The Concordance of Alveolar Bone Deficiency with Severity of Lip Deformity in Microform Cleft Lip
Abstract
1. Introduction
2. Materials and Methods
2.1. Anthropometric Analysis
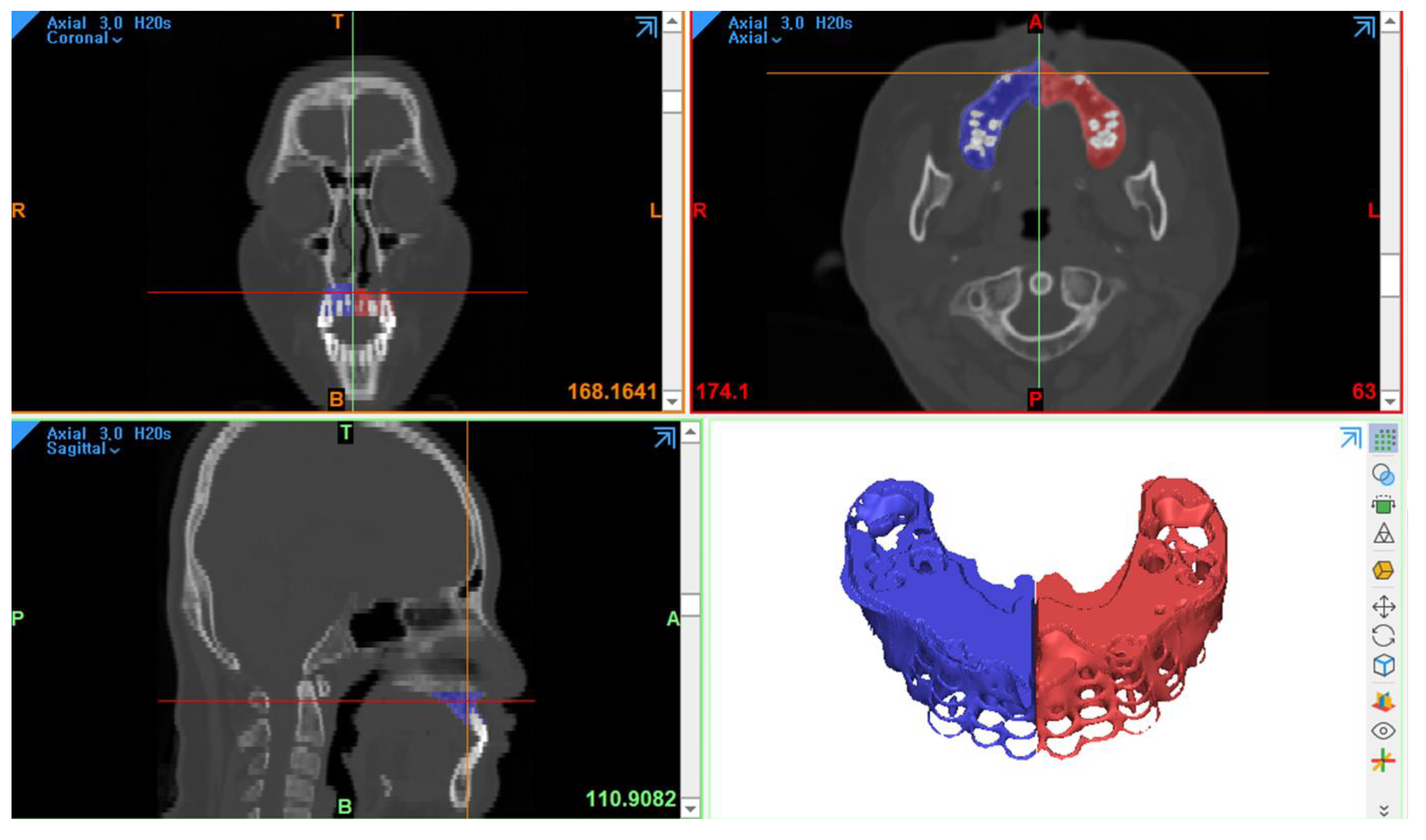
2.2. Three-Dimensional Computed Tomography Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeong, D.K.; Lee, J.W.; Choi, S.J.; Bae, Y.C. Long-term results of unilateral cleft lip repair with multiple infantile hemangiomas including one involving the cleft side of the upper lip. Arch. Plast. Surg. 2020, 47, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Mink van der Molen, A.B.; van Breugel, J.M.M.; Janssen, N.G.; Admiraal, R.J.C.; van Adrichem, L.N.A.; Bierenbroodspot, F.; Bittermann, D.; van den Boogaard, M.-J.H.; Broos, P.H.; Dijkstra-Putkamer, J.J.M.; et al. Clinical practice guidelines on the treatment of patients with cleft lip, alveolus, and palate: An executive summary. J. Clin. Med. 2021, 10, 4813. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, J.; Kim, J.; Jo, T.; Hwang, I.; Han, K.; Jeong, W. An evaluation of muscle repair techniques: Implications in musculoskeletal healing and corollaries in oral-facial clefting. J. Clin. Med. 2021, 10, 4803. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Yoshida, M.; Nakamichi, N.; Saitoh, S.; Takaichi, M.; Ishizaka, R.; Tomihara, K.; Noguchi, M. Mini-microform cleft lip with complete cleft alveolus and palate: A case report. Congenit. Anom. 2021, 61, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Choi, D.H.; Bae, S.G.; Cho, B.C. Correction of minor-form and microform cleft lip using modified muscle overlapping with a minimal skin incision. Arch. Plast. Surg. 2017, 44, 210–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuzuriha, S.; Mulliken, J.B. Minor-form, microform, and mini-microform cleft lip: Anatomical features, operative techniques, and revisions. Plast. Reconstr. Surg. 2008, 122, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeong, W. Secondary bone grafting for alveolar clefts: Surgical timing, graft materials, and evaluation methods. Arch. Craniofac. Surg. 2022, 23, 53–58. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, J.Y.; Hong, K.Y.; Choi, T.H.; Kim, B.J.; Kim, S. The effect of biphasic calcium phosphate and demineralized bone matrix on tooth eruption in mongrel dogs. Arch. Craniofac. Surg. 2021, 22, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Ma, L.; Zhou, X.; Sun, Z. Bony defect of palate and vomer in submucous cleft palate patients. Int. J. Oral. Maxillofac. Surg. 2015, 44, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zheng, Y.; Ma, H.; Yin, N. Muscle flap reconstruction based on muscle tension line groups to repair the philtrum of patients with microform cleft lip or secondary cleft lip. J. Craniofacial Surg. 2022, 33, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.H.; Lo, L.J. Strategic management of the minor-form and microform cleft lip: A long-term outcome assessment. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2021, 74, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Meazzini, M.C.; Corno, M.; Novelli, G.; Autelitano, L.; Tortora, C.; Elsido, D.; Garattini, G.; Brusati, R. Long-term computed tomographic evaluation of alveolar bone formation in patients with unilateral cleft lip and palate after early secondary gingivoalveoloplasty. Plast. Reconstr. Surg. 2016, 137, 365e–374e. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Khang, S.K.; Lee, T.J.; Kim, T.G. Clinical features of the microform cleft lip and the ultrastructural characteristics of the orbicularis oris muscle. Cleft Palate-Craniofacial J. Off. Publ. Am. Cleft Palate-Craniofacial Assoc. 2010, 47, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.; Mayor, R. Collective cell migration in development. J. Cell Biol. 2016, 212, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Bush, J.O.; Lidral, A.C. Development of the upper lip: Morphogenetic and molecular mechanisms. Dev. Dyn. 2006, 235, 1152–1166. [Google Scholar] [CrossRef] [PubMed]
- Couly, G.F.; Coltey, P.M.; Le Douarin, N.M. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development 1992, 114, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Couly, G.F.; Coltey, P.M.; Le Douarin, N.M. The triple origin of skull in higher vertebrates: A study in quail-chick chimeras. Development 1993, 117, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Drolet, B.A.; Baselga, E.; Gosain, A.K.; Levy, M.L.; Esterly, N.B. Preauricular skin defects. A consequence of a persistent ectodermal groove. Arch. Dermatol. 1997, 133, 1551–1554. [Google Scholar] [CrossRef] [PubMed]
- Tangco, I.V.; Bhandari, K.; Yao, C.-F.; Lu, T.-C.; Chen, P.K.-T. Symmetry of the vermillion height after modified rotation-advancement cheiloplasty. J. Clin. Med. 2022, 11, 6744. [Google Scholar] [CrossRef]
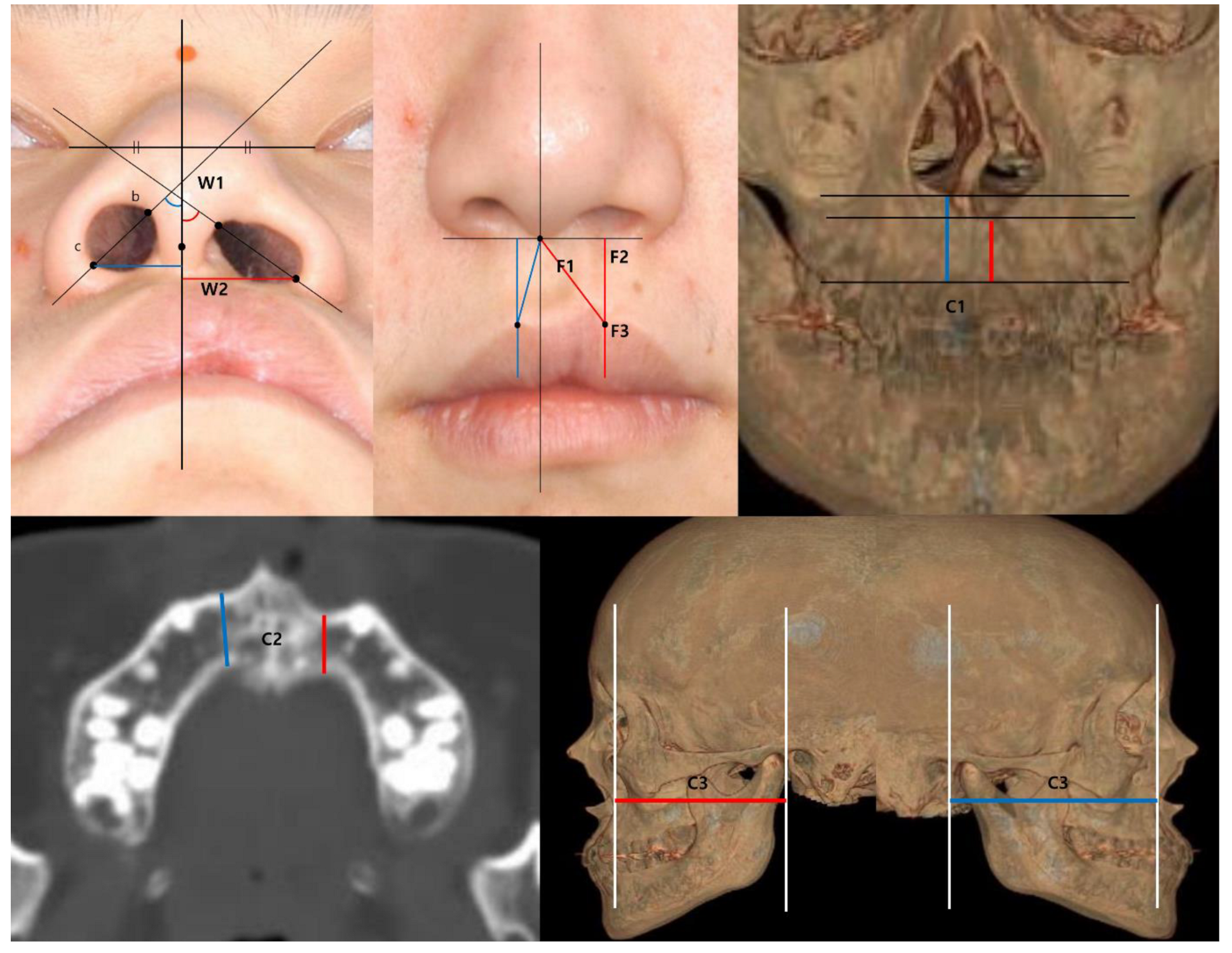




| Measurement Item | Definition |
|---|---|
| Nostril angle (W1) | the angle of the vertical line and the line that crosses the nostril apex (the most upper & medial point) and the alar base (the most lateral & lowest point) |
| Width of alar base (W2) | the shortest distance between the alar base and the vertical line crossing the columella |
| Philtral height (F1) | the distance between the columella and both peaks of Cupid’s bow (the notch on the cleft side and the peak on the normal side) |
| Lip height (F2) | the vertical distance from the horizontal line crossing the columella to both peaks |
| Thickness of dry vermilion (F3) | the distance from the peaks to the redline of the upper lip |
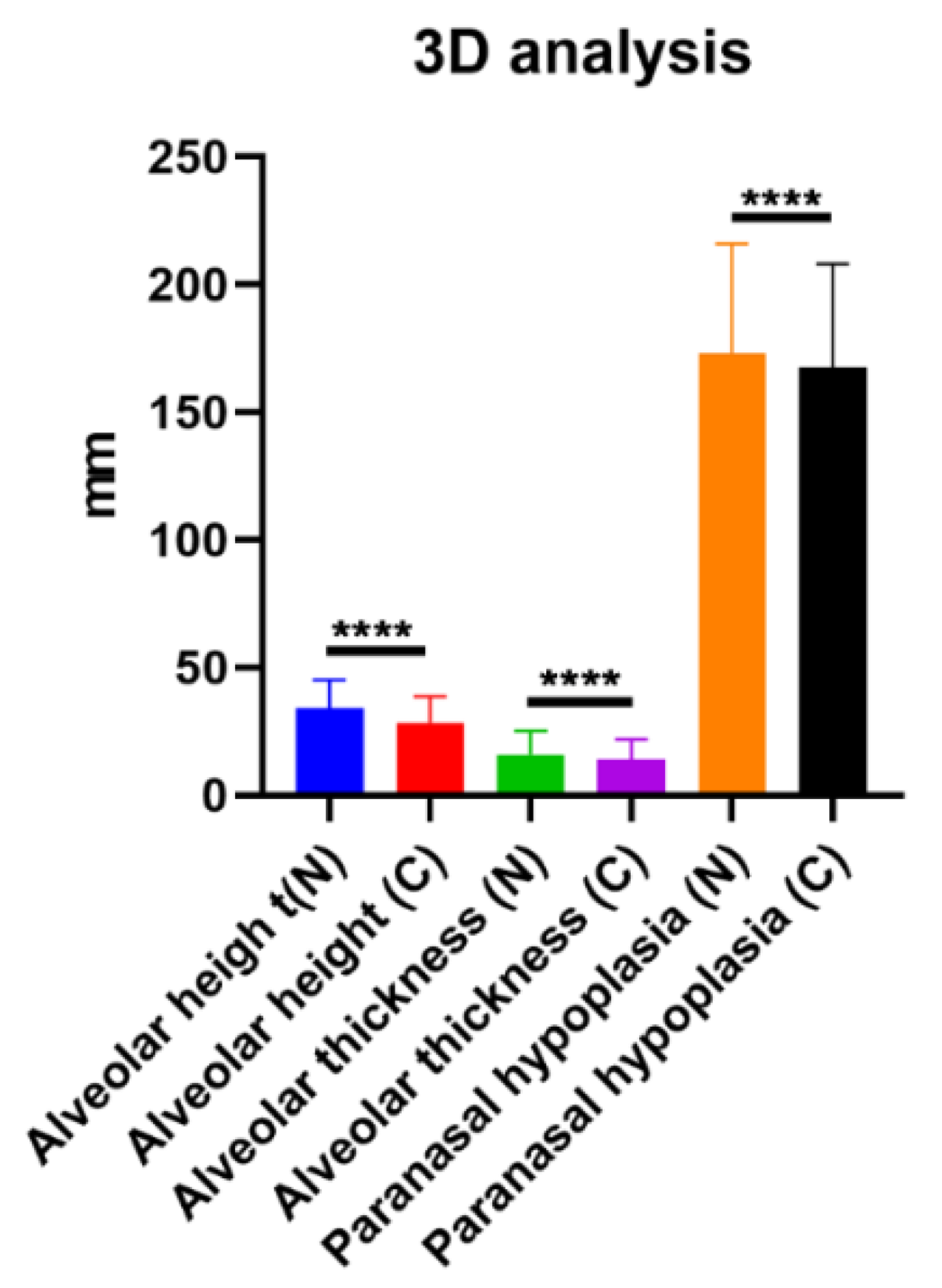
| Alveolar height (C1) | the distance from the lowest part of the anterior aspect of the piriform aperture to the alveolar bone on the cleft side and the normal side |
| Alveolar thickness (C2) | the thickness at the thinnest plane of maxillary bone on the axial cross-section of the CT on the cleft side and the normal side |
| Paranasal hypoplasia (C3) | the distance between the coronal facial plane at the EAC and the most concave point of the maxilla on lateral view of the 3D reconstructed CT |
| Characteristic | Value |
|---|---|
| No. | 23 |
| Mean age ± SD, years (range) | 13.84 ± 12.35 (1.25 to 50) |
| Sex | |
| Male:Female | 14:9 |
| Laterality | |
| Left:Right | 18:5 |
| Concomitant disease | |
| Cleft palate | 0 |
| Submucous cleft palate | 0 |
| Medical disease | VSD (1), TR (1) |
| Family history | 0 |
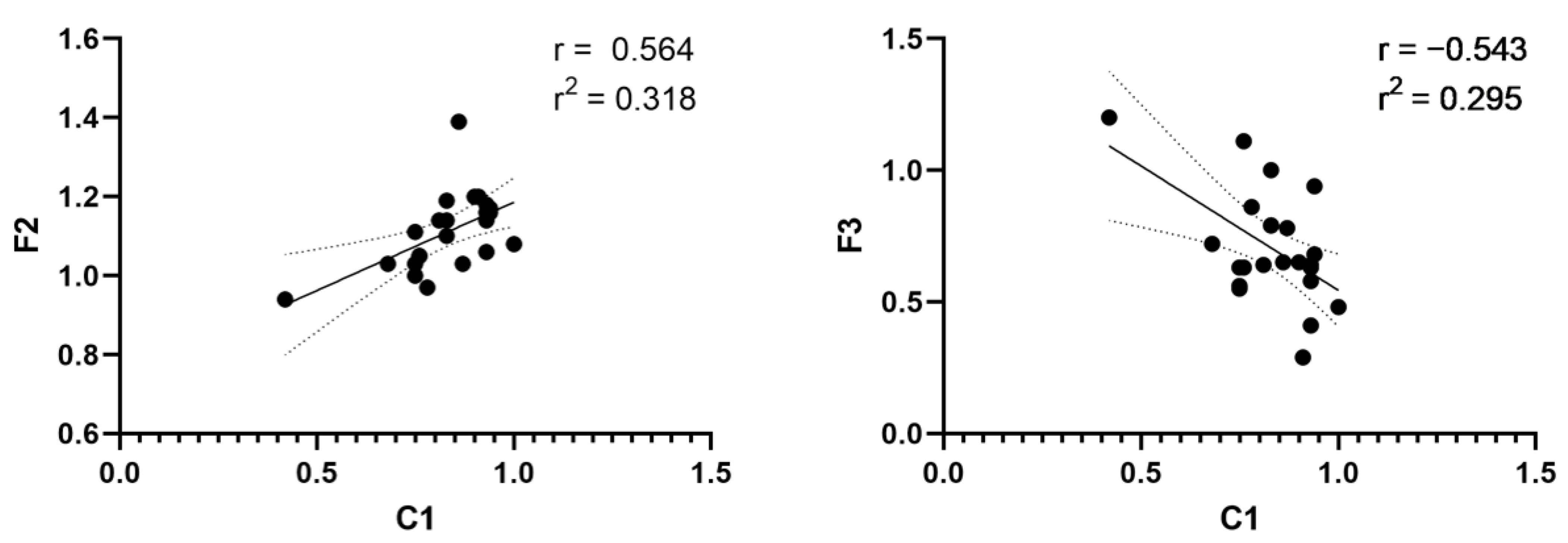
| W1 | W2 | F1 | F2 | F3 | ||
|---|---|---|---|---|---|---|
| C1 | Correlation coefficient | 0.096 | 0.303 | 0.377 | 0.564 ** | −0.543 ** |
| p-value | 0.664 | 0.16 | 0.076 | 0.005 | 0.007 | |
| C2 | Correlation coefficient | 0.050 | 0.101 | 0.035 | −0.021 | 0.219 |
| p-value | 0.821 | 0.646 | 0.872 | 0.926 | 0.316 | |
| C3 | Correlation coefficient | −0.164 | −0.144 | 0.078 | 0.158 | 0.131 |
| p-value | 0.454 | 0.513 | 0.722 | 0.472 | 0.550 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, T.; Choi, K.; Choi, J.; Kim, J.; Han, K.; Jeong, W. The Concordance of Alveolar Bone Deficiency with Severity of Lip Deformity in Microform Cleft Lip. J. Clin. Med. 2023, 12, 39. https://doi.org/10.3390/jcm12010039
Jo T, Choi K, Choi J, Kim J, Han K, Jeong W. The Concordance of Alveolar Bone Deficiency with Severity of Lip Deformity in Microform Cleft Lip. Journal of Clinical Medicine. 2023; 12(1):39. https://doi.org/10.3390/jcm12010039
Chicago/Turabian StyleJo, Taehee, Kyehoon Choi, Jaehoon Choi, Junhyung Kim, Kihwan Han, and Woonhyeok Jeong. 2023. "The Concordance of Alveolar Bone Deficiency with Severity of Lip Deformity in Microform Cleft Lip" Journal of Clinical Medicine 12, no. 1: 39. https://doi.org/10.3390/jcm12010039
APA StyleJo, T., Choi, K., Choi, J., Kim, J., Han, K., & Jeong, W. (2023). The Concordance of Alveolar Bone Deficiency with Severity of Lip Deformity in Microform Cleft Lip. Journal of Clinical Medicine, 12(1), 39. https://doi.org/10.3390/jcm12010039






