Spheno-Orbital Meningioma and Vision Impairment—Case Report and Review of the Literature
Abstract
1. Introduction
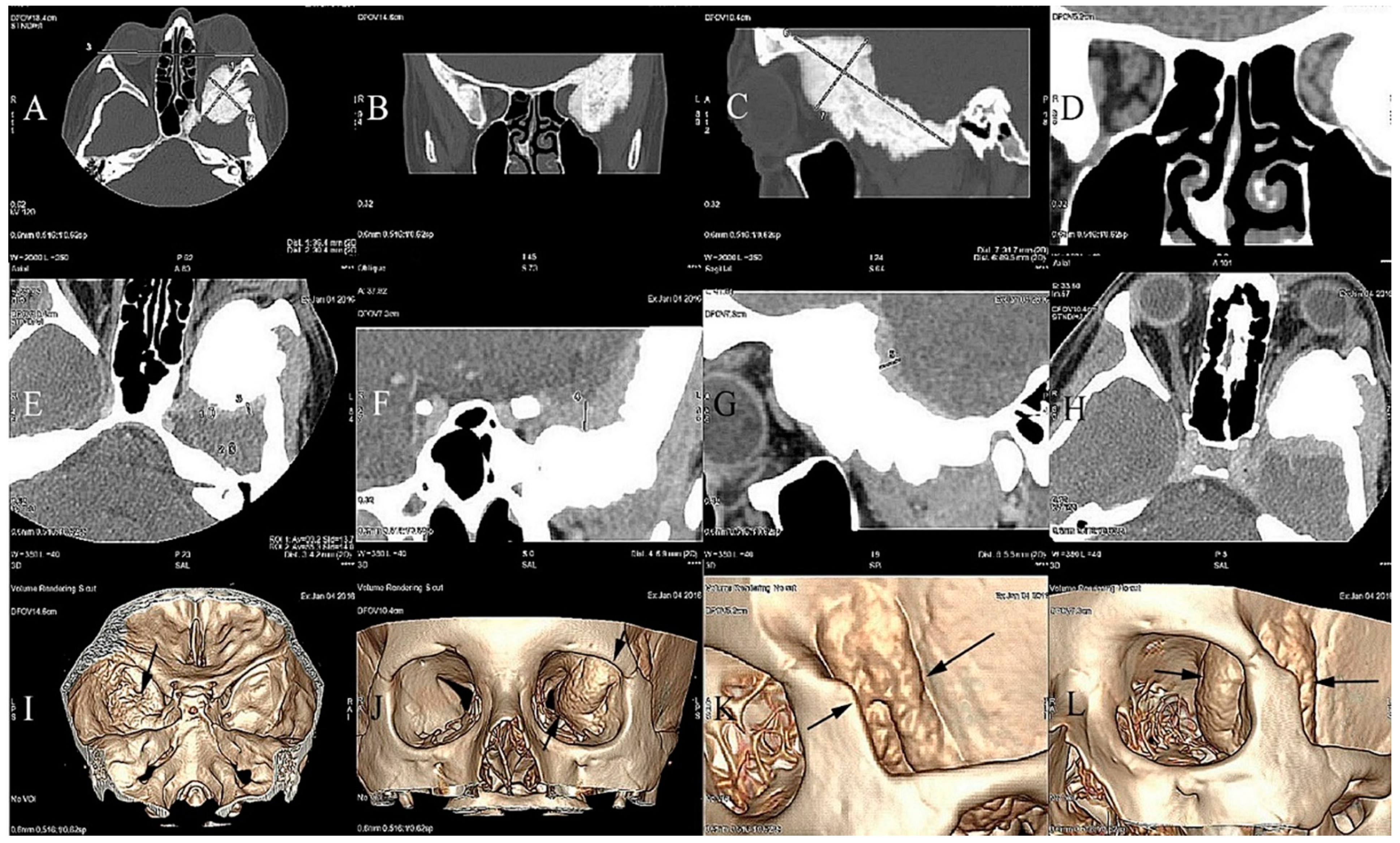
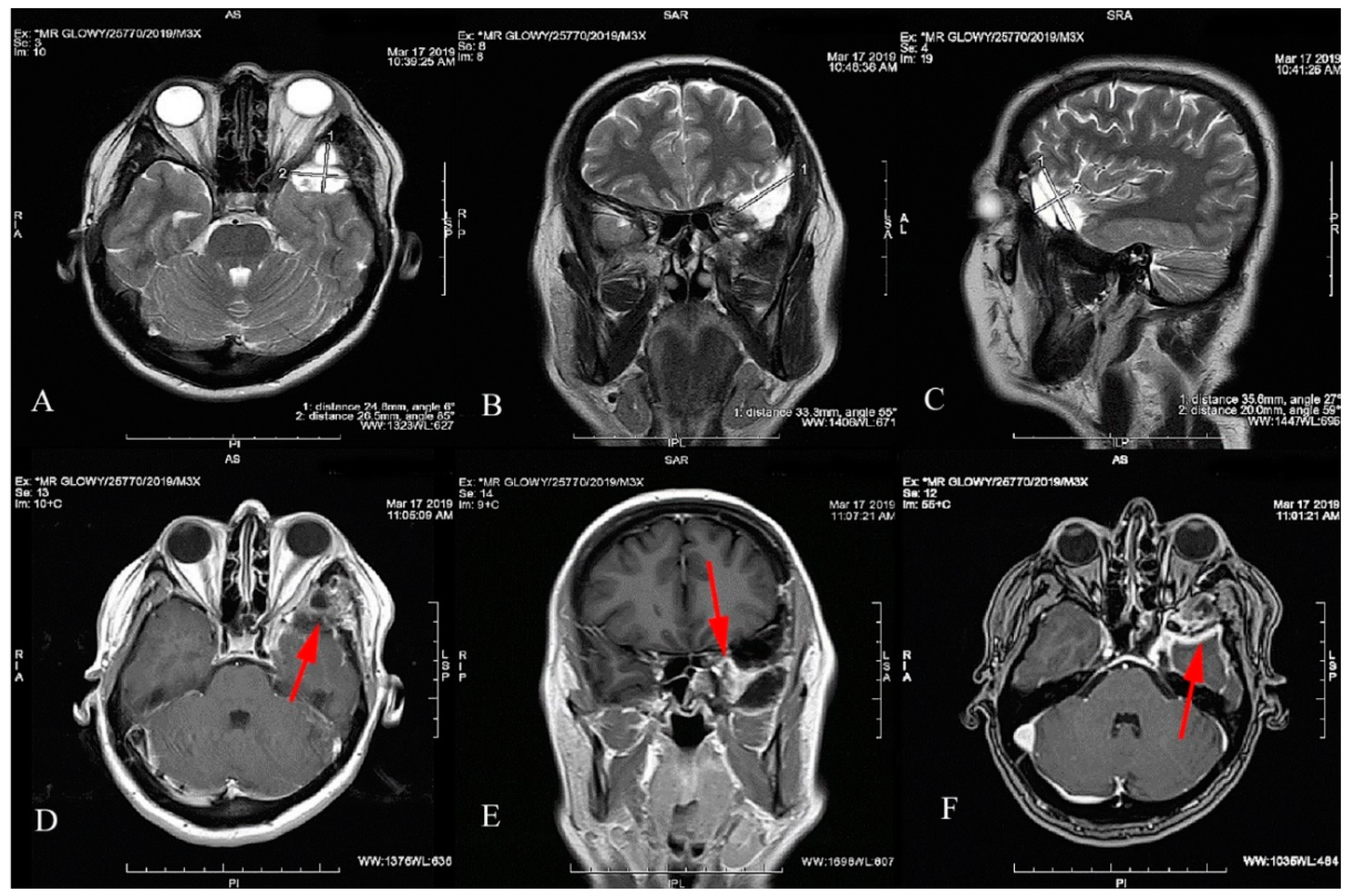
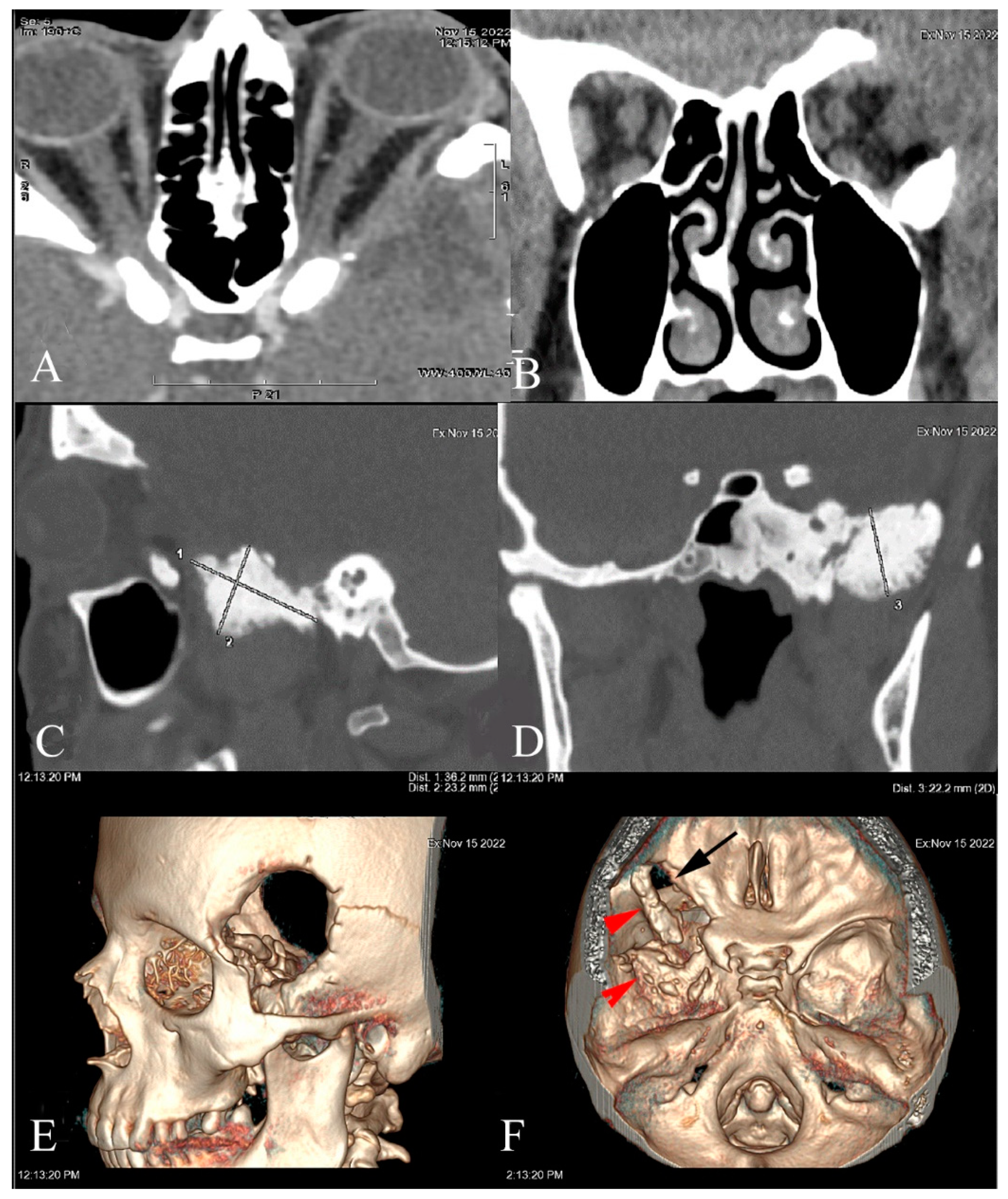
2. Case Report
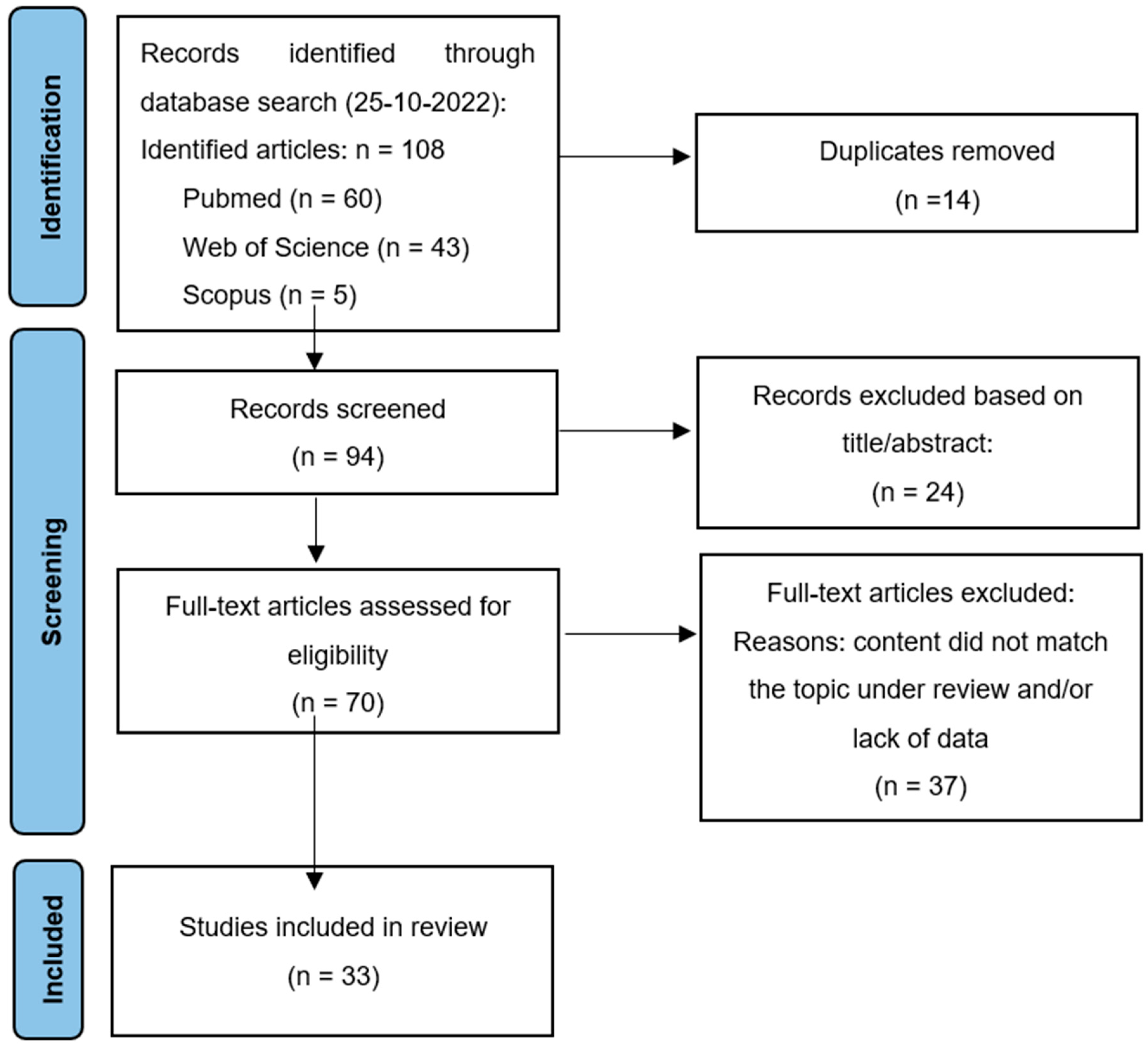
3. Systematic Review
4. Results
5. Discussion
5.1. Preoperative Visual Impairment
5.2. Tumor Surgery and Factors Influencing Postoperative Visual Outcomes
5.3. Rate and Risk Factors of Tumor Recurrence
6. Conclusions
- -
- SOMs are mostly found in women who are around 50 years of age and have a high rate of visual impairment (mean 56.0%).
- -
- A growing body of literature published in the last two decades suggests that early surgical treatment of symptomatic SOMs improves visual outcomes, giving the less vulnerable optic nerve a better chance to recover its function.
- -
- Favorable factors for better visual outcomes after surgery included better preoperative CDVA, a symptom history of less than 2 years, and lateral orbit involvement. Severely compromised visual function before surgery, orbital apex tumors, and the concentric invasion of the optic canal, as well as a lower extent of tumor resection (Simpson grades IV and III), were the predictors for a poorer postoperative visual prognosis.
- -
- SOMs pose a surgical challenge and complete surgical resection is often not possible. Patients with SOMs required a long-term follow-up because of the delayed high rate of recurrence.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bikmaz, K.; Mrak, R.; Al-Mefty, O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J. Neurosurg. 2007, 107, 905–912. [Google Scholar] [CrossRef]
- Gonen, L.; Nov, E.; Shimony, N.; Shofty, B.; Margalit, N. Sphenoorbital meningioma: Surgical series and design of an intraoperative management algorithm. Neurosurg. Rev. 2018, 41, 291–301. [Google Scholar] [CrossRef]
- Freeman, J.L.; Davern, M.S.; Oushy, S.; Sillau, S.; Ormond, D.R.; Youssef, A.S.; Lillehei, K.O. Spheno-Orbital Meningiomas: A 16-Year Surgical Experience. World Neurosurg. 2017, 99, 369–380. [Google Scholar] [CrossRef]
- Shrivastava, R.K.; Sen, C.; Costantino, P.D.; Della Rocca, R. Sphenoorbital meningiomas: Surgical limitations and lessons learned in their long-term management. J. Neurosurg. 2005, 103, 491–497. [Google Scholar] [CrossRef]
- Terrier, L.M.; Fournier, H.D.; Morandi, X.; Velut, S.; Hénaux, P.L.; Amelot, A.; François, P. Spheno-Orbital Meningiomas Surgery: Multicenter Management Study for Complex Extensive Tumors. World Neurosurg. 2018, 112, e145–e156. [Google Scholar] [CrossRef]
- Ringel, F.; Cedzich, C.; Schramm, J. Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery 2007, 60 (Suppl. 2), 214–221, discussion 221–222. [Google Scholar] [CrossRef]
- Nochez, Y.; Francois, P.; Majzoub, S.; Floch, E.; Pisella, P.-J.; Jan, M.; Velut, S. Predictive factors for visual outcome after resection of spheno-orbital meningiomas: A long-term review. Acta Ophthalmol. 2012, 90, e663–e665. [Google Scholar] [CrossRef]
- Mariniello, G.; Bonavolonta, G.; Tranfa, F.; Maiuri, F. Management of the optic canal invasion and visual outcome in spheno-orbital meningiomas. Clin. Neurol. Neurosurg. 2013, 115, 1615–1620. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Mariniello, G.; de Divitiis, O.; Corvino, S.; Strianese, D.; Iuiano, A.; Bonavolonta, G.; Maiuri, F. Recurrrences of Spheno-Orbital Meningiomas: Risk Factors and Management. World Neurosurg. 2022, 161, e514–e522. [Google Scholar] [CrossRef]
- Dos Santos, A.G.; Paiva, W.S.; da Roz, L.M.; Santo, M.P.D.E.; Teixeira, M.J.; Figueiredo, E.G.; da Silva, V.T.G. Spheno-orbital meningiomas: Is orbit reconstruction mandatory? Long-term outcomes and exophthalmos improvement. Surg. Neurol. Int. 2022, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, D.; Restelli, F.; Alfiero, T.; Campione, A.; Pozzi, F.; Balbi, S.; Arosio, A.; Castelnuovo, P. The Role of the Transorbital Superior Eyelid Approach in the Management of Selected Spheno-orbital Meningiomas: In-Depth Analysis of Indications, Technique, and Outcomes from the Study of a Cohort of 35 Patients. J. Neurol. Surg. Part B Skull Base 2020, 83, 145–158. [Google Scholar] [CrossRef]
- Dalle Ore, C.L.; Magill, S.T.; Rodriguez Rubio, R.; Shahin, M.N.; Aghi, M.K.; Theodosopoulos, P.V.; Villanueva-Meyer, J.E.; Kersten, R.C.; Idowu, O.O.; Reza Vagefi, M.; et al. Hyperostosing sphenoid wing meningiomas: Surgical outcomes and strategy for bone resection and multidisciplinary orbital reconstruction. J. Neurosurg. 2021, 134, 711–720. [Google Scholar] [CrossRef]
- Najafabadi, Z.A.H.; Genders, S.W.; van Furth, W.R. Visual outcomes endorse surgery of patients with spheno-orbital meningioma with minimal visual impairment or hyperostosis. Acta Neurochir. 2021, 163, 73–82. [Google Scholar] [CrossRef]
- Menon, S.; Sandesh, O.; Anand, D.; Menon, G. Spheno-Orbital Meningiomas: Optimizing Visual Outcome. J. Neurosci. Rural Pract. 2020, 11, 385–394. [Google Scholar] [CrossRef]
- Samadian, M.; Sharifi, G.; Mousavinejad, S.A.; Amin, A.A.; Ebrahimzadeh, K.; Tavassol, H.H.; Borghei-Razavi, H.; Rezaei, O. Surgical Outcomes of Sphenoorbital En Plaque Meningioma: A 10-Year Experience in 57 Consecutive Cases. World Neurosurg. 2020, 144, e576–e581. [Google Scholar] [CrossRef]
- Pace, S.T.; Koreen, I.V.; Wilson, J.A.; Yeatts, R.P. Orbital Reconstruction via Deformable Titanium Mesh Following Spheno-Orbital Meningioma Resection: Ophthalmic Presentation and Outcomes. Ophthalmic Plast. Reconstr. Surg. 2020, 36, 89–93. [Google Scholar] [CrossRef]
- Young, J.; Mdanat, F.; Dharmasena, A.; Cannon, P.; Leatherbarrow, B.; Hammerbeck-Ward, C.; Rutherford, S.; Ataullah, S. Combined neurosurgical and orbital intervention for spheno-orbital meningiomas—The Manchester experience. Orbit 2020, 39, 251–257. [Google Scholar] [CrossRef]
- Nagahama, A.; Goto, T.; Nagm, A.; Tanoue, Y.; Watanabe, Y.; Arima, H.; Nakajo, K.; Morisako, H.; Uda, T.; Ichinose, T.; et al. Spheno-Orbital Meningioma: Surgical Outcomes and Management of Recurrence. World Neurosurg. 2019, 126, e679–e687. [Google Scholar] [CrossRef]
- Leroy, H.A.; Leroy-Ciocanea, C.I.; Baroncini, M.; Bourgeois, P.; Pellerinm, P.; Labreuche, J.; Duhamel, A.; Lejeune, J.P. Internal and external spheno-orbital meningioma varieties: Different outcomes and prognoses. Acta Neurochir. 2016, 158, 1587–1596. [Google Scholar] [CrossRef]
- Bowers, C.A.; Sorour, M.; Patel, B.C.; Couldwell, W.T. Outcomes after surgical treatment of meningioma-associated proptosis. J. Neurosurg. 2016, 125, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Amirjamshidi, A.; Abbasioun, K.; Shams, A.R.; Ardalan, A.; Ramak, H.S.M. Lateral orbitotomy approach for removing hyperostosing en plaque sphenoid wing meningiomas. Description of surgical strategy and analysis of findings in a series of 88 patients with long-term follow up. Surg. Neurol. Int. 2015, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Talacchi, A.; De Carlo, A.; D’Agostino, A.; Nocini, P. Surgical management of ocular symptoms in spheno-orbital meningiomas. Is orbital reconstruction really necessary? Neurosurg. Rev. 2014, 37, 301–309, discussion 309–310. [Google Scholar] [CrossRef] [PubMed]
- Berhouma, M.; Jacquesson, T.; Abouaf, L.; Vighetto, A.; Jouanneau, E. Endoscopic endonasal optic nerve and orbital apex decompression for nontraumatic optic neuropathy: Surgical nuances and review of the literature. Neurosurg. Focus 2014, 37, E19. [Google Scholar] [CrossRef] [PubMed]
- Boari, N.; Gagliardi, F.; Spina, A.; Bailo, M.; Franzin, A.; Mortini, P. Management of spheno-orbital en plaque meningiomas: Clinical outcome in a consecutive series of 40 patients. Br. J. Neurosurg. 2013, 27, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Marcus, H.; Schwindack, C.; Santarius, T.; Mannion, R.; Kirollos, R. Image-guided resection of spheno-orbital skull-base meningiomas with predominant intraosseous component. Acta Neurochir. 2013, 155, 981–988. [Google Scholar] [CrossRef]
- Saeed, P.; van Furth, W.R.; Tanck, M.; Freling, N.; van der Sprenkel, J.W.; Stalpers, L.J.; van Overbeeke, J.J.; Mourits, M.P. Surgical treatment of sphenoorbital meningiomas. Br. J. Ophthalmol. 2011, 95, 996–1000. [Google Scholar] [CrossRef]
- Oya, S.; Sade, B.; Lee, J.H. Sphenoorbital meningioma: Surgical technique and outcome. J. Neurosurg. 2011, 114, 1241–1249. [Google Scholar] [CrossRef]
- Luetjans, G.; Krauss, J.K.; Branis, A.; Nakamura, M. Bilateral sphenoorbital hyperostotic meningiomas with proptosis and visual impairment: A therapeutic challenge. Report of three patients and review of the literature. Clin. Neurol. Neurosurg. 2011, 113, 859–863. [Google Scholar] [CrossRef]
- Honig, S.; Trantakis, C.; Frerich, B.; Sterker, I.; Schober, R.; Meixensberger, J. Spheno-orbital meningiomas: Outcome after microsurgical treatment: A clinical review of 30 cases. Neurol. Res. 2010, 32, 314–325. [Google Scholar] [CrossRef]
- Mirone, G.; Chibbaro, S.; Schiabello, L.; Tola, S.; George, B. En plaque sphenoid wing meningiomas: Recurrence factors and surgical strategy in a series of 71 patients. Neurosurgery 2009, 65, 100–108, discussion 108–109. [Google Scholar] [CrossRef] [PubMed]
- Heufelder, M.J.; Sterker, I.; Tranakis, C.; Schneider, J.P.; Meixensberger, J.; Hemprich, A.; Frerrich, B. Reconstructive and ophthalmologic outcomes following resection of spheno-orbital meningiomas. Ophthalmic Plast. Reconstr. Surg. 2009, 25, 223–226. [Google Scholar] [CrossRef]
- Cannon, P.S.; Rutherford, S.A.; Richardson, P.L.; King, A.; Leatherbarrow, B. The surgical management and outcomes for spheno-orbital meningiomas: A 7-year review of multi-disciplinary practice. Orbit 2009, 28, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Sandalcioglu, I.E.; Gasser, T.; Mohr, C.; Stolke, D.; Wiedemayer, H. Spheno-orbital meningiomas: Interdisciplinary surgical approach, resectability and long-term results. J. Cranio Maxillofac. Surg. 2005, 33, 260–266. [Google Scholar] [CrossRef]
- De Jesús, O.; Toledo, M.M. Surgical management of meningioma en plaque of the sphenoid ridge. Surg. Neurol. 2001, 55, 265–269. [Google Scholar] [CrossRef]
- Simpson, D. The reccurence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry 1957, 20, 22–39. [Google Scholar] [CrossRef]
- Kamming, D.; Clarke, S. Postoperative visual loss following prone spinal surgery. Br. J. Anesth. 2005, 95, 257–260. [Google Scholar] [CrossRef]
- Park, K.A.; Kim, Y.D.; Woo, K.I. Changes in optical coherence tomography measurements after orbital wall decompression in dysthyroid optic neuropathy. Eye 2018, 32, 1123–1129. [Google Scholar] [CrossRef]
- Poczos, P.; Česák, T.; Jirásková, N.; Macháčková, M.; Čelakovský, P.; Adamkov, J.; Gabalec, F.; Soukup, J.; Kremláček, J. Optical coherence tomography and visual evoked potentials in evaluation of optic chiasm decompression. Sci. Rep. 2022, 12, 2102. [Google Scholar] [CrossRef]
- Semela, L.; Yang, E.B.; Hedges, T.R.; Vuong, L.; Odel, J.G.; Hood, D.C. Multifocal visual-evoked potential in unilateral compressive optic neuropathy. Br. J. Ophthalmol. 2007, 91, 445–448. [Google Scholar] [CrossRef]
- Meredith, J.T.; Celesia, G.G. Pattern-reversal visual evoked potentials and retinal eccentricity. Electroencephalogr. Clin. Neurophysiol. 1982, 53, 243–253. [Google Scholar] [CrossRef] [PubMed]

| Study | Study Period | Study Size | Age Mean (Range) | Female % | Visual Impairment | Visual Improvement * | Visual Deterioration * | Follow-Up Mean (Months) | Tumor Recurrence % | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VA n (%) | VF n (%) | VA n (%) | VF n (%) | VA n (%) | VF n (%) | |||||||
| Mariniello et al., 2022 [10] | 1990–2014 | 80 | 47 (26–75) | 82.5 | 47 (59) | NS | 24 (51) | NS | 7 (15) | NS | 136 | 37.5 |
| Dos Santos et al., 2022 [11] | 2008–2018 | 40 | 49.5 | 87.5 | 26 (65) | NS | 13 (41) | NS | 7 (22) | NS | 39 | 25 |
| Locatelli et al., 2022 [12] | 2011–2021 | 35 | 57 (38–80) | 77 | 11 (32) | 6 (17) | (64) | 2 (33) | 0 (0) | 0 (0) | 31.5 | 14 |
| Dalle Ore et al., 2021 [13] | NS | 54 | 52 (30–79) | 83 | 28 (52) | 28 (52) | 12 (43) | 12 (43) | 1 (3.5) | 0 (0) | 31 | 22 |
| Najafabadi et al., 2021 [14] | 2015–2019 | 19 | 47 (45–50) | 95 | 10 (53) | 8 (42) | 8 (80) | 8 (100) | 2 (20) | 0 (0) | 29 | 10.5 |
| Menon et al., 2021 [15] | 10 years | 17 | 51 (17–72) | 76 | 14 (82) | NS | 4 (29) | NS | 2 (14) | NS | 56 | 59 |
| Samadian et al., 2020 [16] | 2007–2017 | 57 | 48 (22–76) | 93 | 16 (28) | 13 (23) | 7 (44) | NS | 1 (6) | NS | 46 | 17 |
| Pace et al., 2020 [17] | 1996–2017 | 20 | 56 (19–89) | 80 | 11 (55) | 15 (75) | 9 (82) | 11 (73) | 3 (20) | 1 (7) | 47 | 20 |
| Young et al., 2019 [18] | 2000–2017 | 24 | 49.5 | 92 | 17 (71) | 16 (67) | 12 (71) | 7 (44) | 5 (29) | 5 (31) | 82 | 33 |
| Nagahama et al., 2019 [19] | 1996–2017 | 12 | 49 (20–71) | 58 | 3 (25) | NS | NS | NS | 2 (66) | NS | 74 | 33 |
| Terrier et al., 2018 [5] | 20 years | 130 | 51 (28–74) | 91,5 | 49 (38) | NS | 22 (45) | NS | 8 (16) | NS | 77 | 25 |
| Gonen et al., 2018 [2] | 2005–2014 | 27 | 53 (27–78) | 89 | 10 (37) | 4 (15) | 8 (80) | NS | 0 (0) | NS | 41 | 11 |
| Freeman et al., 2017 [3] | 2000–2016 | 25 | 51 (39–71) | 92 | 19 (76) | NS | 4 (21) | NS | 1 (5) | NS | 45 | 48 |
| Leroy et al., 2016 [20] | 2001–2006 | 70 | 52 (21–80) | 91 | 27 (39) | NS | 10 (37) | NS | 6 (22) | NS | 57 | 29 |
| Bowers et al., 2016 [21] | 2002–2015 | 33 | 52 (12–76) | 73 | 17 (51.5) | 12 (36) | 15 (68) | NS | 0 (0) | NS | 54 | 6 |
| Amirjamshidi et al., 2015 [22] | 1979–2013 | 88 | 46 (12–70) | 74 | 65 (74) | NS | 39 (60) | NS | 13 (20) | NS | 135 | 22 |
| Talacchi et al., 2014 [23] | 1992–2012 | 47 | 57 (21–77) | 55,5 | 24 (51) | 6 (13) | 10 (42) | 3 (50) | 3 (12.5) | 1 (17) | 52 | 30 |
| Berhouma et al., 2014 [24] | 2012–2014 | 4 | 58 (49–67) | 75 | 4 (100) | 3 (75) | 3 (75) | 1 (15) | 1 (15) | 0 (0) | 6 | NS |
| Boari et al., 2013 [25] | 2000–2010 | 40 | 53 | 88 | 35 (88) | NS | 27 (67) | NS | NS | NS | 73 | 10 |
| Marcus et al., 2013 [26] | 2004–2012 | 19 | 44 (26–64) | 90 | 11 (58) | 1 (6) | 10 (91) | 1 (100) | 1 (9) | 0 | 60 | 0 |
| Nochez et al., 2012 [7] | 1986–2006 | 37 | 50 (33–76) | 92 | 22 (54.5) | NS | 12 (71) | NS | 5 (23) | NS | 89 | 42.5 |
| Saeed et al., 2011 [27] | 1980–2006 | 66 | 46 (26–68) | 92 | 51 (77) | 51 (77) | 20 (39) | NS | 6 (12.5) | NS | 102 | 17 |
| Oya et al., 2011 [28] | 1994–2009 | 39 | 48 (33–68) | 87 | 21 (54) | NS | 14 (67) | NS | 0 (0) | NS | 41 | 18 |
| Luetjens et al., 2011 [29] | NS | 3 | 62 (49–70) | 100 | 3 (100) | 3 (100) | 3 (50) | 3 (50) | 0 (0) | 0 (0) | 28 | NS |
| Honig et al., 2010 [30] | 2001–2006 | 30 | 54 (25–74) | 73 | 22 (73) | 12 (40) | 15 (68) | NS | 2 (9) | NS | 34 | 27 |
| Mirone et al., 2009 [31] | 1986–2006 | 71 | 53 (12–79) | 87 | 41 (58) | 27 (38) | 30 (73) | NS | 3 (7) | NS | 77 | 5 |
| Heufelder et al., 2009 [32] | 1997–2006 | 21 | 61 (47–81) | 95 | 10 (48) | 7 (33) | 2 (20) | 2 (29) | 2 (20) | 1 (14) | 66 | 33 |
| Cannon et al., 2003 [33] | 2000–2007 | 12 | 51 (34–64) | 92 | 5 (42) | 10 (83) | 2 (40) | 1 (10) | 3 (60) | 3 (30) | 31 | 33 |
| Ringel et al., 2007 [6] | 1983–2003 | 63 | 51 (21–77) | 85 | 28 (47) | 20 (32) | 18 (64) | 11 (55) | 2 (7) | 2 (10) | 54 | 39 |
| Bikmaz et al., 2007 [1] | 1994–2004 | 17 | 52 (36–70) | 88 | 10 (59) | NS | 7 (70) | NS | 0 (0) | NS | 36 | 6 |
| Shrivastava et al., 2005 [4] | 1991–2003 | 25 | 51 (22–76) | 88 | 20 (80) | 9 (36) | 7 (39) | 8 (89) | 0 (0) | 0 (0) | 60 | 8 |
| Sandalcioglu et al., 2005 [34] | 1998–2002 | 16 | 53 (37–76) | 94 | 7 (44) | NS | 6 (86) | NS | 1 (14) | NS | 68 | 56 |
| De Jesus et al., 2001 [35] | 1990–1997 | 6 | 51 (39–64) | 100 | 3 (50) | 3 (50) | 3 (100) | 3 (100) | 0 (0) | 0 (0) | 48 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbowska, J.; Zegadło, A.; Patyk, M.; Rękas, M. Spheno-Orbital Meningioma and Vision Impairment—Case Report and Review of the Literature. J. Clin. Med. 2023, 12, 74. https://doi.org/10.3390/jcm12010074
Wierzbowska J, Zegadło A, Patyk M, Rękas M. Spheno-Orbital Meningioma and Vision Impairment—Case Report and Review of the Literature. Journal of Clinical Medicine. 2023; 12(1):74. https://doi.org/10.3390/jcm12010074
Chicago/Turabian StyleWierzbowska, Joanna, Arkadiusz Zegadło, Michał Patyk, and Marek Rękas. 2023. "Spheno-Orbital Meningioma and Vision Impairment—Case Report and Review of the Literature" Journal of Clinical Medicine 12, no. 1: 74. https://doi.org/10.3390/jcm12010074
APA StyleWierzbowska, J., Zegadło, A., Patyk, M., & Rękas, M. (2023). Spheno-Orbital Meningioma and Vision Impairment—Case Report and Review of the Literature. Journal of Clinical Medicine, 12(1), 74. https://doi.org/10.3390/jcm12010074





