Intravitreal Dexamethasone Implant (IDI) Alone and Combined with Navigated 577 nm Subthreshold Micropulse Laser (SML) for Diabetic Macular Oedema
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Protocol
2.3. SD-OCT Analysis
2.4. Procedures
2.5. Main and Secondary Outcomes
- (1)
- Central macular thickness, BCVA, SCHT, and CVI changes during follow-up in both groups;
- (2)
- Time to retreatment between first and second IDI in both groups in order to assess the SML impact on retreatment.
2.6. Statistical Analysis
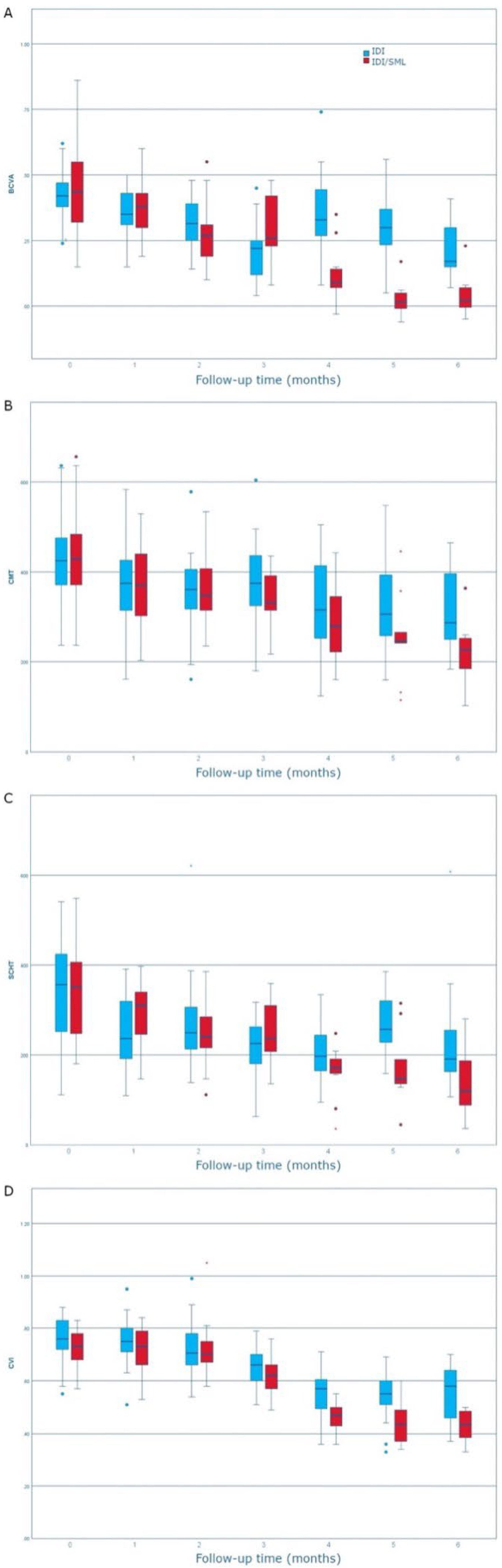
3. Results
Characteristics of Enrolled Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Midena, E.; Bini, S.; Martini, F.; Enrica, C.; Pilotto, E.; Micera, A.; Esposito, G.; Vujosevic, S. Changes of Aqueous Humor Müller Cells’ Biomarkers in Human Patients Affected by Diabetic Macular Edema after Subthreshold Micropulse Laser Treatment. Retina 2020, 40, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Dehghani, A.; Pourmohammadi, R.; Asadpour, L.; Pourazizi, M. Effects of subthreshold diode micropulse laser photocoagulation on treating patients with refractory diabetic macular edema. J. Curr. Ophthalmol. 2019, 31, 157–160. [Google Scholar] [CrossRef]
- Frizziero, L.; Calciati, A.; Torresin, T.; Midena, G.; Parrozzani, R.; Pilotto, E.; Midena, E. Diabetic Macular Edema Treated with 577-nm Subthreshold Micropulse Laser: A Real-Life, Long-Term Study. J. Pers. Med. 2021, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Virgili, G.; Menchini, F.; Casazza, G.; Hogg, R.; Das, R.R.; Wang, X.; Michelessi, M. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst. Rev. 2015, 1, CD008081. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Bressler, N.M.; Doan, Q.V.; Gleeson, M.; Danese, M.; Bower, J.K.; Selvin, E.; Dolan, C.; Fine, J.; Colman, S.; et al. Prevalence of and Risk Factors for Diabetic Macular Edema in the United States. JAMA Ophthalmol. 2014, 132, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Moisseiev, E.; Abbassi, S.; Thinda, S.; Yoon, J.; Yiu, G.; Morse, L.S. Subthreshold micropulse laser reduces anti-VEGF injection burden in patients with diabetic macular edema. Eur. J. Ophthalmol. 2018, 28, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, L.; Calciati, A.; Midena, G.; Torresin, T.; Parrozzani, R.; Pilotto, E.; Midena, E. Subthreshold Micropulse Laser Modulates Retinal Neuroinflammatory Biomarkers in Diabetic Macular Edema. J. Clin. Med. 2021, 10, 3134. [Google Scholar] [CrossRef]
- Midena, E.; Micera, A.; Frizziero, L.; Pilotto, E.; Esposito, G.; Bini, S. Sub-threshold micropulse laser treatment reduces inflammatory biomarkers in aqueous humour of diabetic patients with macular edema. Sci. Rep. 2019, 9, 10034. [Google Scholar] [CrossRef]
- Starace, V.; Battista, M.; Brambati, M.; Cavalleri, M.; Bertuzzi, F.; Amato, A.; Lattanzio, R.; Bandello, F.; Cicinelli, M.V. The role of inflammation and neurodegeneration in diabetic macular edema. Ther. Adv. Ophthalmol. 2021, 13, 25158414211055963. [Google Scholar] [CrossRef]
- Capitão, M.; Soares, R. Angiogenesis and Inflammation Crosstalk in Diabetic Retinopathy. J. Cell. Biochem. 2016, 117, 2443–2453. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985, 103, 1796–1806. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef]
- Gawęcki, M. Subthreshold Diode Micropulse Laser Combined with Intravitreal Therapy for Macular Edema—A Systematized Review and Critical Approach. J. Clin. Med. 2021, 10, 1394. [Google Scholar] [CrossRef]
- Scholz, P.; Altay, L.; Fauser, S. A Review of Subthreshold Micropulse Laser for Treatment of Macular Disorders. Adv. Ther. 2017, 34, 1528–1555. [Google Scholar] [CrossRef]
- Donati, M.C.; Murro, V.; Mucciolo, D.P.; Giorgio, D.; Cinotti, G.; Virgili, G.; Rizzo, S. Subthreshold yellow micropulse laser for treatment of diabetic macular edema: Comparison between fixed and variable treatment regimen. Eur. J. Ophthalmol. 2021, 31, 1254–1260. [Google Scholar] [CrossRef]
- Dorin, G. Evolution of retinal laser therapy: Minimum intensity photocoagulation (MIP). Can the laser heal the retina without harming it? Semin. Ophthalmol. 2004, 19, 62–68. [Google Scholar] [CrossRef]
- Müller, B.; Tatsios, J.; Klonner, J.; Pilger, D.; Joussen, A.M. Navigated laser photocoagulation in patients with non-resolving and chronic central serous chorioretinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1581–1588. [Google Scholar] [CrossRef]
- Usui, S.; Ikuno, Y.; Akiba, M.; Maruko, I.; Sekiryu, T.; Nishida, K.; Iida, T. Circadian Changes in Subfoveal Choroidal Thickness and the Relationship with Circulatory Factors in Healthy Subjects. Investig. Opthalmol. Vis. Sci. 2012, 53, 2300–2307. [Google Scholar] [CrossRef]
- Sonoda, S.; Sakamoto, T.; Yamashita, T.; Uchino, E.; Kawano, H.; Yoshihara, N.; Terasaki, H.; Shirasawa, M.; Tomita, M.; Ishibashi, T. Luminal and Stromal Areas of Choroid Determined by Binarization Method of Optical Coherence Tomographic Images. Am. J. Ophthalmol. 2015, 159, 1123–1131.e1. [Google Scholar] [CrossRef]
- Haller, J.A.; Kuppermann, B.D.; Blumenkranz, M.S.; Williams, G.A.; Weinberg, D.; Chou, C.; Whitcup, S.M. Randomized Controlled Trial of an Intravitreous Dexamethasone Drug Delivery System in Patients with Diabetic Macular Edema. Arch. Ophthalmol. 2010, 128, 289–296. [Google Scholar] [CrossRef]
- Boyer, D.S.; Yoon, Y.H.; Belfort, R., Jr.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.-Y.; Cui, H.; Hashad, Y.; Whitcup, S.M. Three-Year, Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Diabetic Macular Edema. Ophthalmology 2014, 121, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Bellocq, D.; Akesbi, J.; Matonti, F.; Vartin, C.; Despreaux, R.; Comet, A.; Voirin, N.; Denis, P.; Mathis, T.; Kodjikian, L. The Pattern of Recurrence in Diabetic Macular Edema Treated by Dexamethasone Implant: The PREDIAMEX Study. Ophthalmol. Retin. 2018, 2, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Ertan, E.; Duman, R.; Duman, R. Comparison of pain during intravitreal dexamethasone, ranibizumab and aflibercept injection. Clin. Exp. Optom. 2020, 103, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Gupta, V.; Gupta, A.; Dogra, M.R.; Ram, J. Efficacy of Ozurdex implant in recalcitrant diabetic macular edema—A sin-gle-center experience. Int. Ophthalmol. 2016, 36, 207–216. [Google Scholar] [CrossRef]
- Vujosevic, S.; Martini, F.; Convento, E.; Longhin, E.; Kotsafti, O.; Parrozzani, R.; Midena, E. Subthreshold Laser Therapy for Diabetic Macular Edema: Metabolic and Safety Issues. Curr. Med. Chem. 2013, 20, 3267–3271. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Musch, D.C.; Mainster, M.A. Subthreshold diode micropulse photocoagulation for the treatment of clinically signifi-cant diabetic macular oedema. Br. J. Ophthalmol. 2005, 89, 74–80. [Google Scholar] [CrossRef]
- Nicolò, M.; Musetti, D.; Traverso, C.E. Yellow Micropulse Laser in Diabetic Macular Edema: A Short-Term Pilot Study. Eur. J. Ophthalmol. 2014, 24, 885–889. [Google Scholar] [CrossRef]
- Elhamid, A.H.A. Combined Intravitreal Dexamethasone Implant and Micropulse Yellow Laser for Treatment of Anti-VEGF Resistant Diabetic Macular Edema. Open Ophthalmol. J. 2017, 11, 164–172. [Google Scholar] [CrossRef]
- Mansouri, A.; Sampat, K.M.; Malik, K.J.; Steiner, J.N.; Glaser, B.M. Efficacy of subthreshold micropulse laser in the treatment of diabetic macular edema is influenced by pre-treatment central foveal thickness. Eye 2014, 28, 1418–1424. [Google Scholar] [CrossRef]
- Citirik, M. The impact of central foveal thickness on the efficacy of subthreshold micropulse yellow laser photocoagulation in diabetic macular edema. Lasers Med. Sci. 2019, 34, 907–912. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Spink, C.J. Serial Optical Coherence Tomography of Subthreshold Diode Laser Micropulse Photocoagulation for Diabetic Macular Edema. Ophthalmic Surg. Lasers Imaging Retin. 2006, 37, 370–377. [Google Scholar] [CrossRef]
- Abouhussein, M.A.; Gomaa, A.R. Aflibercept plus micropulse laser versus aflibercept monotherapy for diabetic macular edema: 1-year results of a randomized clinical trial. Int. Ophthalmol. 2020, 40, 1147–1154. [Google Scholar] [CrossRef]
- Kanar, H.S.; Arsan, A.; Altun, A.; Akı, S.F.; Hacısalihoglu, A. Can subthreshold micropulse yellow laser treatment change the an-ti-vascular endothelial growth factor algorithm in diabetic macular edema? A randomized clinical trial. Indian J. Ophthalmol. 2020, 68, 14. [Google Scholar] [CrossRef]
- Best, A.-L.; Fajnkuchen, F.; Nghiem-Buffet, S.; Grenet, T.; Quentel, G.; Delahaye-Mazza, C.; Cohen, S.Y.; Giocanti-Aurégan, A. Treatment Efficacy and Compliance in Patients with Diabetic Macular Edema Treated with Ranibizumab in a Real-Life Setting. J. Ophthalmol. 2018, 2018, 4610129. [Google Scholar] [CrossRef]
- Altun, A.; Hacimustafaoglu, A.M. Effect of Dexamethasone Implant on Subfoveal Choroidal Thickness in Early Period in Vitrectomized Eyes with Diabetic Macular Edema. J. Ophthalmol. 2021, 2021, 8840689. [Google Scholar] [CrossRef]
- Aksoy, M.; Yilmaz, G.; Vardarli, I.; Akkoyun, I. Choroidal Thickness After Dexamethasone Implant or Aflibercept in Patients with Diabetic Macular Edema Persistent to Ranibizumab. J. Ocul. Pharmacol. Ther. 2020, 36, 629–635. [Google Scholar] [CrossRef]
- Kocamiş, Ö.; Temel, E.; Özcan, G.; Aşikgarip, N.; Örnek, K. Choroidal vascularity index after a single dose of intravitreal dexame-thasone implant in patients with refractory diabetic macular oedema. Photodiagnosis Photodyn. Ther. 2022, 39, 102996. [Google Scholar] [CrossRef]

| IDI/SML | IDI | p-Value | |
|---|---|---|---|
| Number of eyes | 30 | 30 | - |
| Age, (years) | 70.5 ± 11.8 | 72.8 ± 9.9 | 0.417 |
| Sex, (M/F) | 18/12 | 14/16 | 0.438 |
| Diabetes duration, (years) | 14.7 ± 8.9 | 15.5 ± 8.5 | 0.723 |
| BCVA, (logMAR) | 0.46 ± 0.17 | 0.43 ± 0.09 | 0.403 |
| CMT, (µm) | 430.73 ± 103.39 | 422.97 ± 95.90 | 0.766 |
| SCHT, (µm) | 342.63 ± 103.56 | 340.66 ± 111.02 | 0.944 |
| CVI | 0.73 ± 0.07 | 0.76 ± 0.08 | 0.096 |
| T1 | T2 | T3 | T4 | T5 | T6 | |
|---|---|---|---|---|---|---|
| IDI | ||||||
| BCVA, (logMAR) | 12.40 ± 24.99 | 25.76 ± 16.57 | 53.29 ± 16.98 | −8.97 ± 32.81 | 7.96 ± 37.28 | 28.61 ± 27.23 |
| p | 0.003 | <0.001 | <0.001 | 0.016 | <0.001 | <0.001 |
| CMT, (µm) | 7.41 ± 24.73 | 15.41 ± 16.97 | 9.73 ± 26.04 | -3.28 ± 31.08 | 5.08 ± 32.80 | 10.14 ± 29.11 |
| p | 0.004 | <0.001 | 0.031 | <0.001 | 0.001 | <0.001 |
| SCHT, (µm) | 17.19 ± 45.27 | 15.03 ± 34.56 | 28.73 ± 38.10 | 19.96 ± 56.64 | 16.70 ± 42.31 | 32.58 ± 35.11 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 |
| CVI | 1.09 ± 16.13 | 4.17 ± 17.88 | 13.58 ± 15.55 | 0.70 ± 15.16 | 9.01 ± 25.61 | 7.63 ± 30.04 |
| p | 0.443 | 0.132 | <0.001 | <0.001 | <0.001 | <0.001 |
| IDI/SML | ||||||
| BCVA, (logMAR) | 13.44 ± 41.34 | 37.01 ± 36.20 | 39.29 ± 25.55 | 56.70 ± 15.80 | 77.17 ± 7.61 | 73.49 ± 7.11 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CMT, (µm) | 11.97 ± 24.02 | 15.79 ± 16.58 | 22.79 ± 19.15 | 14.88 ± 17.57 | 23.78 ± 15.71 | 29.01 ± 5.38 |
| p | 0.002 | 0.001 | 0.001 | <0.001 | <0.001 | <0.001 |
| SCHT, (µm) | 14.86 ± 23.05 | 27.60 ± 16.05 | 30.38 ± 8.73 | 22.43 ± 18.99 | 22.69 ± 7.10 | 27.89 ± 3.73 |
| p | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CVI | 0.87 ± 13.79 | −0.70 ± 26.18 | 14.12 ± 11.46 | −4.96 ± 14.26 | −2.57 ± 19.45 | −1.28 ± 11.86 |
| p | 0.566 | 0.789 | 0.001 | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toto, L.; D’Aloisio, R.; Quarta, A.; Libertini, D.; D’Onofrio, G.; De Nicola, C.; Romano, A.; Mastropasqua, R. Intravitreal Dexamethasone Implant (IDI) Alone and Combined with Navigated 577 nm Subthreshold Micropulse Laser (SML) for Diabetic Macular Oedema. J. Clin. Med. 2022, 11, 5200. https://doi.org/10.3390/jcm11175200
Toto L, D’Aloisio R, Quarta A, Libertini D, D’Onofrio G, De Nicola C, Romano A, Mastropasqua R. Intravitreal Dexamethasone Implant (IDI) Alone and Combined with Navigated 577 nm Subthreshold Micropulse Laser (SML) for Diabetic Macular Oedema. Journal of Clinical Medicine. 2022; 11(17):5200. https://doi.org/10.3390/jcm11175200
Chicago/Turabian StyleToto, Lisa, Rossella D’Aloisio, Alberto Quarta, Daniele Libertini, Giada D’Onofrio, Chiara De Nicola, Anna Romano, and Rodolfo Mastropasqua. 2022. "Intravitreal Dexamethasone Implant (IDI) Alone and Combined with Navigated 577 nm Subthreshold Micropulse Laser (SML) for Diabetic Macular Oedema" Journal of Clinical Medicine 11, no. 17: 5200. https://doi.org/10.3390/jcm11175200
APA StyleToto, L., D’Aloisio, R., Quarta, A., Libertini, D., D’Onofrio, G., De Nicola, C., Romano, A., & Mastropasqua, R. (2022). Intravitreal Dexamethasone Implant (IDI) Alone and Combined with Navigated 577 nm Subthreshold Micropulse Laser (SML) for Diabetic Macular Oedema. Journal of Clinical Medicine, 11(17), 5200. https://doi.org/10.3390/jcm11175200









