Cardiovascular Outcomes of ST-Elevation Myocardial Infarction (STEMI) Patients without Standard Modifiable Risk Factors (SMuRF-Less): The Intermountain Healthcare Experience
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vernon, S.T.; Coffey, S.; Bhindi, R.; Soo Hoo, S.Y.; Nelson, G.I.; Ward, M.R.; Hansen, P.S.; Asrress, K.N.; Chow, C.K.; Celermajer, D.S.; et al. Increasing proportion of ST elevation myocardial infaction patients with coronary atherosclerosis poorly explained by standard modifiable risk factors. Eur. J. Prev. Cardiol. 2017, 24, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.T.; Coffey, S.; D’Souza, M.; Chow, C.K.; Kilian, J.; Hyun, K.; Shaw, J.A.; Adams, M.; Roberts-Thomson, P.; Brieger, D.; et al. ST-segment-elevation myocardial infarction (STEMI) patients without standard modifiable cardiovascular risk factors—How common are they, and what are their outcomes? J. Am. Heart Assoc. 2019, 3, e013296. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Goodman, S.G.; Saltzman, I.; Wong, G.C.; Huynh, T.; Dery, J.P.; Leiter, L.A.; Bhatt, D.L.; Welsh, R.C.; Spencer, F.A.; et al. Cardiovascular risk factors and in-hospital mortality in acute coronary syndromes.: Insights from the Canadian Global Registry of Acute Coronary Events. Can. J. Cardiol. 2015, 31, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Figtree, G.A.; Vernon, S.T.; Hadziosmanovic, N.; Sundström, J.; Alfredsson, J.; Arnott, C.; Delatour, V.; Leósdóttir, M.; Hagström, E. Mortality in STEMI patients without standard modifiable risk factors: A sex-disaggregated analysis of SWEDEHEART registry data. Lancet 2021, 397, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Canto, J.G.; Kiefe, C.I.; Rogers, W.J.; Peterson, E.D.; Frederick, P.D.; French, W.J.; Gibson, C.M.; Pollack, C.V.; Ornato, J.P.; Zalenski, R.J.; et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA 2011, 306, 2120–2127. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study)P case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Pope, C.A., III; Muhlestein, J.B.; May, H.T.; Renlund, D.G.; Anderson, J.L.; Horne, B.D. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation 2006, 114, 2443–2448. [Google Scholar] [CrossRef]
- Tsimikas, S. A test in context: Lipoprotein(a): Diagnosis, prognosis, controversies, and emerging therapies. J. Am. Coll. Cardiol. 2017, 69, 692–711. [Google Scholar] [CrossRef]
- Marston, N.A.; Giugliano, R.P.; Melloni, G.E.; Park, J.G.; Morrill, V.; Blazing, M.A.; Ference, B.; Stein, E.; Stroes, E.S.; Braunwald, E.; et al. Association of apolipoprotein B-containing lipoproteins and risk of myocardial infarction in individuals with and without atherosclerosis. JAMA Cardiol. 2022, 7, 250–256. [Google Scholar] [CrossRef]
- Mehta, A.; Vasquez, N.; Ayers, C.R.; Patel, J.; Hooda, A.; Khera, A.; Blumenthal, R.S.; Shapiro, M.D.; Rodriguez, C.J.; Tsai, M.Y.; et al. Independent association of lipoprotein(a) and coronary artery calcification with atherosclerotic cardiovascular risk. J. Am. Coll. Cardiol. 2022, 79, 757–768. [Google Scholar] [CrossRef]
- Blankenberg, S.; Zeller, T.; Saarela, O.; Havulinna, A.S.; Kee, F.; Tunstall-Pedoe, H.; Kuulasmaa, K.; Yarnell, J.; Schnabel, R.B.; Wild, P.S.; et al. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: The MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation 2010, 121, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Folsom, A.R.; Chambless, L.E.; Ballantyne, C.M.; Coresh, J.; Heiss, G.; Wu, K.K.; Boerwinkle, E.; Mosley, T.H.; Sorlie, P.; Diao, G.; et al. An assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: The Atherosclerosis Risk in Communities Study. Arch. Intern. Med. 2006, 166, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Chaffin, M.; Zekavat, S.M.; Collins, R.L.; Roselli, C.; Natarajan, P.; Lichtman, J.H.; D’onofrio, G.; Mattera, J.; Dreyer, R.; et al. Whole-genome sequencing to characterize monogenic and polygenic contribution in patients hospitalized with early-onset myocardial infarction. Circulation 2019, 139, 1593–1602. [Google Scholar] [CrossRef]
- Inouye, M.; Abraham, G.; Nelson, C.P.; Wood, A.M.; Sweeting, M.J.; Dudbridge, F.; Lai, F.Y.; Kaptoge, S.; Brozynska, M.; Wang, T.; et al. Genomic risk prediction of coronary artery disease in 480,000 adults; implications for primary prevention. J. Am. Coll. Cardiol. 2018, 72, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Hasbani, N.R.; Ligthart, S.; Brown, M.R.; Heath, A.S.; Bebo, A.; Ashley, K.E.; Boerwinkle, E.; Morrison, A.C.; Folsom, A.R.; Aguilar, D.; et al. American Heart Association’s Life’s Simple 7: Lifestyle recommendations, polygenic risk, and lifetime risk of coronary heart disease. Circulation 2022, 145, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain. J. Am. Coll. Cardiol. 2021, 78, e187–e285. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]
- Visseren, F.L.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3237. [Google Scholar] [CrossRef]
- Muhlestein, J.B.; Knowlton, K.U.; Le, V.T.; Lappe, D.L.; May, H.T.; Min, D.B.; Johnson, K.M.; Cripps, S.T.; Schwab, L.H.; Braun, S.B.; et al. Comparison of coronary artery calcium score versus pooled cohort equations score for primary prevention guidance: Randomized feasibility trial. JACC-Cardiovasc. Imaging 2021, 9, S1936. [Google Scholar]
- Spinu, M.; Onea, L.H.; Homorodean, C.; Olinic, M.; Ober, M.C.; Olinic, D.M. Optical coherence tomography—OCT for characterization of non-atherosclerotic coronary lesions in acute coronary syndromes. J. Clin. Med. 2022, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Paccone, A.; Iovine, M.; Cavalcanti, E.; Berretta, M.; Maurea, C.; Canale, M.L.; Maurea, N. Interleukin-1 blocking agents as promising strategy for prevention of anticancer drug-induced cardiotoxicities: Possible implications in cancer patients with COVID-19. Rev. Med. Pharmacol. Sci. 2021, 25, 6797–6812. [Google Scholar]
- Quagliariello, V.; De Laurentiis, M.; Rea, D.; Barbieri, A.; Monti, M.G.; Carbone, A.; Paccone, A.; Altucci, L.; Conte, M.; Canale, M.L.; et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and proinflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc. Diabetol. 2021, 20, 150. [Google Scholar] [CrossRef] [PubMed]
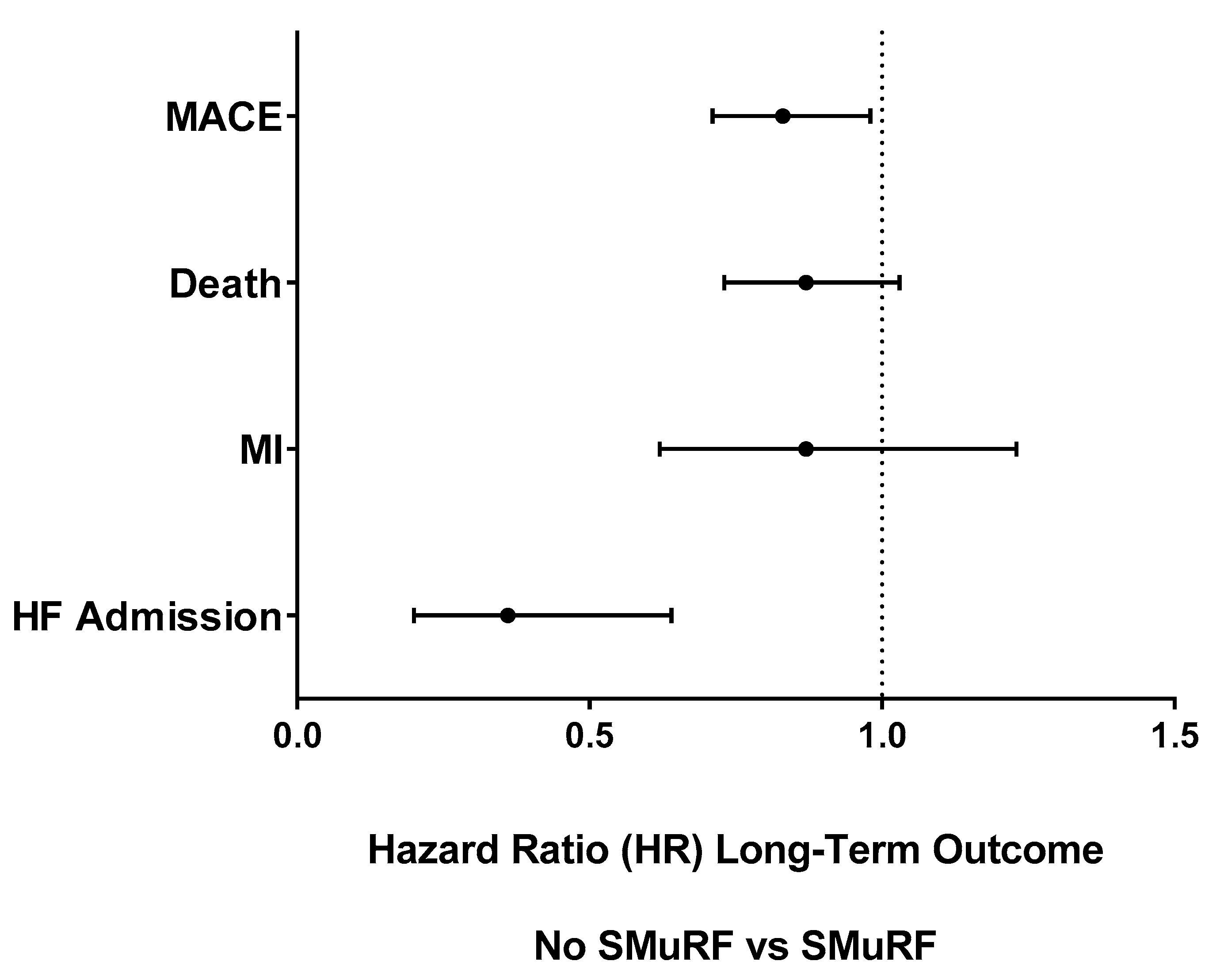

| SMuRF | No SMuRF | ||||
|---|---|---|---|---|---|
| Demographics and Clinical Characteristics | n = 2591 | n = 919 | p-Value | ||
| n | % | n | % | ||
| Age, median (IQR) | 61 (53, 71) | 61 (52, 70) | 0.09 | ||
| Age groups | 0.02 | ||||
| <40 | 85 | 3.3% | 49 | 5.3% | |
| 40–49 | 360 | 13.9% | 140 | 15.2% | |
| 50–59 | 720 | 27.8% | 228 | 24.8% | |
| 60–69 | 717 | 27.7% | 271 | 29.5% | |
| 70–79 | 471 | 18.2% | 150 | 16.3% | |
| >79 | 238 | 9.2% | 80 | 8.7% | |
| Sex | 0.009 | ||||
| Male | 1885 | 72.8% | 709 | 77.3% | |
| Female | 706 | 27.3% | 210 | 22.9% | |
| Race | 0.28 | ||||
| White/Caucasian | 2260 | 87.2% | 818 | 89.0% | |
| African American | 14 | 0.5% | 8 | 0.9% | |
| Asian | 57 | 2.2% | 15 | 1.6% | |
| Pacific Islander | 5 | 0.3% | 3 | 0.3% | |
| Unknown | 255 | 9.8% | 75 | 8.2% | |
| Family history of heart disease | 849 | 32.8% | 137 | 14.9% | <0.0001 |
| Comorbidities | |||||
| Atrial Fibrillation (AF) | 420 | 16.2% | 112 | 12.2% | 0.004 |
| COPD | 234 | 9.0% | 52 | 5.7% | 0.001 |
| Depression | 438 | 16.9% | 136 | 14.8% | 0.14 |
| Heart Failure (HF) | 126 | 4.9% | 33 | 3.6% | 0.11 |
| Stroke | 37 | 1.4% | 12 | 1.3% | 0.7 |
| SMURF criteria | |||||
| Diabetes | 1044 | 40.3% | 0 | NA | |
| Hyperlipidemia | 1613 | 62.3% | 0 | NA | |
| Hypertension | 1736 | 67.0% | 0 | NA | |
| Smoking history | NA | ||||
| Never | 1785 | 68.9% | 919 | ||
| Former | 285 | 11.0% | 0 | ||
| Current | 521 | 20.1% | 0 | ||
| SMuRF | No SMuRF | ||||
|---|---|---|---|---|---|
| Treatments and Medications | n = 2591 | n = 919 | |||
| n | % | n | % | p-Value | |
| PCI performed | 2267 | 87.5% | 788 | 85.8% | 0.17 |
| CABG | 210 | 8.1% | 58 | 6.3% | 0.08 |
| Discharge Medications | |||||
| Beta Blocker | 2177 | 84.0% | 787 | 85.6% | 0.25 |
| ACE-I/ARB | 1739 | 67.1% | 576 | 62.7% | 0.01 |
| Anticoagulant | 887 | 34.2% | 333 | 36.2% | 0.27 |
| Antiplatelet | 2464 | 95.1% | 887 | 96.5% | 0.50 |
| Aspirin | 2454 | 94.7% | 875 | 95.2% | 0.65 |
| CCB | 314 | 12.1% | 100 | 10.9% | 0.32 |
| SMuRF | No SMuRF | |||||||
|---|---|---|---|---|---|---|---|---|
| n = 2591 | n = 919 | |||||||
| n | % | n | % | Unadjusted p-Values | Adj a HR | 95% CI | p-Value | |
| 60-day Outcomes | ||||||||
| MACE | 210 | 8.1% | 71 | 7.7% | 0.76 | 0.95 | (0.72, 1.25) | 0.72 |
| Death | 170 | 6.6% | 65 | 7.1% | 0.57 | 1.06 | (0.79, 1.42) | 0.69 |
| MI | 14 | 0.5% | 4 | 0.4% | 0.70 | NA b | ||
| HF Hospitalization | 27 | 1.0% | 2 | 0.2% | 0.03 | NA b | ||
| Long-term Outcomes | ||||||||
| MACE | 813 | 31.4% | 197 | 21.4% | 0.02 | 0.83 | (0.71, 0.98) | 0.02 |
| Death | 646 | 24.9% | 160 | 17.4% | 0.11 | 0.87 | (0.73, 1.03) | 0.11 |
| MI | 178 | 6.9% | 42 | 4.6% | 0.29 | 0.87 | (0.62, 1.23) | 0.44 |
| HF Hospitalization | 125 | 4.8% | 13 | 1.4% | 0.0003 | 0.36 | (0.20, 0.64) | 0.0005 |
| SMuRF Count | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||||||||
| n = 919 | n = 834 | n = 1007 | n = 649 | n = 101 | ||||||||||
| n | % | n | % | n | % | n | % | n | % | Unadjusted | Adj ** HR | 95% CI | p-Value | |
| p-Values | ||||||||||||||
| 60-day Outcomes | ||||||||||||||
| MACE | 71 | 7.7% | 61 | 7.3% | 78 | 7.8% | 65 | 10.0% | 6 | 5.9% | 0.30 | 1.07 | (0.96, 1.19) | 0.23 |
| Death | 65 | 7.1% | 50 | 6.0% | 64 | 6.4% | 52 | 8.0% | 4 | 4.0% | 0.92 | 1.03 | (0.91, 1.15) | 0.68 |
| MI | 4 | 0.4% | 3 | 0.4% | 8 | 0.8% | 2 | 0.3% | 1 | 1.0% | 0.63 | NA * | ||
| HF Hospitalization | 2 | 0.2% | 8 | 0.2% | 7 | 0.7% | 11 | 1.7% | 1 | 1.0% | 0.01 | NA * | ||
| Long-term Outcomes | ||||||||||||||
| MACE | 197 | 21.4% | 244 | 29.3% | 292 | 29.0% | 228 | 35.1% | 49 | 48.5% | <0.0001 | 1.16 | (1.09, 1.23) | <0.0001 |
| Death | 160 | 17.4% | 193 | 23.1% | 229 | 22.7% | 184 | 28.4% | 40 | 39.6% | <0.0001 | 1.17 | (1.09, 1.25) | <0.0001 |
| MI | 42 | 4.6% | 62 | 7.4% | 59 | 5.9% | 46 | 7.1% | 11 | 10.9% | 0.08 | 1.11 | (0.98, 1.25) | 0.11 |
| HF Hospitalization | 13 | 1.4% | 34 | 4.1% | 44 | 4.4% | 38 | 5.9% | 9 | 8.9% | <0.0001 | 1.41 | (1.21, 1.64) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, J.L.; Knight, S.; May, H.T.; Le, V.T.; Almajed, J.; Bair, T.L.; Knowlton, K.U.; Muhlestein, J.B. Cardiovascular Outcomes of ST-Elevation Myocardial Infarction (STEMI) Patients without Standard Modifiable Risk Factors (SMuRF-Less): The Intermountain Healthcare Experience. J. Clin. Med. 2023, 12, 75. https://doi.org/10.3390/jcm12010075
Anderson JL, Knight S, May HT, Le VT, Almajed J, Bair TL, Knowlton KU, Muhlestein JB. Cardiovascular Outcomes of ST-Elevation Myocardial Infarction (STEMI) Patients without Standard Modifiable Risk Factors (SMuRF-Less): The Intermountain Healthcare Experience. Journal of Clinical Medicine. 2023; 12(1):75. https://doi.org/10.3390/jcm12010075
Chicago/Turabian StyleAnderson, Jeffrey L., Stacey Knight, Heidi T. May, Viet T. Le, Jawad Almajed, Tami L. Bair, Kirk U. Knowlton, and Joseph B. Muhlestein. 2023. "Cardiovascular Outcomes of ST-Elevation Myocardial Infarction (STEMI) Patients without Standard Modifiable Risk Factors (SMuRF-Less): The Intermountain Healthcare Experience" Journal of Clinical Medicine 12, no. 1: 75. https://doi.org/10.3390/jcm12010075
APA StyleAnderson, J. L., Knight, S., May, H. T., Le, V. T., Almajed, J., Bair, T. L., Knowlton, K. U., & Muhlestein, J. B. (2023). Cardiovascular Outcomes of ST-Elevation Myocardial Infarction (STEMI) Patients without Standard Modifiable Risk Factors (SMuRF-Less): The Intermountain Healthcare Experience. Journal of Clinical Medicine, 12(1), 75. https://doi.org/10.3390/jcm12010075







