Autonomic Nerve Involvement in Post-Acute Sequelae of SARS-CoV-2 Syndrome (PASC)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Tilt Table Testing
2.3. Heart Rate Variability during Deep Breathing (HRVDB)
2.4. Valsalva Maneuver
2.5. Cutaneous Nerve Biopsy
2.6. Antibody Testing
2.7. Statistical Analysis
3. Results
3.1. Demographics of Post-Acute Sequelae of SARS-CoV-2 Syndrome (PASC) and Postural Orthostatic Tachycardia Syndrome (POTS)
3.2. Clinical Symptoms
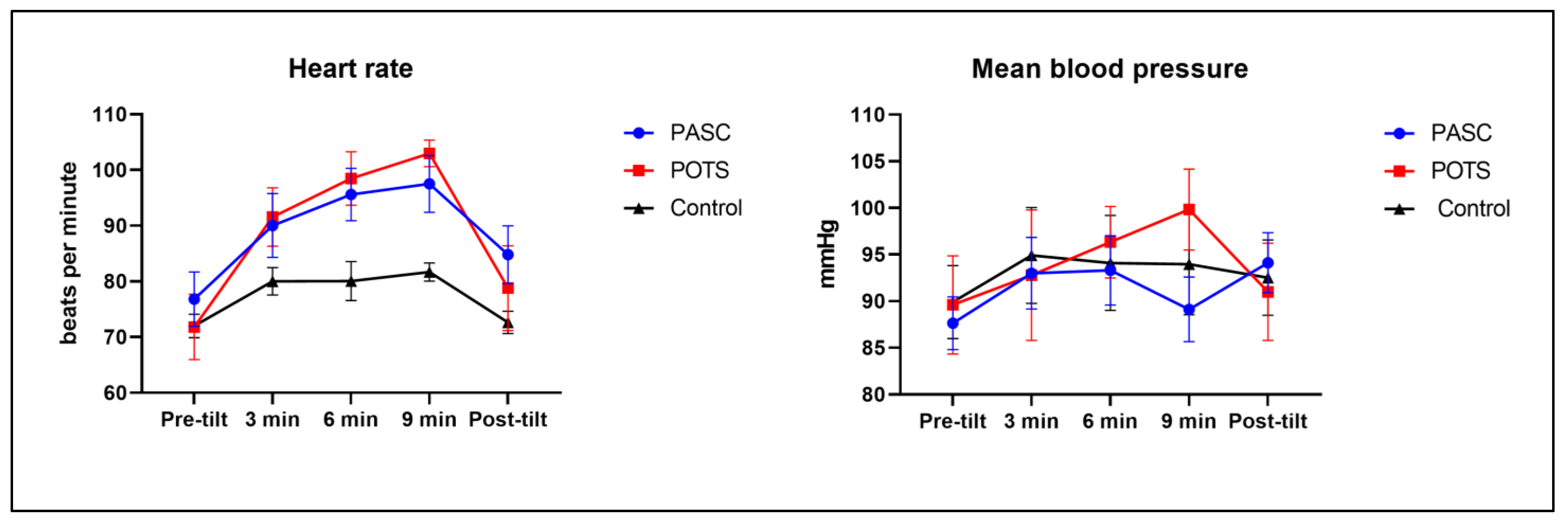
3.3. Tilt Table Test Response
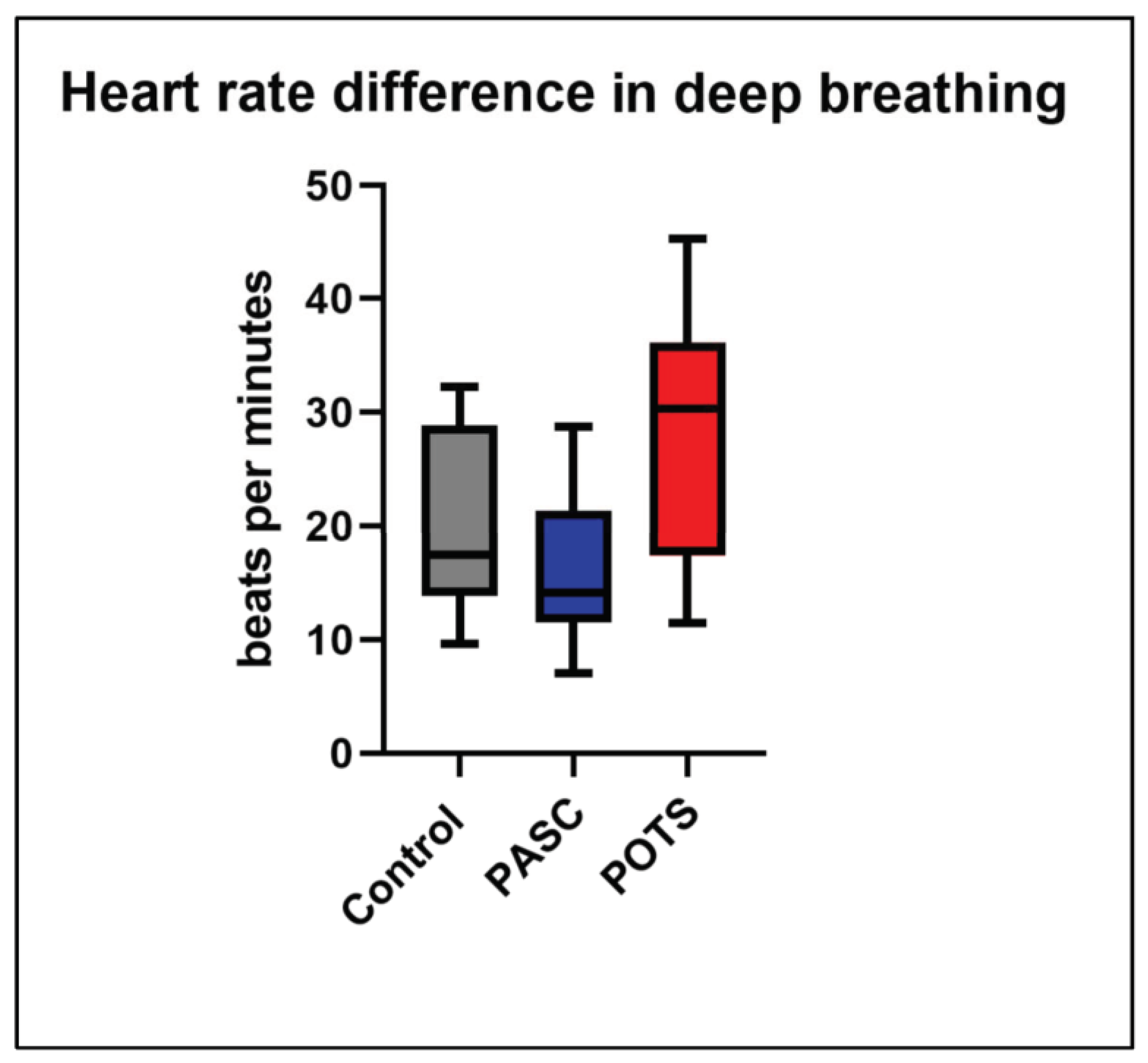
3.4. Heart Rate Variability during Deep Breathing
3.5. Valsalva Ratio
3.6. Cutaneous Nerve Biopsy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alimohamadi, Y.; Tola, H.H.; Abbasi-Ghahramanloo, A.; Janani, M.; Sepandi, M. Case fatality rate of COVID-19: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2021, 62, E311–E320. [Google Scholar] [CrossRef] [PubMed]
- Nka, A.D.; Ka’E, A.C.; Bouba, Y.; Semengue, E.N.J.; Tchouaket, M.C.T.; Takou, D.; Pabo, W.; Fainguem, N.; Sosso, S.M.; Colizzi, V.; et al. Global burden of SARS-CoV-2 infection, hospitalization and case fatality rate among COVID-19 vaccinated individuals and its associated factors: A systematic review and meta-analysis protocol. PLoS ONE 2022, 17, e0272839. [Google Scholar] [CrossRef] [PubMed]
- Finelli, L.; Gupta, V.; Petigara, T.; Yu, K.; Bauer, K.A.; Puzniak, L.A. Mortality Among US Patients Hospitalized With SARS-CoV-2 Infection in 2020. JAMA Netw. Open 2021, 4, e216556. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Long COVID Collaborators; Hanson, S.W.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; et al. Estimated Global Proportions of Individuals with Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; de Noordhout, C.M.; Jong, C.P.-D.; Cleemput, I.; Heede, K.V.D. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- Gualano, M.R.; Rossi, M.F.; Borrelli, I.; Santoro, P.E.; Amantea, C.; Daniele, A.; Tumminello, A.; Moscato, U. Returning to work and the impact of post COVID-19 condition: A systematic review. Work 2022, 73, 405–413. [Google Scholar] [CrossRef]
- Miglis, M.G.; Prieto, T.; Shaik, R.; Muppidi, S.; Sinn, D.-I.; Jaradeh, S. A case report of postural tachycardia syndrome after COVID-19. Clin. Auton. Res. 2020, 30, 449–451. [Google Scholar] [CrossRef]
- Novak, P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: A case report. Eneurologicalsci 2020, 21, 100276. [Google Scholar] [CrossRef]
- Blitshteyn, S. Is postural orthostatic tachycardia syndrome (POTS) a central nervous system disorder? J. Neurol. 2021, 269, 725–732. [Google Scholar] [CrossRef]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021, 31, 385–394. [Google Scholar] [CrossRef]
- Goodman, B.P.; Khoury, J.A.; Blair, J.E.; Grill, M.F. COVID-19 Dysautonomia. Front. Neurol. 2021, 12, 624968. [Google Scholar] [CrossRef] [PubMed]
- Jacob, G.; Costa, F.; Shannon, J.R.; Robertson, R.M.; Wathen, M.; Stein, M.; Biaggioni, I.; Ertl, A.; Black, B.; Robertson, D. The Neuropathic Postural Tachycardia Syndrome. N. Engl. J. Med. 2000, 343, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Bonyhay, I.; Freeman, R. Sympathetic Nerve Activity in Response to Hypotensive Stress in the Postural Tachycardia Syndrome. Circulation 2004, 110, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Mukerji, S.S.; Alabsi, H.S.; Systrom, D.; Marciano, S.P.; Felsenstein, D.; Mullally, W.J.; Pilgrim, D.M. Multisystem Involvement in Post-Acute Sequelae of Coronavirus Disease 19. Ann. Neurol. 2021, 91, 367–379. [Google Scholar] [CrossRef]
- Definition of Post-Acute Sequelae of SARS CoV-2 Infection (PASC) by Center of Disease Control and Prevention (CDC). Updated 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html#print (accessed on 1 September 2022).
- Lauria, G.; Cornblath, D.R.; Johansson, O.; McArthur, J.C.; Mellgren, S.I.; Nolano, M.; Rosenberg, N.; Sommer, C. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur. J. Neurol. 2005, 12, 747–758. [Google Scholar] [CrossRef]
- Jamal, S.M.; Landers, D.B.; Hollenberg, S.M.; Turi, Z.G.; Glotzer, T.V.; Tancredi, J.; Parrillo, J.E. Prospective Evaluation of Autonomic Dysfunction in Post-Acute Sequela of COVID-19. J. Am. Coll. Cardiol. 2022, 79, 2325–2330. [Google Scholar] [CrossRef]
- Billig, S.C.; Schauermann, J.C.; Rolke, R.; Katona, I.; Schulz, J.B.; Maier, A. Quantitative sensory testing predicts histological small fiber neuropathy in postural tachycardia syndrome. Neurol. Clin. Pract. 2020, 10, 428–434. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Sneller, M.C.; Liang, C.J.; Marques, A.R.; Chung, J.Y.; Shanbhag, S.M.; Fontana, J.R.; Raza, D.H.; Okeke, O.; Dewar, R.L.; Higgins, R.B.P.; et al. A Longitudinal Study of COVID-19 Sequelae and Immunity: Baseline Findings. Ann. Intern. Med. 2022, 175, 969–979. [Google Scholar] [CrossRef]
- Bryarly, M.; Raj, S.R.; Phillips, L.; Hynan, L.S.; Okamoto, L.E.; Arnold, A.C.; Paranjape, S.Y.; Vernino, M.; Black, B.K.; Vernino, S. Ganglionic Acetylcholine Receptor Antibodies in Postural Tachycardia Syndrome. Neurol. Clin. Pract. 2021, 11, e397–e401. [Google Scholar] [CrossRef]
- Cashman, C.R.; Höke, A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci. Lett. 2015, 596, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Maccio, U.; Zinkernagel, A.S.; Schuepbach, R.; Probst-Mueller, E.; Frontzek, K.; Brugger, S.D.; Hofmaenner, D.A.; Moch, H.; Varga, Z. Long-Term Persisting SARS-CoV-2 RNA and Pathological Findings: Lessons Learnt from a Series of 35 COVID-19 Autopsies. Front. Med. 2022, 9, 778489. [Google Scholar] [CrossRef] [PubMed]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef]
- Fabbri, V.P.; Riefolo, M.; Lazzarotto, T.; Gabrielli, L.; Cenacchi, G.; Gallo, C.; Aspide, R.; Frascaroli, G.; Liguori, R.; Lodi, R.; et al. COVID-19 and the Brain: The Neuropathological Italian Experience on 33 Adult Autopsies. Biomolecules 2022, 12, 629. [Google Scholar] [CrossRef]
- Maiese, A.; Manetti, A.C.; Bosetti, C.; Del Duca, F.; La Russa, R.; Frati, P.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. SARS-CoV-2 and the brain: A review of the current knowledge on neuropathology in COVID-19. Brain Pathol. 2021, 31, e13013. [Google Scholar] [CrossRef]
- Thakur, K.T.; Miller, E.H.; Glendinning, M.D.; Al-Dalahmah, O.; A Banu, M.; Boehme, A.K.; Boubour, A.L.; Bruce, S.S.; Chong, A.M.; Claassen, J.; et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain 2021, 144, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Oaklander, A.L.; Mills, A.J.; Kelley, M.; Toran, L.S.; Smith, B.; Dalakas, M.C.; Nath, A. Peripheral Neuropathy Evaluations of Patients with Prolonged Long COVID. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1146. [Google Scholar] [CrossRef]
| Mean Age | Sex | Ethnicity | Mean BMI | |
|---|---|---|---|---|
| Post-Acute Sequelae of SARS-CoV-2 Syndrome (PASC) (N = 13) | 47.39 (±14.01) | 11/13 (84.62%) | White—10/13 (76.92%) Hispanic—1/13 (7.69%) Asian—2/13 (15.38%) | 26.93 (±6.79)) |
| Postural Orthostatic Tachycardia Syndrome (POTS) (N = 6) | 30.17 (±7.47) | 5/6 (83.33%) | White—5/6 (83.33%) Asian—1/6 (16.67%) | 26.62 (±7.66)) |
| Control (N = 7) | 44 (±16.16) | 5/7 (71.43%) | White—5/7 (71.43%) Asian—2/7 (28.57%) | 25.69 (±4.81) |
| Post-Acute Sequelae of SARS-CoV-2 Syndrome (PASC) | Postural Orthostatic Tachycardia Syndrome (POTS) | p Values for t-Test between PASC and POTS | Control | |
|---|---|---|---|---|
| Mean duration of symptoms in months | 21.15 (± 5.15) | 138.83 (± 123.16) | <0.01 | N/A |
| Severity of COVID-19 infection | Mild—11/13 (84.62%) Moderate—2/13 (15.38%) | N/A | N/A | N/A |
| Fatigue | 13/13 (100%) | 6/6 (100%) | N/A | None |
| Orthostatic intolerance | 13/13 (100%) | 6/6 (100%) | N/A | None |
| Palpitations | 8/13 (61.54%) | 3/6 (50%) | 0.64 | None |
| Shortness of breath | 9/13 (69.23%) | 4/6 (66.67%) | 0.90 | None |
| Chest pain | 3/13 (23.08%) | 0/6 (0%) | 0.20 | None |
| Headache | 5/13 (38.46%) | 3/6 (50%) | 0.64 | None |
| Brain fog | 12/13 (92.30%) | 6/6 (100%) | 0.48 | None |
| Diffuse pain | 7/13 (53.85%) | 2/6 (33.33%) | 0.41 | None |
| Gastrointesstinal symptoms | 9/13 (69.23%) | 3/6 (50%) | 0.42 | None |
| Post-exertional malaise | 11/13 (92.30%) | 6/6 (100%) | 0.48 | None |
| Insomnia | 10/13 (76.92%) | 4/6 (66.67%) | 0.64 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, T.H.; Azar, A. Autonomic Nerve Involvement in Post-Acute Sequelae of SARS-CoV-2 Syndrome (PASC). J. Clin. Med. 2023, 12, 73. https://doi.org/10.3390/jcm12010073
Chung TH, Azar A. Autonomic Nerve Involvement in Post-Acute Sequelae of SARS-CoV-2 Syndrome (PASC). Journal of Clinical Medicine. 2023; 12(1):73. https://doi.org/10.3390/jcm12010073
Chicago/Turabian StyleChung, Tae Hwan, and Antoine Azar. 2023. "Autonomic Nerve Involvement in Post-Acute Sequelae of SARS-CoV-2 Syndrome (PASC)" Journal of Clinical Medicine 12, no. 1: 73. https://doi.org/10.3390/jcm12010073
APA StyleChung, T. H., & Azar, A. (2023). Autonomic Nerve Involvement in Post-Acute Sequelae of SARS-CoV-2 Syndrome (PASC). Journal of Clinical Medicine, 12(1), 73. https://doi.org/10.3390/jcm12010073






