Influence of Intravitreal Therapy on Choroidal Thickness in Patients with Diabetic Macular Edema
Abstract
1. Introduction
- To demonstrate whether there are differences in the variation in macular and choroidal thicknesses in patients treated with anti-VEGF compared to patients treated with corticosteroids.
- To analyze whether there is a variation in CMT and GCSF in untreated eyes after intravitreal treatment in the contralateral eyes.
- To evaluate the influence of the following variables on the response to intravitreal therapy with anti-VEGF or corticosteroids:
- -
- Age;
- -
- Gender;
- -
- Previous intravitreal therapy;
- -
- Type of antidiabetic treatment;
- -
- Time course of type 2 diabetes.
2. Material and Methods
2.1. Design
2.2. Participants and Methods
2.3. Statistical Analysis
2.3.1. Within-Group Analysis
- -
- The variable “Pretreatment” was dichotomized into yes (there was pretreatment) and no (there was not) groups.
- -
- The variable “Thinning in CMT or SFCT after treatment” was categorized into three levels:
- ▪
- 50 μm;
- ▪
- 50–100 μm;
- ▪
- >100 μm.
- -
- The variable “Antidiabetic treatment” was categorized into three levels:
- ▪
- INS (insulin);
- ▪
- ANI (non-insulin antidiabetic);
- ▪
- COM (combined treatment with insulin and non-insulin antidiabetics).
2.3.2. Comparison of Treatment Groups
2.3.3. Comparison with Untreated Oleander Eyes
3. Results
3.1. Aflibercept-Treated Group
3.1.1. Differences in CMT with Treatment
3.1.2. Differences in SFCT with Treatment
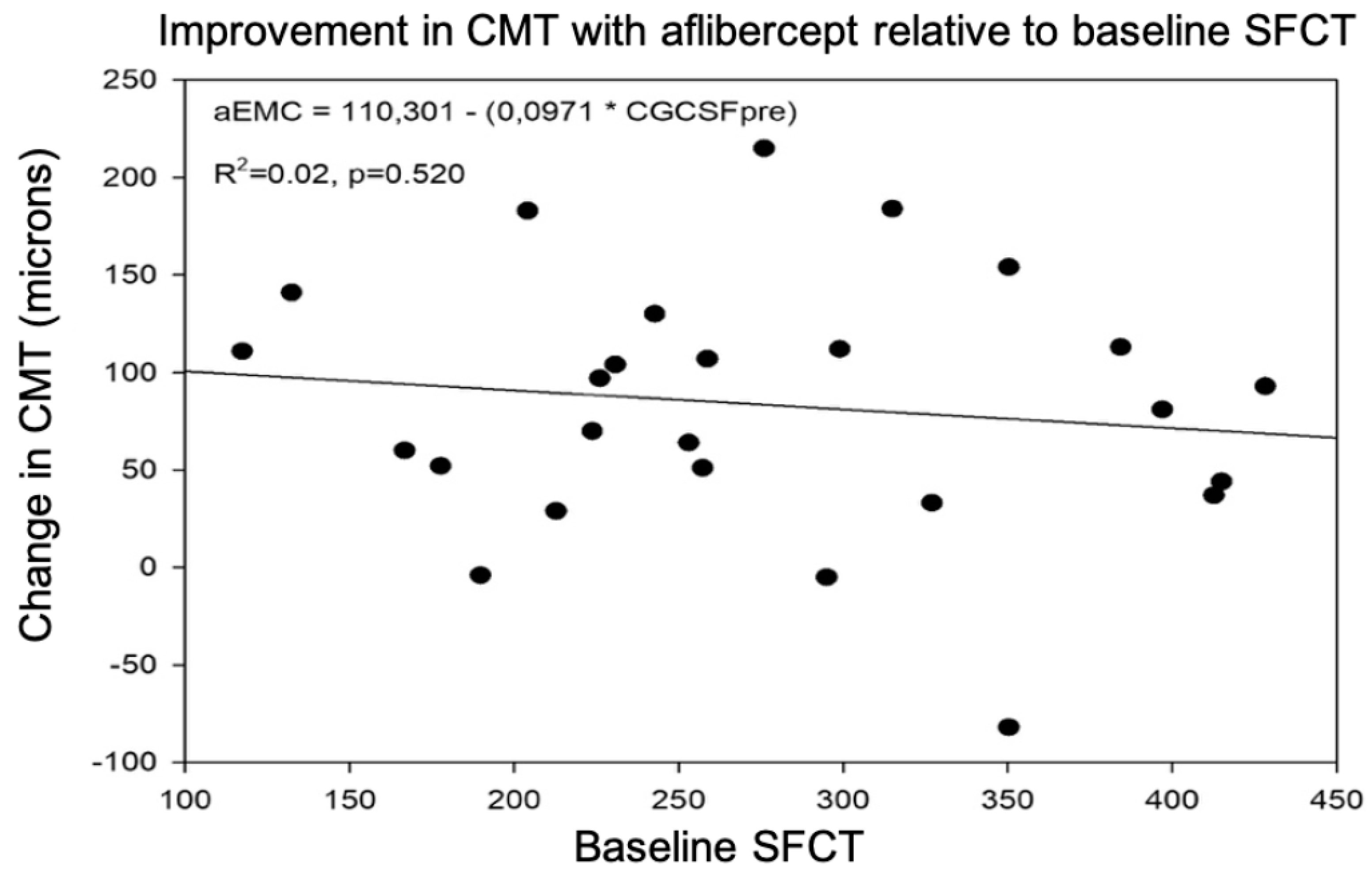
3.1.3. Predictive Value of SFCT for CMT Response after Treatment
3.2. Dexamethasone-Treated Group
3.2.1. Differences in CMT with Treatment
3.2.2. Differences in SFCT with Treatment
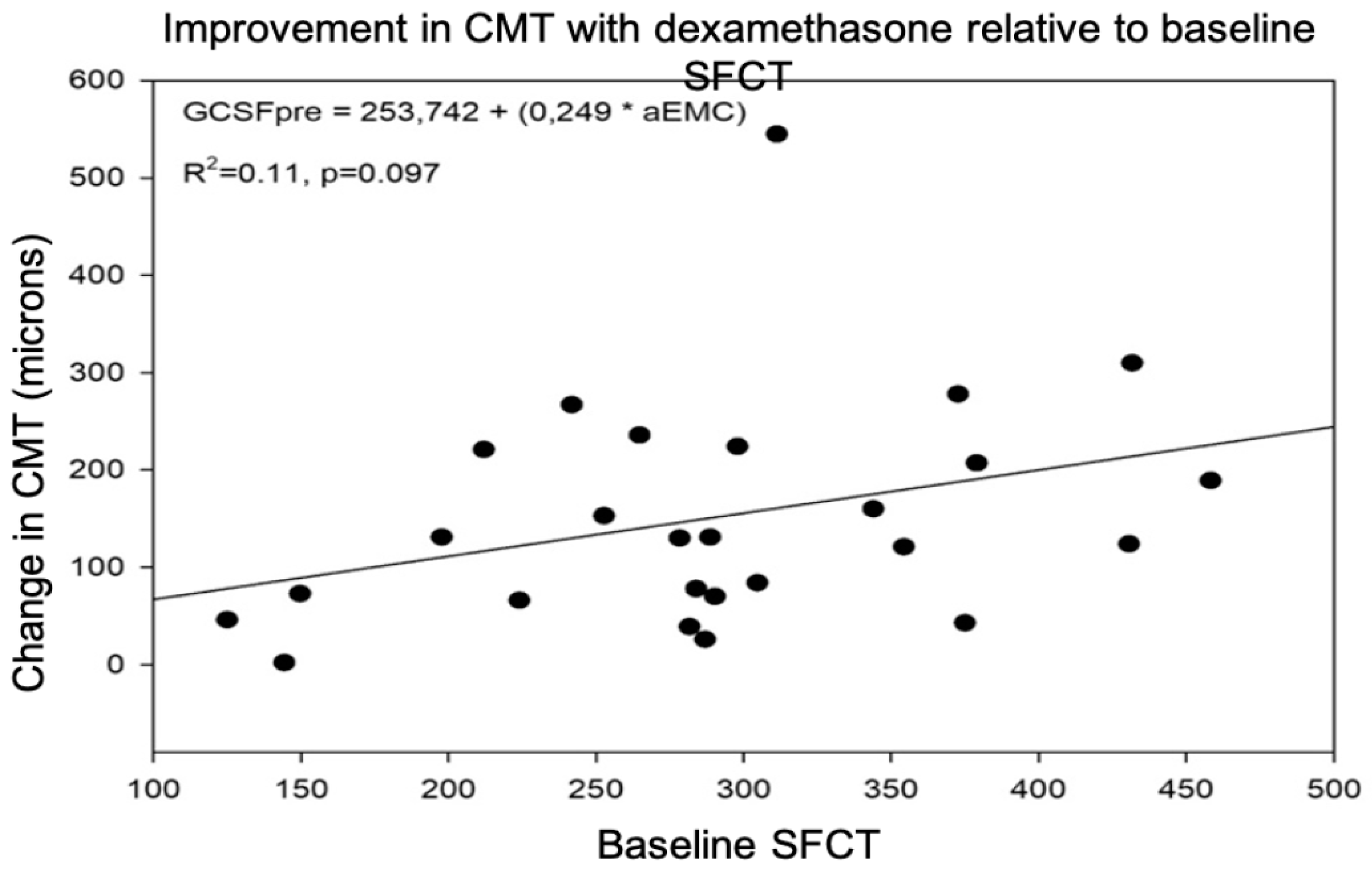
3.2.3. Predictive Value of SFCT for Post-Treatment DME Response
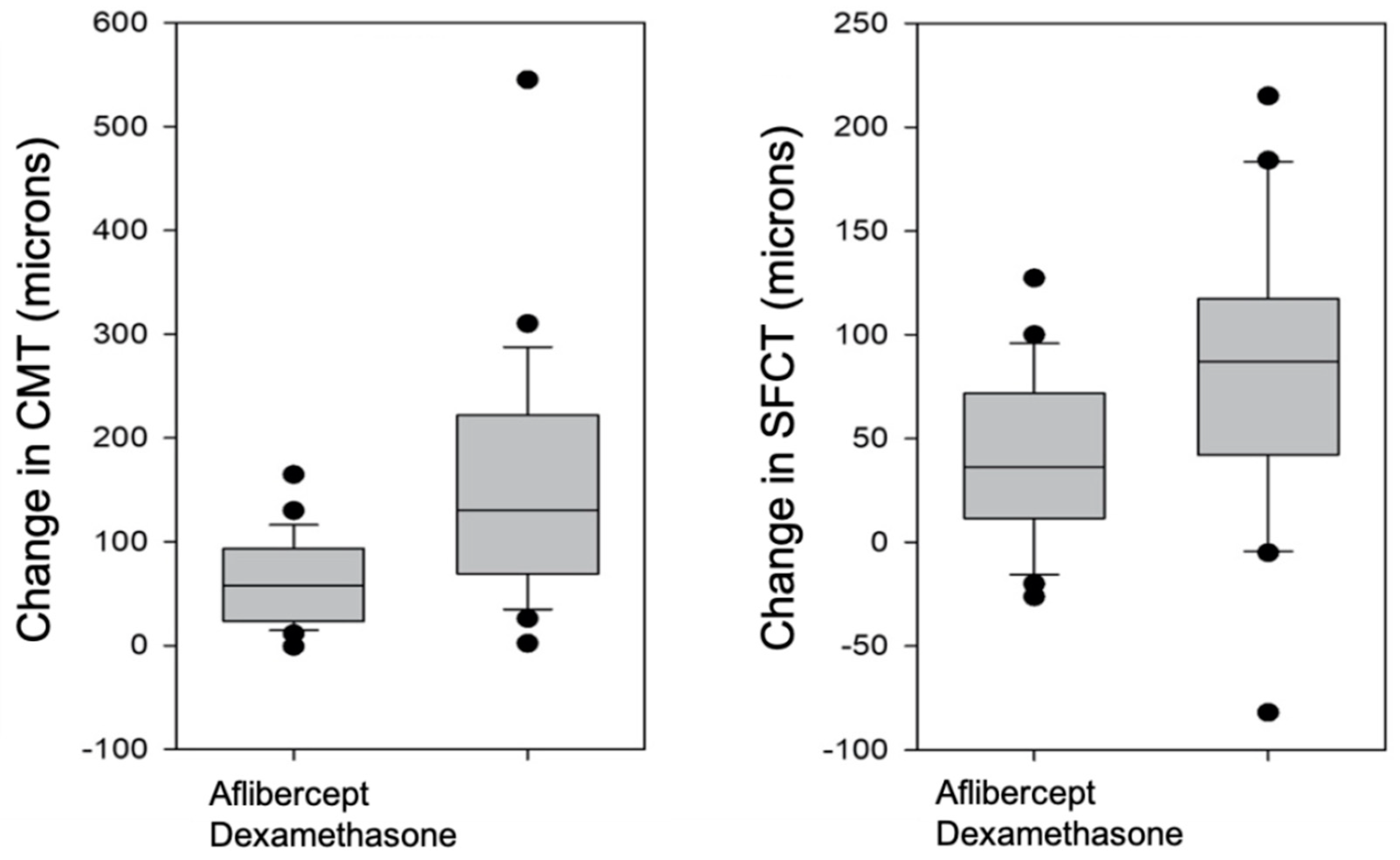
3.3. Comparison of Aflibercept and Dexamethasone Groups
3.4. Control Group
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, R.; Airey, M.; Baxter, H.; Forrester, J.; Kennedy-Martin, T.; Girach, A. Epidemiology of diabetic retinopathy and macular oedema: A systematic review. Eye 2004, 18, 963–983. [Google Scholar] [CrossRef] [PubMed]
- Martín-Manzano, J.L. Complicaciones Crónicas: Ojo. SAMFyC. 2010. Available online: http://www.grupodiabetessamfyc.es/index.php/guia-clinica/guia-clinica/complicaciones-cronicas/ojo.html (accessed on 26 October 2022).
- Browning, D.; Stewart, M.; Lee, C. Diabetic macular edema: Evidence-based management. Indian J. Ophthalmol. 2018, 66, 1736–1750. [Google Scholar]
- Andonegui, J.; Jiménez Lasanta, L. Edema macular diabético. An. Del Sist. Sanit. De Navar. 2008, 31, 35–44. [Google Scholar]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.; Kowalski, J.W.; Bek, T.; Chen, S.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, F.C.; Yolcu, U.; Akay, F.; Ilhan, A.; Ozge, G.; Uzun, S. Diabetic macular edema. Pak. J. Med. Sci. 2016, 32, 505–510. [Google Scholar]
- Miller, K.; Fortun, J.A. Diabetic macular edema: Current understanding, pharmacologic treatment options, and developing therapies. Asia-Pac. J. Ophthalmol. 2018, 7, 28–35. [Google Scholar]
- Zur, D.; Iglicki, M.; Busch, C.; Invernizzi, A.; Mariussi, M.; Loewenstein, A.; Cebeci, Z.; Chhablani, J.K.; Chaikitmongkol, V.; Couturier, A.; et al. OCT biomarkers as functional outcome predictors in diabetic macular edema treated with dexamethasone implant. Ophthalmology 2018, 125, 267–275. [Google Scholar] [CrossRef]
- Gerendas, B.S.; Prager, S.; Deak, G.; Simader, C.; Lammer, J.; Waldstein, S.M.; Guerin, T.; Kundi, M.; Schmidt-Erfurth, U.M. Predictive imaging biomarkers relevant for functional and anatomical outcomes during ranibizumab therapy of diabetic macular oedema. Br. J. Ophthalmol. 2018, 102, 195–203. [Google Scholar] [CrossRef]
- Regatieri, C.V.; Branchini, L.; Carmody, J.; Fujimoto, J.G.; Duker, J.S. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina 2012, 32, 563–568. [Google Scholar] [CrossRef]
- Querques, G.; Lattanzio, R.; Querques, L.; Del Turco, C.; Forte, R.; Pierro, L.; Souied, E.H.; Bandello, F. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6017–6024. [Google Scholar] [CrossRef]
- Ünsal, E.; Eltutar, K.; Zirtiloğlu, S.; Dinçer, N.; Erkul, S.Ö.; Güngel, H. Choroidal thickness in patients with diabetic retinopathy. Clin. Ophthalmol. 2014, 8, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, S.; Qiu, Z.; He, M.; Wang, L.; Li, Y.; Huang, W. Choroidal thickness in diabetes and diabetic retinopathy: A swept source OCT study. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef]
- López Gálvez, M.I. Escala internacional de severidad de la retinopatía y del edema macular diabético. Arch. Soc. Esp. Oftalmol. 2004, 79, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef] [PubMed]
- Panozzo, G.; Cicinelli, M.V.; Augustin, A.J.; Parodi, M.B.; Cunha-Vaz, J.; Guarnaccia, G.; Kodjikian, L.; Jampol, L.M.; Jünemann, A.; Lanzetta, P.; et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: The European School for Advanced Studies in Ophthalmology classification. Eur. J. Ophthalmol. 2020, 30, 8–18. [Google Scholar] [CrossRef]
- Udaondo, P.; Adan, A.; Arias-Barquet, L.; Ascaso, F.J.; Cabrera-López, F.; Castro-Navarro, V.; Donate-López, J.; García-Layana, A.; Lavid, F.J.; Rodríguez-Maqueda, M.; et al. Challenges in Diabetic Macular Edema Management: An Expert Consensus Report. Clin. Ophthalmol. 2021, 15, 3183–3195. [Google Scholar] [CrossRef]
- Rewbury, R.; Want, A.; Varughese, R.; Chong, V. Subfoveal choroidal thickness in patients with diabetic retinopathy and diabetic macular oedema. Eye 2016, 30, 1568–1572. [Google Scholar] [CrossRef]
- Gupta, C.; Tan, R.; Mishra, C.; Khandelwal, N.; Raman, R.; Kim, R.; Agrawal, R.; Sen, P. Choroidal structural analysis in eyes with diabetic retinopathy and diabetic macular edema-A novel OCT based imaging biomarker. PLoS ONE 2018, 13, e0207435. [Google Scholar] [CrossRef]
- Esmaeelpour, M.; Považay, B.; Hermann, B.; Hofer, B.; Kajic, V.; Hale, S.L.; North, R.V.; Drexler, W.; Sheen, N.J. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2011, 52, 5311–5316. [Google Scholar] [CrossRef]
- Gerendas, B.S.; Waldstein, S.M.; Simader, C.; Deak, G.; Hajnajeeb, B.; Zhang, L.; Bogunovic, H.; Abramoff, M.D.; Kundi, M.; Sonka, M.; et al. Three-dimensional automated choroidal volume assessment on standard spectral-domain optical coherence tomography and correlation with the level of diabetic macular edema. Am. J. Ophthalmol. 2014, 158, 1039–1048. [Google Scholar] [CrossRef]
- Hua, R.; Liu, L.; Wang, X.; Chen, L. Imaging evidence of diabetic choroidopathy in vivo: Angiographic pathoanatomy and choroidal-enhanced depth imaging. PLoS ONE 2013, 8, e83494. [Google Scholar] [CrossRef]
- Kim, J.T.; Lee, D.H.; Joe, S.G.; Kim, J.G.; Yoon, Y.H. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Retina 2013, 54, 3378–3384. [Google Scholar] [CrossRef]
- Gómez-Resa, M.; Corcóstegui, B. Tomografía de coherencia óptica en el edema macular diabético. Ann. Ophthalmol. 2014, 22, 15–23. [Google Scholar]
- McCourt, E.A.; Cadena, B.C.; Barnett, C.J.; Ciardella, A.P.; Mandava, N.; Kahook, M.Y. Measurement of subfoveal choroidal thickness using spectral domain optical coherence tomography. Ophthalmic Surg. Lasers Imaging Retin. 2010, 41, 28–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tan, C.S.; Cheong, K.X.; Lim, L.W.; Li, K.Z. Topographic variation of choroidal and retinal thicknesses at the macula in healthy adults. Br. J. Ophthalmol. 2014, 98, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, A.A.; Fine, B.S. Diabetic choroidopathy: Light and electron microscopic observations of seven cases. Ophthalmology 1985, 92, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Fryczkowski, A.W.; Sato, S.E.; Hodes, B.L. Changes in the diabetic choroidal vasculature: Scanning electron microscopy findings. Ann. Ophthalmol. 1988, 20, 299–305. [Google Scholar] [PubMed]
- Satoru, K.; Hiroaki, E.; Mitsuo, T.; Yuki, I.; Michiyuki, S.; Masahiko, Y. Alteration of choroidal vascular structure in diabetic macular edema. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 971–977. [Google Scholar]
- De, S.; Saxena, S.; Kaur, A.; Mahdi, A.A.; Misra, A. Sequential restoration of external limiting membrane and ellipsoid zone after intravitreal anti-VEGF therapy in diabetic macular oedema. Eye 2020, 35, 1490–1495. [Google Scholar] [CrossRef]
- Sen, S.; Ramasamy, K.; Sivaprasad, S. Indicators of visual prognosis in diabetic macular oedema. J. Pers. Med. 2021, 11, 449. [Google Scholar] [CrossRef]
- Campos, A.; Campos, E.J.; Carmo, A.d.; Patrício, M.; de Sousa, J.P.C.; Ambrósio, A.F.; Silva, R. Choroidal thickness changes stratified by outcome in real-world treatment of diabetic macular edema. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1857–1865. [Google Scholar] [CrossRef]
- Yiu, G.; Manjunath, V.; Chiu, S.J.; Farsiu, S.; Mahmoud, T.H. Effect of anti-vascular endothelial growth factor therapy on choroidal thickness in diabetic macular edema. Am. J. Ophthalmol. 2014, 158, 745–751.e2. [Google Scholar] [CrossRef] [PubMed]
- Kniggendorf, V.F.; Novais, E.A.; Kniggendorf, S.L.; Xavier, C.; Cole, E.D.; Regatieri, C. Effect of intravitreal anti-VEGF on choroidal thickness in patients with diabetic macular edema using spectral domain OCT. Arq. Bras. Oftalmol. 2016, 79, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group. Early Treatment Diabetic Retinopathy Study Design and Baseline Patient Characteristics: ETDRS Report Number 7. Ophthalmology 1991, 98, 741–756. [Google Scholar] [CrossRef]
- Kim, M.; Cho, Y.J.; Lee, C.H.; Lee, S.C. Effect of intravitreal dexamethasone implant on retinal and choroidal thickness in refractory diabetic macular oedema after multiple anti-VEGF injections. Eye 2016, 30, 718–725. [Google Scholar] [CrossRef]
- Aksoy, M.; Yilmaz, G.; Vardarli, I.; Akkoyun, I. Choroidal thickness after Dexamethasoneimplant or Aflibercept in patients with diabetic macular edema oersistent to Ranibizumab. J. Ocul. Pharmacol. Ther. 2020, 36, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Horozoğlu, F.; Sever, Ö. Early retinal and choroidal coat thickness changes after intravitreal dexamethasone implant injection for diabetic macular edema. Balkan Med. J. 2018, 35, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Altun, A.; Hacimustafaoglu, A.M. Effect of dexamethasone implant on subfoveal choroidal thickness in early period in vitrectomized eyes with diabetic macular edema. J. Ophthalmol. 2021, 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mathis, T.; Mendes, M.; Dot, C.; Bouteleux, V.; Machkour-Bentaleb, Z.; El Chehab, H.; Agard, E.; Denis, P.; Kodjikian, L. Increased choroidal thickness: A new indicator for monitoring diabetic macular oedema recurrence. Acta Ophthalmol. 2020, 98, 968–974. [Google Scholar] [CrossRef]
- Jonas, J.B.; Degenring, R.F.; Kamppeter, B.A.; Kreissig, I.; Akkoyun, I. Duration of the effect of intravitreal triamcinolone acetonide as treatment for diffuse diabetic macular edema. Am. J. Ophthalmol. 2004, 138, 158–160. [Google Scholar] [CrossRef]
- Park, N.; Lee, I.; Kim, J. Changes in choroidal thickness in advanced diabetic retinopathy treated with pan-retinal photocoagulation using a pattern scanning laser versus a conventional laser. BMC Ophthalmol. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chay, I.W.; Tan, C.S. Independent Factors of Choroidal Thickness. Ocul. Immunol. Inflamm. 2019, 27, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Ouyang, Y.; Ruiz, H.; Sadda, S.R. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 261–266. [Google Scholar] [CrossRef] [PubMed]
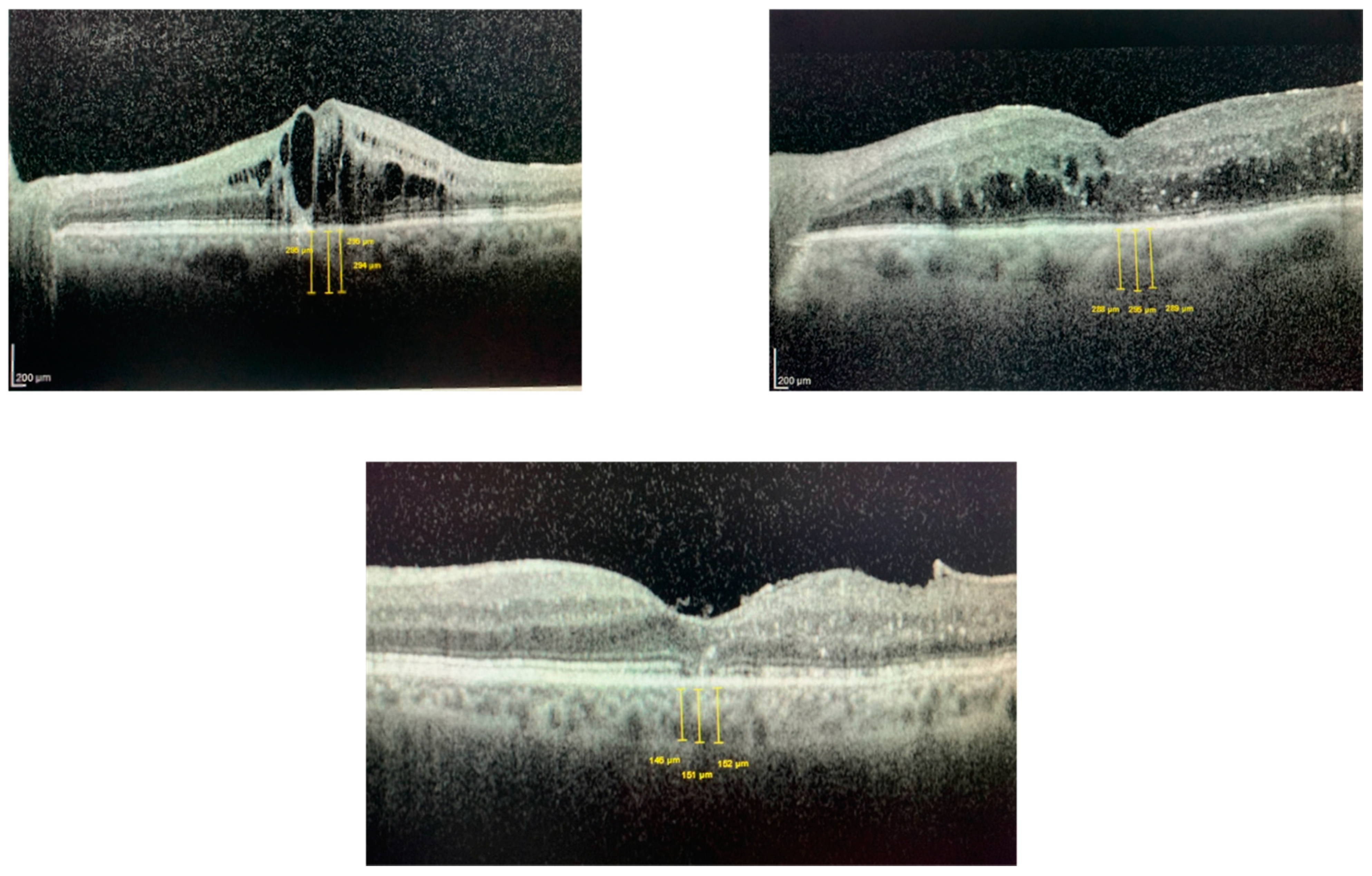
| CMT Aflibercept | SFCT AFLIBERCEPT | CMT Dexamethasone Implant | SFCT Dexamethasone Implant | CMT Reduction Aflibercept versus Dexamethasone | |
|---|---|---|---|---|---|
| Pre-treatment | 441.4 ± 75.9 μm | 274.7 ± 88.8 μm | 480.1 ± 97.3 μm | 291.6 ± 87.1 μm | |
| Post-treatment | 357.8 ± 87.2 μm | 235.6 ± 83.4 μm | 328.0 ± 72.2 μm | 229.8 ± 58.8 μm | |
| p-value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udaondo Mirete, P.; Muñoz-Morata, C.; Albarrán-Diego, C.; España-Gregori, E. Influence of Intravitreal Therapy on Choroidal Thickness in Patients with Diabetic Macular Edema. J. Clin. Med. 2023, 12, 348. https://doi.org/10.3390/jcm12010348
Udaondo Mirete P, Muñoz-Morata C, Albarrán-Diego C, España-Gregori E. Influence of Intravitreal Therapy on Choroidal Thickness in Patients with Diabetic Macular Edema. Journal of Clinical Medicine. 2023; 12(1):348. https://doi.org/10.3390/jcm12010348
Chicago/Turabian StyleUdaondo Mirete, Patricia, Carmen Muñoz-Morata, César Albarrán-Diego, and Enrique España-Gregori. 2023. "Influence of Intravitreal Therapy on Choroidal Thickness in Patients with Diabetic Macular Edema" Journal of Clinical Medicine 12, no. 1: 348. https://doi.org/10.3390/jcm12010348
APA StyleUdaondo Mirete, P., Muñoz-Morata, C., Albarrán-Diego, C., & España-Gregori, E. (2023). Influence of Intravitreal Therapy on Choroidal Thickness in Patients with Diabetic Macular Edema. Journal of Clinical Medicine, 12(1), 348. https://doi.org/10.3390/jcm12010348






