Ultra Short Heart Rate Variability Predicts Clinical Outcomes in Patients with a Clinical Presentation Consistent with Myocarditis: A Derivation Cohort Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. ECG and HRV Analysis
2.4. Endpoints
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
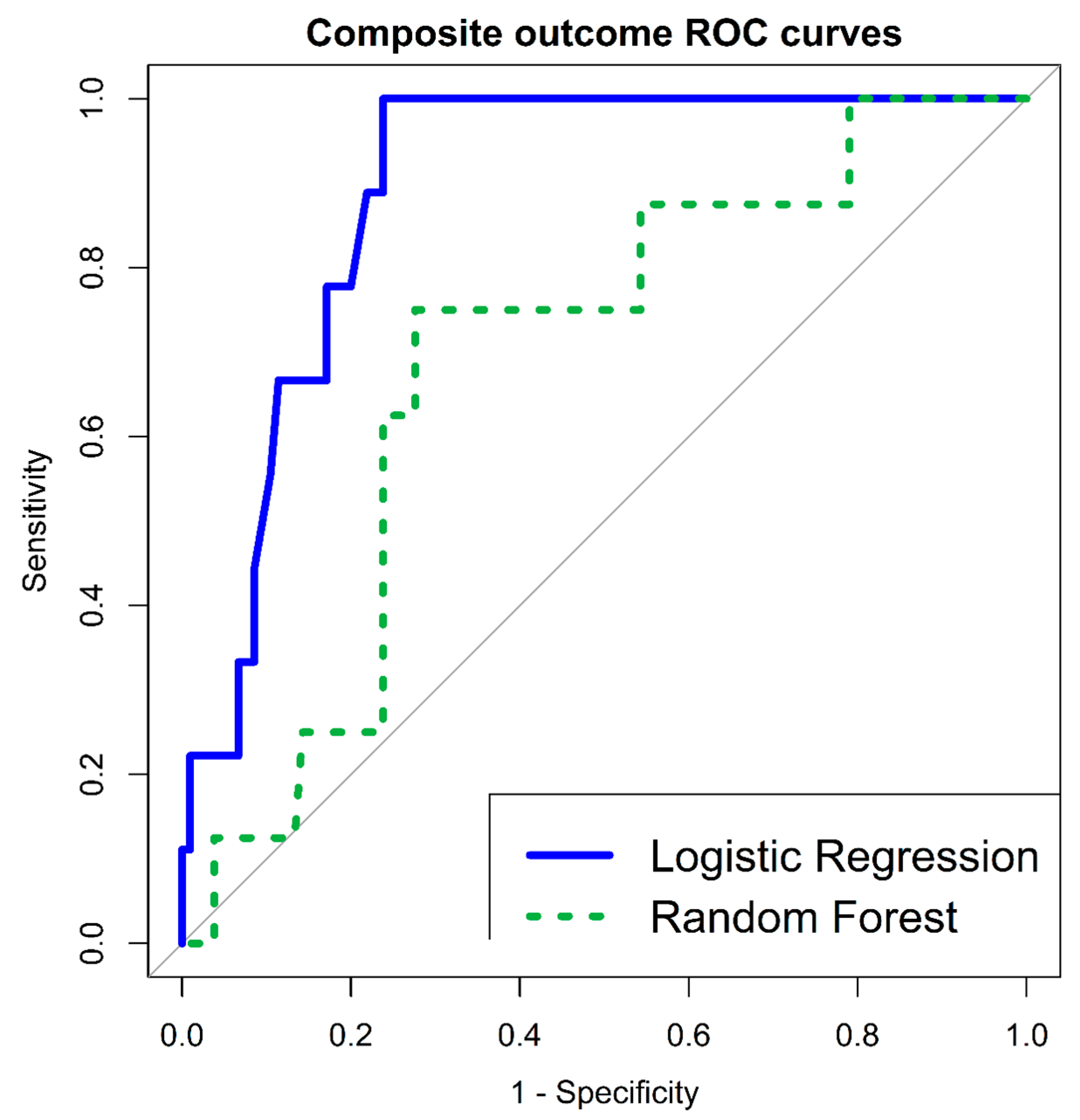
3.2. Heart Failure
3.3. Dilated Cardiomyopathy
3.4. Ventricular Arrhythmia
3.5. Mortality
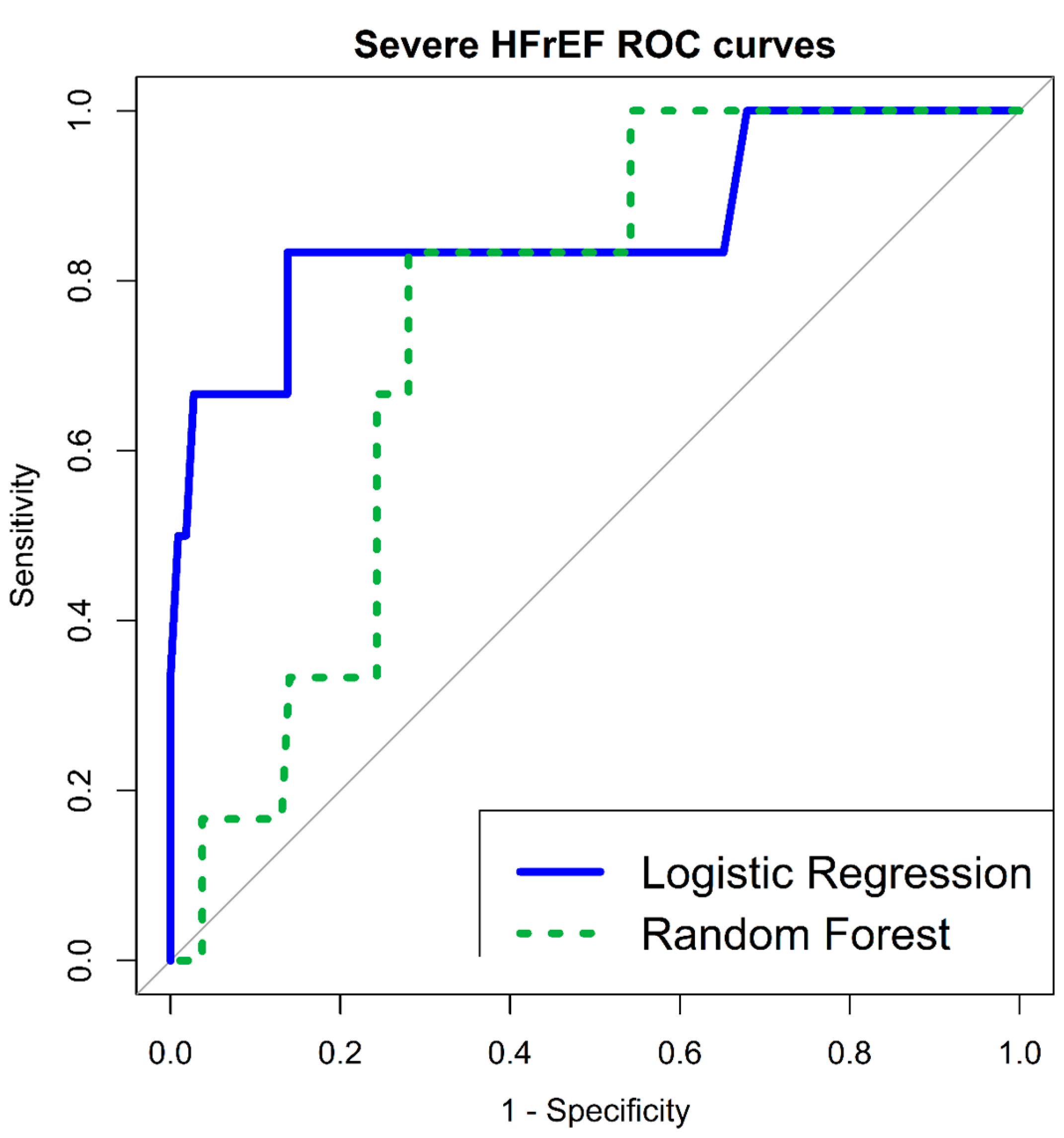
3.6. Severe Short-Term Disease-Specific Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T.J. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Inaba, O.; Satoh, Y.; Isobe, M.; Yamamoto, T.; Nagao, K.; Takayama, M. Factors and Values at Admission That Predict a Fulminant Course of Acute Myocarditis: Data from Tokyo CCU Network Database. Heart Vessel. 2017, 32, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Tsai, W.C.; Hsu, C.H.; Liu, P.Y.; Lin, L.J.; Chen, J.H. Predictive Factors of a Fulminant Course in Acute Myocarditis. Int. J. Cardiol. 2006, 109, 142–145. [Google Scholar] [CrossRef]
- Fischer, K.; Marggraf, M.; Stark, A.W.; Kaneko, K.; Aghayev, A.; Guensch, D.P.; Huber, A.T.; Steigner, M.; Blankstein, R.; Reichlin, T.; et al. Association of ECG Parameters with Late Gadolinium Enhancement and Outcome in Patients with Clinical Suspicion of Acute or Subacute Myocarditis Referred for CMR Imaging. PLoS ONE 2020, 15, e0227134. [Google Scholar] [CrossRef] [PubMed]
- Sanguineti, F.; Garot, P.; Mana, M.; O’h-Ici, D.; Hovasse, T.; Unterseeh, T.; Louvard, Y.; Troussier, X.; Morice, M.C.; Garot, J. Cardiovascular Magnetic Resonance Predictors of Clinical Outcome in Patients with Suspected Acute Myocarditis. J. Cardiovasc. Magn. Reson. 2015, 17, 78. [Google Scholar] [CrossRef]
- Grn, S.; Schumm, J.; Greulich, S.; Wagner, A.; Schneider, S.; Bruder, O.; Kispert, E.M.; Hill, S.; Ong, P.; Klingel, K.; et al. Long-Term Follow-Up of Biopsy-Proven Viral Myocarditis: Predictors of Mortality and Incomplete Recovery. J. Am. Coll. Cardiol. 2012, 59, 1604–1615. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdés-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-Synthesizing T Cells Relay Neural Signals in a Vagus Nerve Circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef]
- McCraty, R.; Shaffer, F. Heart Rate Variability: New Perspectives on Physiological Mechanisms, Assessment of Self-Regulatory Capacity, and Health Risk. Glob. Adv. Health Med. 2015, 4, 46–61. [Google Scholar] [CrossRef]
- Kuo, T.B.J.; Lai, C.J.; Huang, Y.-T.; Yang, C.C.H. Regression Analysis between Heart Rate Variability and Baroreflex-Related Vagus Nerve Activity in Rats. J. Cardiovasc. Electrophysiol. 2005, 16, 864–869. [Google Scholar] [CrossRef]
- Nussinovitch, U.; Elishkevitz, K.P.; Kaminer, K.; Nussinovitch, M.; Segev, S.; Volovitz, B.; Nussinovitch, N. The Efficiency of 10-Second Resting Heart Rate for the Evaluation of Short-Term Heart Rate Variability Indices. Pacing Clin. Electrophysiol. 2011, 34, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, U.; Elishkevitz, K.P.; Katz, K.; Nussinovitch, M.; Segev, S.; Volovitz, B.; Nussinovitch, N. Reliability of Ultra-Short ECG Indices for Heart Rate Variability. Ann. Noninvasive Electrocardiol. 2011, 16, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, U.; Cohen, O.; Kaminer, K.; Ilani, J.; Nussinovitch, N. Evaluating Reliability of Ultra-Short ECG Indices of Heart Rate Variability in Diabetes Mellitus Patients. J. Diabetes Complications 2012, 26, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.J.; Cho, C.H.; Cho, J.; Woo, J.M. Reliability of Ultra-Short-Term Analysis as a Surrogate of Standard 5-Min Analysis of Heart Rate Variability. Telemed. E-Health 2015, 21, 404–414. [Google Scholar] [CrossRef]
- Nussinovitch, U. Reliability of Ultra-Short Indices for Autonomic Dysfunction in Dyslipidemia. Clin. Physiol. Funct. Imaging 2020, 40, 423–433. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Karp, E.; Shiyovich, A.; Zahger, D.; Gilutz, H.; Grosbard, A.; Katz, A. Ultra-Short-Term Heart Rate Variability for Early Risk Stratification Following Acute St-Elevation Myocardial Infarction. Cardiology 2009, 114, 275–283. [Google Scholar] [CrossRef]
- Buccelletti, E.; Gilardi, E.; Scaini, E.; Galiuto, L.; Persiani, R.; Biondi, A.; Basile, F.; Silveri, N.G. Heart Rate Variability and Myocardial Infarction: Systematic Literature Review and Metanalysis. Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 299–307. [Google Scholar]
- Mol, M.B.A.; Strous, M.T.A.; van Osch, F.H.M.; Vogelaar, F.J.; Barten, D.G.; Farchi, M.; Foudraine, N.A.; Gidron, Y. Heart-Rate-Variability (HRV), Predicts Outcomes in COVID-19. PLoS ONE 2021, 16, e0258841. [Google Scholar] [CrossRef]
- Boehm, K.; Duckheim, M.; Mizera, L.; Groga-Bada, P.; Malek, N.; Kreth, F.; Gawaz, M.; Zuern, C.S.; Eick, C. Heart Rate Variability for Rapid Risk Stratification of Emergency Patients with Malignant Disease. Support. Care Cancer 2018, 26, 3289–3296. [Google Scholar] [CrossRef]
- De Couck, M.; Maréchal, R.; Moorthamers, S.; Van Laethem, J.-L.; Gidron, Y. Vagal Nerve Activity Predicts Overall Survival in Metastatic Pancreatic Cancer, Mediated by Inflammation. Cancer Epidemiol. 2016, 40, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Li, C.L.; Wang, Z.Z.; Zhang, H.N.; Xu, H.; An, X.J. Heart Rate Variability in Children with Myocarditis Presenting with Ventricular Arrhythmias. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1102–1105. [Google Scholar] [PubMed]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, M.E.; Rijnbeek, P.R.; Niemeijer, M.N.; Hofman, A.; van Herpen, G.; Bots, M.L.; Hillege, H.; Swenne, C.A.; Eijgelsheim, M.; Stricker, B.H.; et al. Normal Values of Corrected Heart-Rate Variability in 10-Second Electrocardiograms for All Ages. Front. Physiol. 2018, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Zagrosek, A.; Abdel-Aty, H.; Boyé, P.; Wassmuth, R.; Messroghli, D.; Utz, W.; Rudolph, A.; Bohl, S.; Dietz, R.; Schulz-Menger, J. Cardiac Magnetic Resonance Monitors Reversible and Irreversible Myocardial Injury in Myocarditis. JACC. Cardiovasc. Imaging 2009, 2, 131–138. [Google Scholar] [CrossRef]
- Cappola, T.P.; Felker, G.M.; Kao, W.H.L.; Hare, J.M.; Baughman, K.L.; Kasper, E.K. Pulmonary Hypertension and Risk of Death in Cardiomyopathy: Patients with Myocarditis Are at Higher Risk. Circulation 2002, 105, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Nishii, M.; Inomata, T.; Takehana, H.; Takeuchi, I.; Nakano, H.; Koitabashi, T.; Nakahata, J.; Aoyama, N.; Izumi, T. Serum Levels of Interleukin-10 on Admission as a Prognostic Predictor of Human Fulminant Myocarditis. J. Am. Coll. Cardiol. 2004, 44, 1292–1297. [Google Scholar] [CrossRef]
- Magnani, J.W.; Suk Danik, H.J.; Dec, G.W.; DiSalvo, T.G. Survival in Biopsy-Proven Myocarditis: A Long-Term Retrospective Analysis of the Histopathologic, Clinical, and Hemodynamic Predictors. Am. Heart J. 2006, 151, 463–470. [Google Scholar] [CrossRef]
- Piazza, I.; Ferrero, P.; Marra, A.; Cosentini, R. Early Diagnosis of Acute Myocarditis in the ED: Proposal of a New ECG-Based Protocol. Diagnostics 2022, 12, 481. [Google Scholar] [CrossRef]
- Ferrero, P.; Piazza, I.; Kühl, U.; Grosu, A.; Tschöpe, C.; Senni, M. QRS Fragmentation as a Possible Electrocardiographic Diagnostic Marker in Patients with Acute Myocarditis: Preliminary Histopathological Validation. ESC Heart Fail. 2020, 7, 2527–2533. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [PubMed]
- Balanescu, S.; Corlan, A.D.; Dorobantu, M.; Gherasim, L. Prognostic Value of Heart Rate Variability after Acute Myocardial Infarction. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2004, 10, CR307-15. [Google Scholar]
- Salahuddin, N.; Shafquat, A.; Marashly, Q.; Zaza, K.J.; Sharshir, M.; Khurshid, M.; Ali, Z.; Malgapo, M.; Jamil, M.G.; Shoukri, M.; et al. Increases in Heart Rate Variability Signal Improved Outcomes in Rapid Response Team Consultations: A Cohort Study. Cardiol. Res. Pract. 2018, 2018, 1590217. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Camm, A.J.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart Rate Variability. Standards of Measurement, Physiological Interpretation, and Clinical Use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 354–381. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T.; Moss, A.J. Decreased Heart Rate Variability and Its Association with Increased Mortality after Acute Myocardial Infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef]
- Leone, O.; Pieroni, M.; Rapezzi, C.; Olivotto, I. The Spectrum of Myocarditis: From Pathology to the Clinics. Virchows Arch. 2019, 475, 279–301. [Google Scholar] [CrossRef]
- Krejčí, J.; Botek, M.; McKune, A.J. Stabilization Period before Capturing an Ultra- Short Vagal Index Can Be Shortened to 60 s in Endurance Athletes and to 90 s in University Students. PLoS ONE 2018, 13, e0205115. [Google Scholar] [CrossRef]
- Kim, J.W.; Seok, H.S.; Shin, H. Is Ultra-Short-Term Heart Rate Variability Valid in Non-Static Conditions? Front. Physiol. 2021, 12, 596060. [Google Scholar] [CrossRef]

| Mean (±Standard Deviation)/Number (Percentage of Group) | |
|---|---|
| Age (years) | 34 (±13) |
| Gender-Male | 102 (89%) |
| Emergency Department Vital Signs | |
| MAP (mmHg) | 93 (±14) |
| Pulse (BPM) | 86 (±17) |
| Saturation (%) | 98 (±2) |
| Temperature (°C) | 37 (±1) |
| Emergency Department Labs | |
| WBC (10³/µL) | 9.7 (±3.5) |
| Neutrophils (10³/µL) | 6.5 (±3.3) |
| Lymphocytes (10³/µL) | 1.8 (±0.7) |
| Hemoglobin (g/dL) | 14.2 (±1.4) |
| Platelets (10³/µL) | 219 (±73) |
| Troponin (ng/mL) | 4.5 (±5.9) |
| Creatinine (mg/dL) | 0.9 (±0.2) |
| Emergency Department ECG & HRV | |
| PR interval (ms) | 149 (±24) |
| QRS interval (ms) | 88 (±11) |
| QTC interval (ms; Bazzet) | 403 (±32) |
| SDNN (ms) | 30.1 (±33.7) |
| RMSSD (ms) | 32.3 (±46.2) |
| MRI & Echo Findings | |
| Pericardial Effusion | 10 (9%) |
| Wall Motion Abnormalities | 31 (27%) |
| Late Gadolinium Enhancement | 35 (30%) |
| Logistic Regression—Univariate | Random Forest—Multivariate | ||||
|---|---|---|---|---|---|
| OR | 95% CI | p-Value | Mean Decrease Accuracy | Mean Decrease Gini | |
| Age (years) | 1.060 | 1.008–1.114 | 0.022 * | −0.776 | 0.982 |
| Gender−Male | 0.618 | 0.066–5.747 | 0.673 | 0.016 | 0.020 |
| Emergency Department Vital Signs | |||||
| MAP (mmHg) | 1.098 | 1.033–1.166 | 0.002 * | 3.879 | 1.553 |
| Pulse (BPM) | 1.042 | 0.995–1.091 | 0.075 | −1.628 | 0.782 |
| Saturation (%) | 0.717 | 0.477–1.078 | 0.111 | −1.466 | 0.454 |
| Temperature (°C) | 1.316 | 0.390–4.439 | 0.657 | −0.188 | 0.374 |
| Emergency Department Labs | |||||
| WBC (10³/µL) | 0.938 | 0.723–1.216 | 0.630 | 1.376 | 0.780 |
| Neutrophils (10³/µL) | 1.002 | 0.781–1.285 | 0.985 | −0.024 | 0.522 |
| Lymphocytes (10³/µL) | 0.405 | 0.095–1.717 | 0.220 | −1.133 | 0.837 |
| Hemoglobin (g/dL) | 0.559 | 0.311–1.003 | 0.051 | 0.559 | 0.942 |
| Platelets (10³/µL) | 1.005 | 0.997–1.013 | 0.181 | −1.679 | 0.535 |
| Troponin (ng/mL) | 0.163 | 0.008–3.102 | 0.227 | −0.434 | 0.846 |
| Creatinine (mg/dL) | 7.701 | 0.264–224.286 | 0.235 | 1.411 | 0.924 |
| Emergency Department HRV | |||||
| SDNN (ms) | 0.971 | 0.913–1.031 | 0.342 | 5.216 | 1.023 |
| RMSSD (ms) | 0.988 | 0.949–1.029 | 0.579 | 1.722 | 0.920 |
| SDNN < 11.25 ms | 6.719 | 1.161–38.869 | 0.033 * | / | / |
| RMSSD < 10.72 ms | 17.708 | 1.973–158.910 | 0.010 * | / | / |
| Logistic Regression—Univariate | Random Forest—Multivariate | ||||
|---|---|---|---|---|---|
| OR | 95% CI | p-Value | Mean Decrease Accuracy | Mean Decrease Gini | |
| Age (years) | 1.067 | 1.021–1.116 | 0.003 * | 2.349 | 1.455 |
| Gender-Male | 0.208 | 0.045–0.963 | 0.044 * | 0.267 | 0.092 |
| Emergency Department Vital Signs | |||||
| MAP (mmHg) | 1.057 | 1.009–1.108 | 0.018 * | 1.859 | 1.945 |
| Pulse (BPM) | 1.036 | 0.996–1.077 | 0.071 | 0.172 | 0.776 |
| Saturation (%) | 0.806 | 0.557–1.167 | 0.254 | −0.970 | 0.489 |
| Temperature (°C) | 1.549 | 0.571–4.201 | 0.389 | 0.345 | 0.514 |
| Emergency Department Labs | |||||
| WBC (10³/µL) | 1.016 | 0.842–1.226 | 0.860 | 2.269 | 0.999 |
| Neutrophils (10³/µL) | 1.154 | 0.985–1.353 | 0.075 | 1.489 | 0.955 |
| Lymphocytes (10³/µL) | 0.247 | 0.062–0.975 | 0.045 * | 0.034 | 1.077 |
| Hemoglobin (g/dL) | 0.562 | 0.342–0.923 | 0.022 * | 0.496 | 1.340 |
| Platelets (10³/µL) | 1.001 | 0.993–1.010 | 0.708 | −0.404 | 0.912 |
| Troponin (ng/mL) | 0.802 | 0.605–1.061 | 0.124 | −0.508 | 0.690 |
| Creatinine (mg/dL) | 2.152 | 0.088–52.634 | 0.638 | 0.986 | 0.974 |
| Emergency Department HRV | |||||
| SDNN (ms) | 0.967 | 0.918–1.019 | 0.215 | 5.267 | 1.225 |
| RMSSD (ms) | 0.989 | 0.958–1.021 | 0.524 | 4.403 | 1.363 |
| SDNN < 11.25 ms | 4.270 | 1.062–17.166 | 0.040 * | / | / |
| RMSSD < 10.72 ms | 7.217 | 1.674–31.103 | 0.008 * | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perek, S.; Nussinovitch, U.; Cohen, R.; Gidron, Y.; Raz-Pasteur, A. Ultra Short Heart Rate Variability Predicts Clinical Outcomes in Patients with a Clinical Presentation Consistent with Myocarditis: A Derivation Cohort Analysis. J. Clin. Med. 2023, 12, 89. https://doi.org/10.3390/jcm12010089
Perek S, Nussinovitch U, Cohen R, Gidron Y, Raz-Pasteur A. Ultra Short Heart Rate Variability Predicts Clinical Outcomes in Patients with a Clinical Presentation Consistent with Myocarditis: A Derivation Cohort Analysis. Journal of Clinical Medicine. 2023; 12(1):89. https://doi.org/10.3390/jcm12010089
Chicago/Turabian StylePerek, Shay, Udi Nussinovitch, Reut Cohen, Yori Gidron, and Ayelet Raz-Pasteur. 2023. "Ultra Short Heart Rate Variability Predicts Clinical Outcomes in Patients with a Clinical Presentation Consistent with Myocarditis: A Derivation Cohort Analysis" Journal of Clinical Medicine 12, no. 1: 89. https://doi.org/10.3390/jcm12010089
APA StylePerek, S., Nussinovitch, U., Cohen, R., Gidron, Y., & Raz-Pasteur, A. (2023). Ultra Short Heart Rate Variability Predicts Clinical Outcomes in Patients with a Clinical Presentation Consistent with Myocarditis: A Derivation Cohort Analysis. Journal of Clinical Medicine, 12(1), 89. https://doi.org/10.3390/jcm12010089






