, S. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery 2015, 157, 1088–1098. [Google Scholar] [CrossRef]
- Zhou, C.-J.; Zhang, F.-M.; Zhang, F.-Y.; Yu, Z.; Chen, X.-L.; Shen, X.; Zhuang, C.-L. Sarcopenia: A new predictor of postoperative complications for elderly gastric cancer patients who underwent radical gastrectomy. J. Surg. Res. 2017, 211, 137–146. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Baba, Y.; Sakamoto, Y.; Ohuchi, M.; Tokunaga, R.; Kurashige, J.; Hiyoshi, Y.; Iwagami, S.; Yoshida, N.; Yoshida, M.; et al. Sarcopenia is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann. Surg. Oncol. 2015, 22, 2663–2668. [Google Scholar] [CrossRef]
- Harada, K.; Ida, S.; Baba, Y.; Ishimoto, T.; Kosumi, K.; Tokunaga, R.; Izumi, D.; Ohuchi, M.; Nakamura, K.; Kiyozumi, Y.; et al. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis. Esophagus 2016, 29, 627–633. [Google Scholar] [CrossRef]
- Benmassaoud, A.; Roccarina, D.; Arico, F.; Leandro, G.; Yu, B.; Cheng, F.; Yu, D.; Patch, D.; Tsochatzis, E. Sarcopenia Does Not Worsen Survival in Patients With Cirrhosis Undergoing Transjugular Intrahepatic Portosystemic Shunt for Refractory Ascites. Am. J. Gastroenterol. 2020, 115, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Ando, Y.; Gyawali, B.; Shimokata, T.; Maeda, O.; Fukaya, M.; Goto, H.; Nagino, M.; Kodera, Y. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol. Rep. 2016, 35, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Grotenhuis, B.A.; Shapiro, J.; Van Adrichem, S.; De Vries, M.; Koek, M.; Wijnhoven, B.P.L.; Van Lanschot, J.J.B. Sarcopenia/Muscle Mass is not a Prognostic Factor for Short- and Long-Term Outcome After Esophagectomy for Cancer. World J. Surg. 2016, 40, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Watanabe, M.; Yoshida, N.; Baba, Y.; Umezaki, N.; Harada, K.; Karashima, R.; Imamura, Y.; Iwagami, S.; Baba, H. Sarcopenia is a Predictor of Postoperative Respiratory Complications in Patients with Esophageal Cancer. Ann. Surg. Oncol. 2015, 22, 4432–4437. [Google Scholar] [CrossRef]
- Biolo, G.; Zorat, F.; Antonione, R.; Ciocchi, B. Muscle glutamine depletion in the intensive care unit. Int. J. Biochem. Cell. Biol. 2005, 37, 2169–2179. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Van Der Werf, A.; Dekker, I.M.; Meijerink, M.R.; Wierdsma, N.J.; De Van Der Schueren, M.A.E.; Langius, J.A.E. Skeletal muscle analyses: Agreement between non-contrast and contrast CT scan measurements of skeletal muscle area and mean muscle attenuation. Clin. Physiol. Funct. Imaging 2018, 38, 366–372. [Google Scholar] [CrossRef]


| L3-SMI | L3-PMI | L3-MRA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | High | Low | p Value | High | Low | p Value | High | Low | p Value | |||||||
| n | 56 | 71 | 26 | 101 | 63 | 64 | ||||||||||
| Sex, n (%) | Female | 20 | (35.7) | 18 | (25.4) | 0.2 | 15 | (57.7) | 23 | (22.8) | 0.001 | 19 | (30.1) | 18 | (28.1) | 1 |
| Male | 36 | (64.3) | 53 | (74.6) | 11 | (42.3) | 78 | (77.2) | 44 | (70.0) | 46 | (71.9) | ||||
| Age, mean (SD) | years | 70.0 | (14.3) | 75.4 | (14.5) | 0.04 | 71.0 | (11.5) | 73.5 | (15.3) | 0.4 | 71.8 | (15.3) | 74.2 | (13.9) | 0.4 |
| Body height, mean (SD) | cm | 159.9 | (9.3) | 161.1 | (9.1) | 0.5 | 157.1 | (7.6) | 161.5 | (9.4) | 0.03 | 160.4 | (8.4) | 161.1 | (10.0) | 0.7 |
| Body weight, mean (SD) | kg | 56.1 | (15.2) | 49.9 | (11.6) | 0.01 | 52.8 | (14.1) | 52.6 | (13.6) | 0.9 | 50.0 | (12.2) | 55.3 | (14.6) | 0.03 |
| BMI, mean (SD) | kg/m2 | 21.7 | (4.3) | 19.1 | (3.7) | <0.001 | 21.2 | (4.9) | 20.0 | (3.4) | 0.2 | 19.2 | (3.7) | 21.2 | (4.4) | 0.01 |
| Pneumonia, n (%) | No | 38 | (67.9) | 34 | (47.9) | 0.03 | 18 | (69.2) | 54 | (53.5) | 0.2 | 33 | (52.4) | 38 | (60.3) | 0.5 |
| Yes | 18 | (32.1) | 37 | (52.1) | 8 | (30.8) | 47 | (46.5) | 30 | (47.6) | 25 | (39.7) | ||||
| Total protein, mean (SD) | g/dL | 6.4 | (0.8) | 6.3 | (0.8) | 0.6 | 6.5 | (0.6) | 6.3 | (0.8) | 0.1 | 6.4 | (0.8) | 6.2 | (0.8) | 0.4 |
| Serum albumin, mean (SD) | g/dL | 3.0 | (0.6) | 2.9 | (0.6) | 0.2 | 3.2 | (0.5) | 2.9 | (0.6) | 0.03 | 3.0 | (0.6) | 2.8 | (0.6) | 0.04 |
| Serum TC, mean (SD) | mg/dL | 48.8 | (90.6) | 64.5 | (84.6) | 0.5 | 47.8 | (71.8) | 60.0 | (89.1) | 0.7 | 43.6 | (71.3) | 80.6 | (102.2) | 0.09 |
| Serum ChE, mean (SD) | U/L | 201 | (743.7) | 183.1 | (80) | 0.2 | 2364 | (69) | 180.8 | (76) | 0.002 | 198 | (78) | 183.2 | (77) | 0.3 |
| Serum CRP, mean (SD) | mg/dL | 1.2 | (2.2) | 1.8 | (2.4) | 0.2 | 1.1 | (1.6) | 1.6 | (2.4) | 0.3 | 1.1 | (1.7) | 1.9 | (2.7) | 0.04 |
| PNI, mean (SD) | 36.5 | (6.9) | 35.7 | (7.6) | 0.5 | 38.3 | (5.6) | 35.5 | (7.6) | 0.09 | 36.9 | (7.1) | 35.1 | (7.4) | 0.2 | |
| Hemoglobin, mean (SD) | g/dL | 11.7 | (1.8) | 11.1 | (2.0) | 0.08 | 11.9 | (1.7) | 11.2 | (1.9) | 0.1 | 11.7 | (1.9) | 11.0 | (1.9) | 0.03 |
| Platelet, mean (SD) | ×109/L | 261 | (121) | 233 | (106) | 0.2 | 306 | (146) | 230.1 | (99) | 0.002 | 258 | (134) | 230.9 | (87) | 0.2 |
| WBC, mean (SD) | ×109/L | 6.4 | (2.0) | 9.4 | (21.8) | 0.3 | 6.8 | (1.7) | 8.4 | (18.3) | 0.7 | 6.4 | (2.4) | 9.7 | (23.0) | 0.3 |
| TLC, mean (SD) | ×109/L | 1.3 | (0.5) | 1.4 | (0.7) | 0.3 | 1.4 | (0.5) | 1.4 | (0.7) | 0.8 | 1.3 | (0.6) | 1.4 | (0.7) | 0.5 |
| Univariate Cox Proportional Hazard Regression Analysis | Multivariate Cox Proportional Hazard Regression Analysis * | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p Value | Adjusted HR (95% CI) | p Value |
| L3-SMI, low group | 2.8 (1.6–4.8) | <0.001 | 2.9 (1.6–5.4) | <0.001 |
| L3-PMI, low group | 3.2 (1.5–7.1) | 0.004 | (-) | |
| L3-MRA, low group | 1.4 (0.9–2.4) | 0.2 | (-) | |
| Sex, male | 1.8 (1.0–3.3) | 0.046 | 2.0 (1.1–3.7) | 0.03 |
| Age, per year | 1.9 (1.2–3.2) | 0.01 | (-) | |
| BMI, per kg/mm2 | 0.94 (0.89–1.0) | 0.049 | (-) | |
| Serum albumin, per g/dL | 0.34 (0.21–0.56) | <0.001 | 0.34 (0.21–0.55) | <0.001 |
| TLC, <1.5 ×109/L | 1.8 (1.0–3.0) | 0.04 | (-) | |
| Serum CRP, ≥1.0 mg/dL | 2.5 (1.5–4.1) | 0.0004 | (-) | |
| Underlying pneumonia, yes | 1.9 (1.1–3.2) | 0.02 | (-) | |
| Before-Matching | After-Matching | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | High L3-SMI | Low L3-SMI | SMD | High L3-SMI | Low L3-SMI | SMD | |||||
| n | 56 | 71 | 37 | 37 | |||||||
| Matched variables | |||||||||||
| Sex, n (%) | Female | 20 | (35.7) | 18 | (25.4) | 0.226 | 13.0 | (35.1) | 11.0 | (29.7) | 0.116 |
| Male | 36 | (64.3) | 53 | (74.6) | 24.0 | (64.9) | 26.0 | (70.3) | |||
| Age, mean (SD) | years | 70.0 | (14.3) | 75.4 | (14.5) | 0.374 | 73.1 | (13.0) | 73.2 | (17.5) | 0.009 |
| BMI, mean (SD) | 21.7 | (4.3) | 19.1 | (3.7) | 0.644 | 21.3 | (4.7) | 20.6 | (4.1) | 0.15 | |
| Pneumonia, n (%) | No | 38 | (67.9) | 34 | (47.9) | 0.413 | 22.0 | (59.5) | 24.0 | (64.9) | 0.112 |
| Yes | 18 | (32.1) | 37 | (52.1) | 15.0 | (40.5) | 13.0 | (35.1) | |||
| Albumin, mean (SD) | g/dL | 3.0 | (0.6) | 2.9 | (0.6) | 0.235 | 2.9 | (0.6) | 3.0 | (0.7) | 0.072 |
| CRP, mean (SD) | mg/dL | 1.2 | (2.2) | 1.8 | (2.4) | 0.257 | 1.3 | (2.5) | 1.4 | (2.4) | 0.075 |
| TLC, mean (SD) | ×109/L | 1.3 | (0.5) | 1.4 | (0.7) | 0.176 | 1364.9 | (529.8) | 1224.3 | (530.4) | 0.265 |
| Non-matched variables | |||||||||||
| Body height, mean (SD) | cm | 159.9 | (9.3) | 161.1 | (9.1) | 0.134 | 8.8 | (8.8) | 161.6 | (8.7) | 0.341 |
| Body weight, mean (SD) | kg | 56.1 | (15.2) | 49.9 | (11.6) | 0.462 | 15.2 | (15.2) | 54.0 | (12.7) | 0.005 |
| Total protein, mean (SD) | g/dL | 6.4 | (0.8) | 6.3 | (0.8) | 0.098 | 1.0 | (0.9) | 6.2 | (0.7) | 0.146 |
| PNI, mean (SD) | 36.5 | (6.9) | 35.7 | (7.6) | 0.114 | 6.9 | (6.9) | 35.6 | (7.8) | 0.033 | |
| ChE, mean (SD) | U/L | 201.8 | (73.7) | 183.1 | (79.6) | 0.244 | 64.1 | (64.1) | 200.1 | (88.5) | 0.173 |
| WBC, mean (SD) | ×109/L | 6.4 | (2.0) | 9.4 | (21.8) | 0.193 | 1842.9 | (1842.9) | 11,329.7 | (30,116.8) | 0.241 |
| Hemoglobin, mean (SD) | g/dL | 11.7 | (1.8) | 11.1 | (2.0) | 0.315 | 1.8 | (1.7) | 11.0 | (2) | 0.182 |
| Platelet count, mean (SD) | ×109/L | 261.7 | (121.4) | 233.0 | (105.8) | 0.252 | 13.7 | (13.7) | 23.8 | (8.1) | 0.299 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, S.; Furuhashi, H.; Kisaki, S.; Horiuchi, H.; Matsui, H.; Dobashi, A.; Ojiri, H.; Sumiyama, K. Sarcopenia Is a Prognostic Factor in Patients Undergoing Percutaneous Endoscopic Gastrostomy. J. Clin. Med. 2023, 12, 3360. https://doi.org/10.3390/jcm12103360
Ono S, Furuhashi H, Kisaki S, Horiuchi H, Matsui H, Dobashi A, Ojiri H, Sumiyama K. Sarcopenia Is a Prognostic Factor in Patients Undergoing Percutaneous Endoscopic Gastrostomy. Journal of Clinical Medicine. 2023; 12(10):3360. https://doi.org/10.3390/jcm12103360
Chicago/Turabian StyleOno, Shingo, Hiroto Furuhashi, Shunsuke Kisaki, Hideka Horiuchi, Hiroaki Matsui, Akira Dobashi, Hiroya Ojiri, and Kazuki Sumiyama. 2023. "Sarcopenia Is a Prognostic Factor in Patients Undergoing Percutaneous Endoscopic Gastrostomy" Journal of Clinical Medicine 12, no. 10: 3360. https://doi.org/10.3390/jcm12103360
APA StyleOno, S., Furuhashi, H., Kisaki, S., Horiuchi, H., Matsui, H., Dobashi, A., Ojiri, H., & Sumiyama, K. (2023). Sarcopenia Is a Prognostic Factor in Patients Undergoing Percutaneous Endoscopic Gastrostomy. Journal of Clinical Medicine, 12(10), 3360. https://doi.org/10.3390/jcm12103360






