The Causal Effect of Reproductive Factors on Breast Cancer: A Two-Sample Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
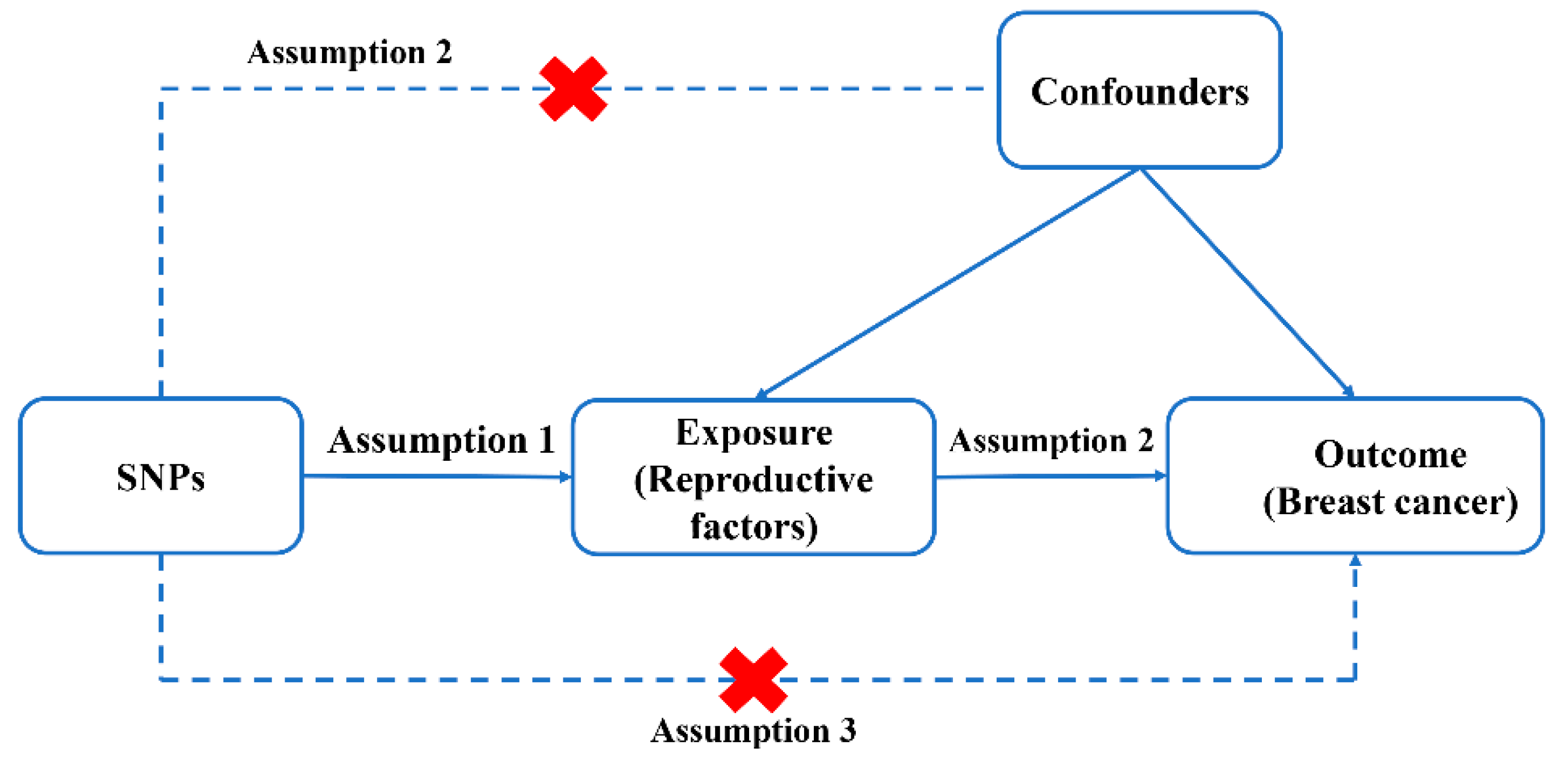
2.1. Study Design
2.2. Data Sources
2.3. IVs Extraction
2.4. Statistical Analysis
3. Results
3.1. Genetics Variants Selection
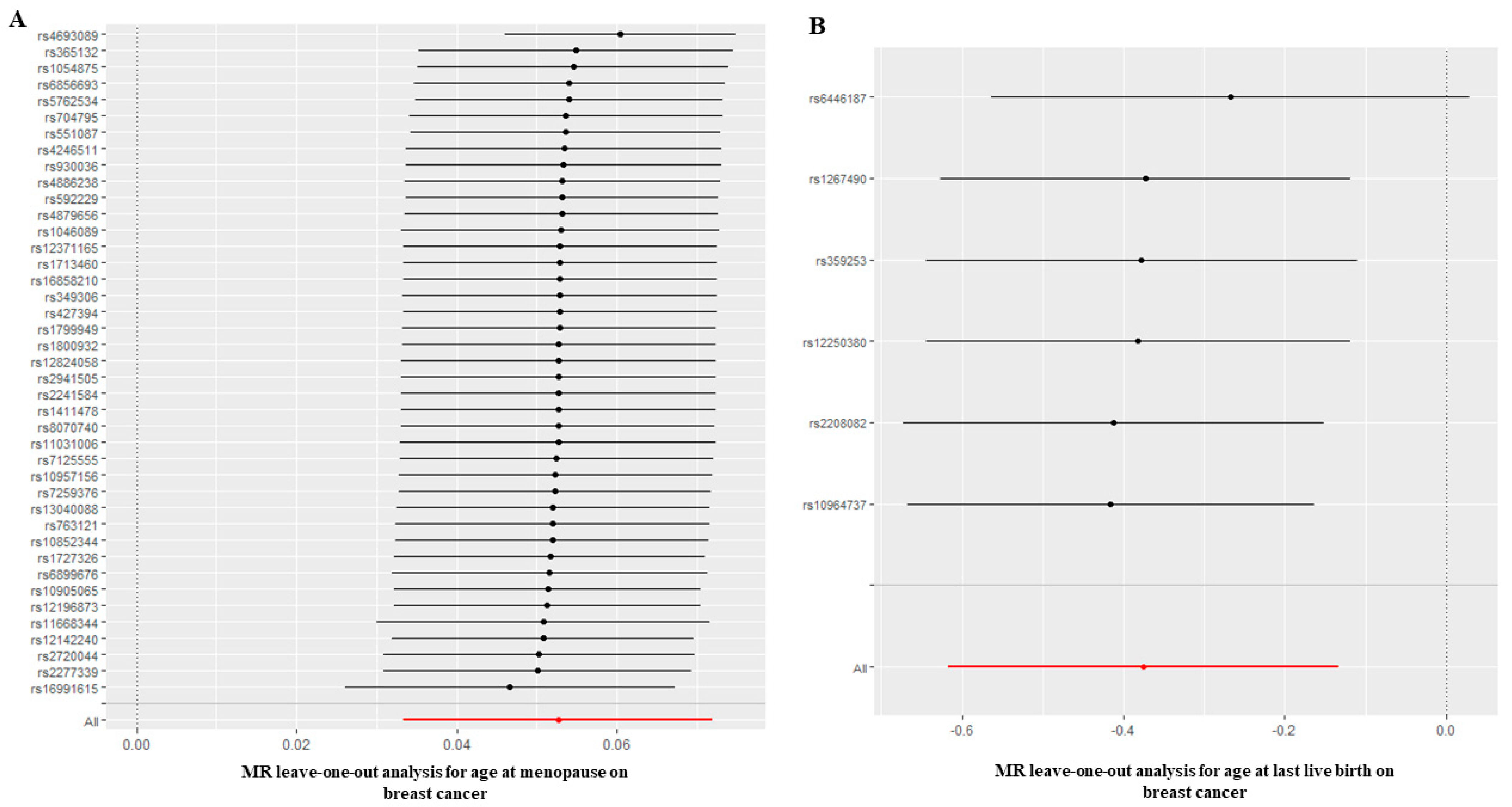
3.2. Causal Effect of Age at Menarche and Menopause on BC
3.3. Causal Effect of AFS on BC
3.4. Causal Effect of Age at First Birth and Last Live Birth on BC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, W.; Chen, H.D.; Yu, Y.W.; Li, N.; Chen, W.Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W. Reproductive health as a sentinel of chronic disease in women. Women’s Health 2009, 5, 101–105. [Google Scholar] [CrossRef]
- Schwartz, A.G.; Ray, R.M.; Cote, M.L.; Abrams, J.; Sokol, R.J.; Hendrix, S.L.; Chen, C.; Chlebowski, R.T.; Hubbell, F.A.; Kooperberg, C.; et al. Hormone Use, Reproductive History, and Risk of Lung Cancer: The Women’s Health Initiative Studies. J. Thorac. Oncol. 2015, 10, 1004–1013. [Google Scholar] [CrossRef]
- Stoer, N.C.; Botteri, E.; Ghiasvand, R.; Busund, M.; Vangen, S.; Lund, E.; Veierod, M.B.; Weiderpass, E. Reproductive factors and risk of melanoma: A population-based cohort study. Br. J. Dermatol. 2019, 181, 282–289. [Google Scholar] [CrossRef]
- Yu, M.W.; Chang, H.C.; Chang, S.C.; Liaw, Y.F.; Lin, S.M.; Liu, C.J.; Lee, S.D.; Lin, C.L.; Chen, P.J.; Lin, S.C.; et al. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology 2003, 38, 1393–1400. [Google Scholar] [CrossRef]
- Yoo, J.E.; Shin, D.W.; Jang, W.; Han, K.; Kim, D.; Won, H.S.; Park, H.S. Female reproductive factors and the risk of Parkinson’s disease: A nationwide cohort study. Eur. J. Epidemiol. 2020, 35, 871–878. [Google Scholar] [CrossRef]
- Ardissino, M.; Slob, E.; Rogne, T.; Burgess, S.; Ng, F.S. Impact of reproductive factors on major cardiovascular disease risk in women: A Mendelian randomization study. Eur. Heart J. 2022, 43, 26–29. [Google Scholar] [CrossRef]
- Khincha, P.P.; Best, A.F.; Fraumeni, J.F., Jr.; Loud, J.T.; Savage, S.A.; Achatz, M.I. Reproductive factors associated with breast cancer risk in Li-Fraumeni syndrome. Eur. J. Cancer 2019, 116, 199–206. [Google Scholar] [CrossRef]
- Khalis, M.; Charbotel, B.; Chajes, V.; Rinaldi, S.; Moskal, A.; Biessy, C.; Dossus, L.; Huybrechts, I.; Fort, E.; Mellas, N.; et al. Menstrual and reproductive factors and risk of breast cancer: A case-control study in the Fez region, Morocco. PLoS ONE 2018, 13, e0191333. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Kitamura, Y.; Kitamura, T.; Sobue, T.; Sado, J.; Sugawara, Y.; Matsuo, K.; Nakayama, T.; Tsuji, I.; Ito, H.; et al. Reproductive and lifestyle factors related to breast cancer among Japanese women: An observational cohort study. Medicine 2019, 98, e18315. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, G.; Ndetan, H.; Das, D.N.; Gupta, G.; Suryavanshi, M.; Mehta, A.; Singh, K.P. Reproductive factors and breast cancer risk: A meta-analysis of case-control studies in Indian women. South Asian J. Cancer 2019, 8, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012, 13, 1141–1151. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Sekula, P.; Del Greco, M.F.; Pattaro, C.; Kottgen, A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J. Am. Soc. Nephrol. 2016, 27, 3253–3265. [Google Scholar] [CrossRef]
- Perry, J.R.; Day, F.; Elks, C.E.; Sulem, P.; Thompson, D.J.; Ferreira, T.; He, C.; Chasman, D.I.; Esko, T.; Thorleifsson, G.; et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 2014, 514, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.C.; Tropf, F.C.; Brazel, D.M.; van Zuydam, N.; Vaez, A.; eQTLGen Consortium; BIOS Consortium; Human Reproductive Behaviour Consortium; Pers, T.H.; Snieder, H.; et al. Identification of 371 genetic variants for age at first sex and birth linked to externalising behaviour. Nat. Hum. Behav. 2021, 5, 1717–1730. [Google Scholar] [CrossRef]
- Day, F.R.; Ruth, K.S.; Thompson, D.J.; Lunetta, K.L.; Pervjakova, N.; Chasman, D.I.; Stolk, L.; Finucane, H.K.; Sulem, P.; Bulik-Sullivan, B.; et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat. Genet. 2015, 47, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, K.; Lindstrom, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemacon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef]
- Myers, T.A.; Chanock, S.J.; Machiela, M.J. LDlinkR: An R Package for Rapidly Calculating Linkage Disequilibrium Statistics in Diverse Populations. Front. Genet. 2020, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Scott, R.A.; Timpson, N.J.; Davey Smith, G.; Thompson, S.G.; Consortium, E.-I. Using published data in Mendelian randomization: A blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 2015, 30, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.; Chalumeau, M.; Cohen, R.; Korevaar, D.A.; Khoshnood, B.; Bossuyt, P.M. Cochran’s Q test was useful to assess heterogeneity in likelihood ratios in studies of diagnostic accuracy. J. Clin. Epidemiol. 2015, 68, 299–306. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Nazarzadeh, M.; Pinho-Gomes, A.C.; Bidel, Z.; Dehghan, A.; Canoy, D.; Hassaine, A.; Ayala Solares, J.R.; Salimi-Khorshidi, G.; Smith, G.D.; Otto, C.M.; et al. Plasma lipids and risk of aortic valve stenosis: A Mendelian randomization study. Eur. Heart J. 2020, 41, 3913–3920. [Google Scholar] [CrossRef]
- Bustamante-Montes, L.P.; Flores-Meza, B.; Hernandez-Valero, M.A.; Cardenas-Lopez, A.; Dolores-Velazquez, R.; Borja-Bustamante, P.; Borja-Aburto, V.H. Night Shift Work and Risk of Breast Cancer in Women. Arch. Med. Res. 2019, 50, 393–399. [Google Scholar] [CrossRef]
- Arthur, R.; Wang, Y.; Ye, K.; Glass, A.G.; Ginsberg, M.; Loudig, O.; Rohan, T. Association between lifestyle, menstrual/reproductive history, and histological factors and risk of breast cancer in women biopsied for benign breast disease. Breast Cancer Res. Treat. 2017, 165, 623–631. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Davis Lynn, B.C.; Edusei, L.; Titiloye, N.; Adjei, E.; Clegg-Lamptey, J.N.; Yarney, J.; Wiafe-Addai, B.; Awuah, B.; Duggan, M.A.; et al. Reproductive factors and risk of breast cancer by tumor subtypes among Ghanaian women: A population-based case-control study. Int. J. Cancer 2020, 147, 1535–1547. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kitamura, Y.; Sobue, T.; Utada, M.; Ozasa, K.; Sugawara, Y.; Tsuji, I.; Hori, M.; Sawada, N.; Tsugane, S.; et al. Impact of reproductive factors on breast cancer incidence: Pooled analysis of nine cohort studies in Japan. Cancer Med. 2021, 10, 2153–2163. [Google Scholar] [CrossRef]
- Lambertini, M.; Santoro, L.; Del Mastro, L.; Nguyen, B.; Livraghi, L.; Ugolini, D.; Peccatori, F.A.; Azim, H.A., Jr. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: A systematic review and meta-analysis of epidemiological studies. Cancer Treat. Rev. 2016, 49, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tobias, D.K.; Sturgeon, K.M.; Rosner, B.; Malik, V.; Cespedes, E.; Joshi, A.D.; Eliassen, A.H.; Colditz, G.A. Physical activity from menarche to first pregnancy and risk of breast cancer. Int. J. Cancer 2016, 139, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Li, C.I.; Malone, K.E.; Daling, J.R.; Potter, J.D.; Bernstein, L.; Marchbanks, P.A.; Strom, B.L.; Simon, M.S.; Press, M.F.; Ursin, G.; et al. Timing of menarche and first full-term birth in relation to breast cancer risk. Am. J. Epidemiol. 2008, 167, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Biswas, L.N.; Manna, B.; Maiti, P.K.; Sengupta, S. Sexual Risk Factors for Cervical Cancer among Rural Indian Women: A Case-Control Study. Int. J. Epidemiol. 1997, 26, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Sull, J.W.; Jee, S.H.; Yi, S.; Lee, J.E.; Park, J.S.; Kim, S.; Ohrr, H. The effect of methylenetetrahydrofolate reductase polymorphism C677T on cervical cancer in Korean women. Gynecol. Oncol. 2004, 95, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.; Gulbech Ording, A.; Grotmol, T.; Glimelius, I.; Engeland, A.; Gissler, M.; Trabert, B.; Ekbom, A.; Madanat-Harjuoja, L.; Sorensen, H.T.; et al. Pregnancy complications and subsequent breast cancer risk in the mother: A Nordic population-based case-control study. Int. J. Cancer 2018, 143, 1904–1913. [Google Scholar] [CrossRef]
- Romieu, I.; Biessy, C.; Carayol, M.; His, M.; Torres-Mejia, G.; Angeles-Llerenas, A.; Sanchez, G.I.; Jaramillo, R.; Navarro, E.; Porras, C.; et al. Reproductive factors and molecular subtypes of breast cancer among premenopausal women in Latin America: The PRECAMA study. Sci. Rep. 2018, 8, 13109. [Google Scholar] [CrossRef]
- Grabrick, D.M.; Vierkant, R.A.; Anderson, K.E.; Cerhan, J.R.; Anderson, V.E.; Seller, T.A. Association of correlates of endogenous hormonal exposure with breast cancer risk in 426 families (United States). Cancer Causes Control 2002, 13, 333–341. [Google Scholar] [CrossRef]
- Shantakumar, S.; Terry, M.B.; Teitelbaum, S.L.; Britton, J.A.; Millikan, R.C.; Moorman, P.G.; Neugut, A.I.; Gammon, M.D. Reproductive factors and breast cancer risk among older women. Breast Cancer Res. Treat. 2007, 102, 365–374. [Google Scholar] [CrossRef]
- Jung, A.Y.; Ahearn, T.U.; Behrens, S.; Middha, P.; Bolla, M.K.; Wang, Q.; Arndt, V.; Aronson, K.J.; Augustinsson, A.; Beane Freeman, L.E.; et al. Distinct reproductive risk profiles for intrinsic-like breast cancer subtypes: Pooled analysis of population-based studies. J. Natl. Cancer Inst. 2022, 114, 1706–1719. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Borges, M.C.; Fraser, A.; Lawlor, D.A. Identifying potential causal effects of age at menopause: A Mendelian randomization phenome-wide association study. Eur. J. Epidemiol. 2022, 37, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Moron, F.J.; Ruiz, A.; Galan, J.J. Genetic and genomic insights into age at natural menopause. Genome Med. 2009, 1, 76. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Trichopoulos, D.; Katsouyanni, K.; Yuasa, S. Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: Associations and interactions in an international case-control study. Int. J. Cancer 1990, 46, 796–800. [Google Scholar] [CrossRef] [PubMed]
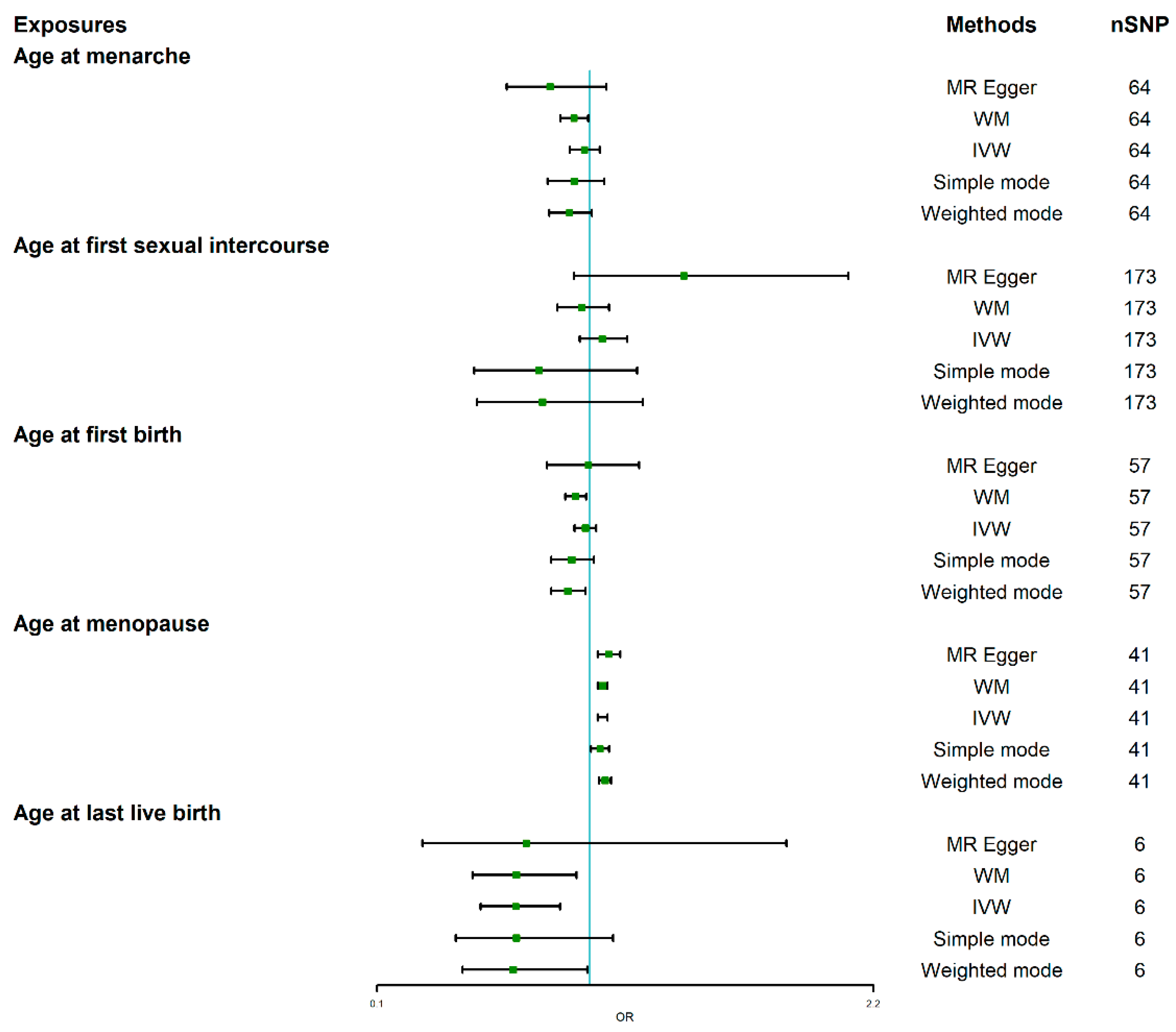
| Exposure | Method | p-Value | OR | 95%LCI | 95%UCI |
|---|---|---|---|---|---|
| Age at menarche | MR Egger | 0.156 | 0.832 | 0.648 | 1.069 |
| WM | 0.030 | 0.933 | 0.876 | 0.993 | |
| IVW | 0.484 | 0.977 | 0.915 | 1.043 | |
| Simple mode | 0.298 | 0.934 | 0.822 | 1.061 | |
| Weighted mode | 0.076 | 0.914 | 0.829 | 1.008 | |
| Age at first sexual intercourse | MR Egger | 0.105 | 1.398 | 0.934 | 2.093 |
| WM | 0.546 | 0.966 | 0.862 | 1.082 | |
| IVW | 0.284 | 1.053 | 0.958 | 1.157 | |
| Simple mode | 0.265 | 0.784 | 0.512 | 1.201 | |
| Weighted mode | 0.305 | 0.800 | 0.523 | 1.224 | |
| Age at first birth | MR Egger | 0.956 | 0.994 | 0.818 | 1.209 |
| WM | 0.009 | 0.939 | 0.896 | 0.984 | |
| IVW | 0.404 | 0.981 | 0.936 | 1.027 | |
| Simple mode | 0.111 | 0.923 | 0.837 | 1.017 | |
| Weighted mode | 0.019 | 0.906 | 0.837 | 0.982 | |
| Age at last live birth | MR Egger | 0.541 | 0.731 | 0.292 | 1.832 |
| WM | 0.020 | 0.690 | 0.504 | 0.944 | |
| IVW | 0.002 | 0.687 | 0.539 | 0.875 | |
| Simple mode | 0.179 | 0.69 | 0.434 | 1.098 | |
| Weighted mode | 0.100 | 0.676 | 0.462 | 0.990 | |
| Age at menopause | MR Egger | 1.404 × 10−3 | 1.080 | 1.034 | 1.129 |
| WM | 7.270 × 10−8 | 1.054 | 1.034 | 1.074 | |
| IVW | 8.010 × 10−8 | 1.054 | 1.034 | 1.075 | |
| Simple mode | 3.359 × 10−2 | 1.043 | 1.005 | 1.082 | |
| Weighted mode | 1.050 × 10−5 | 1.064 | 1.039 | 1.090 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Lv, W.; Liang, L.; Ma, Y.; Ma, X.; Zhang, S.; Zhao, Y. The Causal Effect of Reproductive Factors on Breast Cancer: A Two-Sample Mendelian Randomization Study. J. Clin. Med. 2023, 12, 347. https://doi.org/10.3390/jcm12010347
Jia L, Lv W, Liang L, Ma Y, Ma X, Zhang S, Zhao Y. The Causal Effect of Reproductive Factors on Breast Cancer: A Two-Sample Mendelian Randomization Study. Journal of Clinical Medicine. 2023; 12(1):347. https://doi.org/10.3390/jcm12010347
Chicago/Turabian StyleJia, Lijun, Wei Lv, Liang Liang, Yuguang Ma, Xingcong Ma, Shuqun Zhang, and Yonglin Zhao. 2023. "The Causal Effect of Reproductive Factors on Breast Cancer: A Two-Sample Mendelian Randomization Study" Journal of Clinical Medicine 12, no. 1: 347. https://doi.org/10.3390/jcm12010347
APA StyleJia, L., Lv, W., Liang, L., Ma, Y., Ma, X., Zhang, S., & Zhao, Y. (2023). The Causal Effect of Reproductive Factors on Breast Cancer: A Two-Sample Mendelian Randomization Study. Journal of Clinical Medicine, 12(1), 347. https://doi.org/10.3390/jcm12010347





