Ceramic Crowns and Sleep Bruxism: First Results from a Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
- if p1 ≤ 0.01, terminate the study and conclude that complication rates are different;
- if p1 > 0.20, terminate the study because of low differences between complication rates;
- if 0.01 ≤ p1 ≤ 0.20, continue to stage II with the two groups that show the smallest p-value (pA or pB).Stage II analysis. Calculate the chi-square p-value p2 based on the stage II data.
- if p1 × p2 ≤ 0.013: conclude that complication rates in the selected groups are different;
- otherwise: no difference in complication rates.
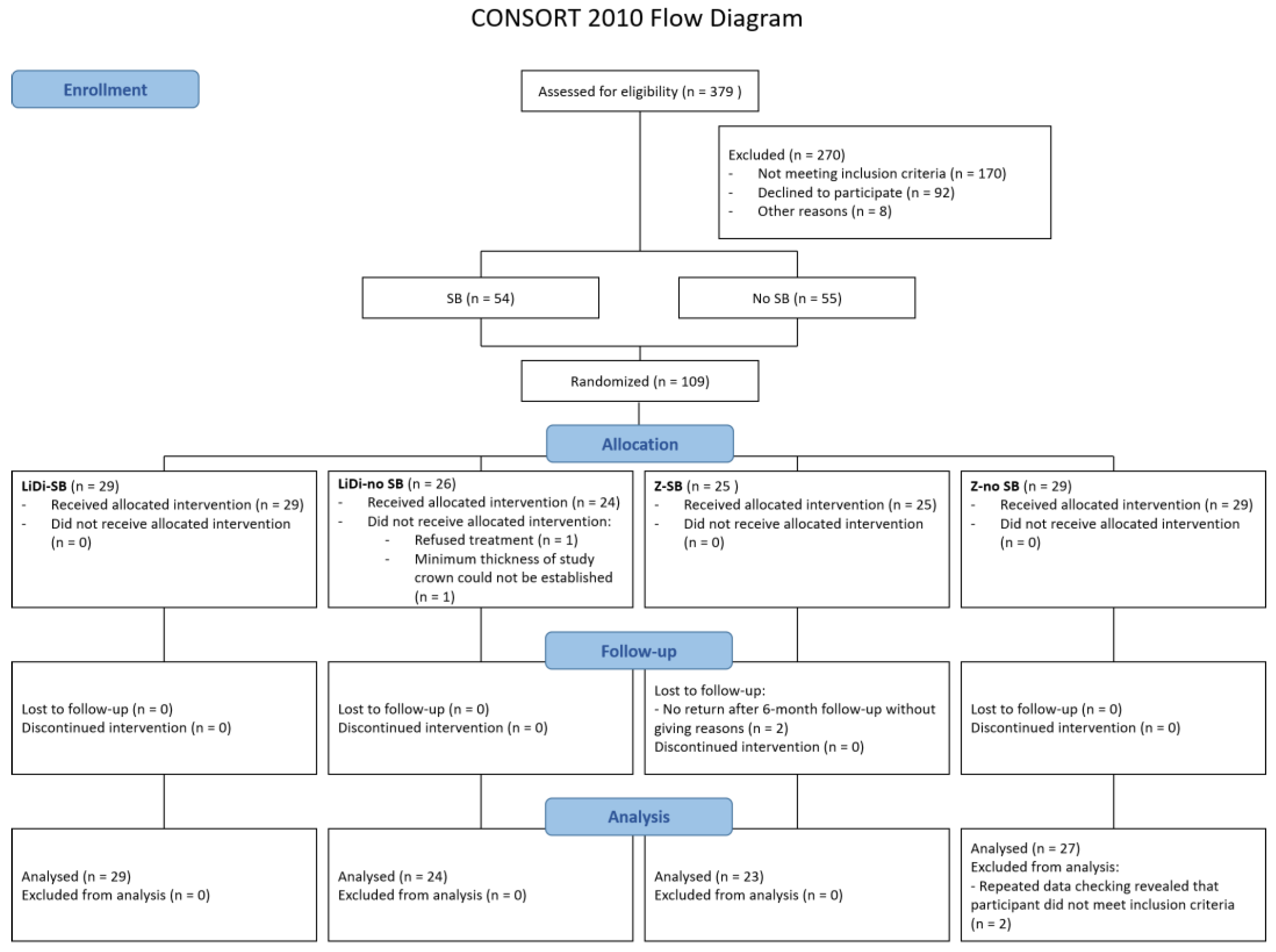
3. Results
3.1. Participants
3.2. Complications and Failures
3.3. Technical Complication, Survival, and Success Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, S.J.; Lawson, N.C.; McLaren, E.E.; Nejat, A.H.; Burgess, J.O. Comparison of the mechanical properties of translucent zirconia and lithium disilicate. J. Prosthet. Dent. 2018, 120, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Laumbacher, H.; Strasser, T.; Knuttel, H.; Rosentritt, M. Long-term clinical performance and complications of zirconia-based tooth- and implant-supported fixed prosthodontic restorations: A summary of systematic reviews. J. Dent. 2021, 111, 103723. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Winocur, E.; Guarda-Nardini, L.; Paesani, D.; Lobbezoo, F. Epidemiology of bruxism in adults: A systematic review of the literature. J. Orofac. Pain 2013, 27, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Nishigawa, K.; Bando, E.; Nakano, M. Quantitative study of bite force during sleep associated bruxism. J. Oral Rehabil. 2001, 28, 485–491. [Google Scholar] [CrossRef]
- Waltimo, A.; Kononen, M. A novel bite force recorder and maximal isometric bite force values for healthy young adults. Scand. J. Dent. Res. 1993, 101, 171–175. [Google Scholar] [CrossRef] [PubMed]
- de Souza Melo, G.; Batistella, E.A.; Bertazzo-Silveira, E.; Simek Vega Goncalves, T.M.; Mendes de Souza, B.D.; Porporatti, A.L.; Flores-Mir, C.; De Luca Canto, G. Association of sleep bruxism with ceramic restoration failure: A systematic review and meta-analysis. J. Prosthet. Dent. 2018, 119, 354–362. [Google Scholar] [CrossRef]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Lavigne, G.; Rompre, P.; Kato, T.; Urade, M.; Huynh, N. Is there a first night effect on sleep bruxism? A sleep laboratory study. J. Clin. Sleep Med. 2013, 9, 1139–1145. [Google Scholar] [CrossRef]
- Kato, T.; Dal-Fabbro, C.; Lavigne, G.J. Current knowledge on awake and sleep bruxism: Overview. Alpha Omegan. 2003, 96, 24–32. [Google Scholar]
- Lavigne, G.J.; Khoury, S.; Abe, S.; Yamaguchi, T.; Raphael, K. Bruxism physiology and pathology: An overview for clinicians. J. Oral Rehabil. 2008, 35, 476–494. [Google Scholar] [CrossRef]
- Castroflorio, T.; Deregibus, A.; Bargellini, A.; Debernardi, C.; Manfredini, D. Detection of sleep bruxism: Comparison between an electromyographic and electrocardiographic portable holter and polysomnography. J. Oral Rehabil. 2014, 41, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Mainieri, V.C.; Saueressig, A.C.; Pattussi, M.P.; Fagondes, S.C.; Grossi, M.L. Validation of the Bitestrip versus polysomnography in the diagnosis of patients with a clinical history of sleep bruxism. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Stuginski-Barbosa, J.; Porporatti, A.L.; Costa, Y.M.; Svensson, P.; Conti, P.C. Diagnostic validity of the use of a portable single-channel electromyography device for sleep bruxism. Sleep Breath 2016, 20, 695–702. [Google Scholar] [CrossRef]
- Castroflorio, T.; Mesin, L.; Tartaglia, G.M.; Sforza, C.; Farina, D. Use of electromyographic and electrocardiographic signals to detect sleep bruxism episodes in a natural environment. IEEE J. Biomed. Health Inform. 2013, 17, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.J.; Huynh, N.; Kato, T.; Okura, K.; Adachi, K.; Yao, D.; Sessle, B. Genesis of sleep bruxism: Motor and autonomic-cardiac interactions. Arch. Oral Biol. 2007, 52, 381–384. [Google Scholar] [CrossRef]
- De Luca Canto, G.; Singh, V.; Bigal, M.E.; Major, P.W.; Flores-Mir, C. Association between tension-type headache and migraine with sleep bruxism: A systematic review. Headache 2014, 54, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. Sleep Medicine. Sleep Related Bruxism. In International Classification of Sleep Disorders. Diagnostic and Coding Manual, 2nd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2005; pp. 51–55. [Google Scholar]
- Paesani, D.A.; Lobbezoo, F.; Gelos, C.; Guarda-Nardini, L.; Ahlberg, J.; Manfredini, D. Correlation between self-reported and clinically based diagnoses of bruxism in temporomandibular disorders patients. J. Oral Rehabil. 2013, 40, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.G.; Sirois, D.A.; Janal, M.N.; Wigren, P.E.; Dubrovsky, B.; Nemelivsky, L.V.; Klausner, J.J.; Krieger, A.C.; Lavigne, G.J. Sleep bruxism and myofascial temporomandibular disorders: A laboratory-based polysomnographic investigation. J. Am. Dent. Assoc. 2012, 143, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Faus-Matoses, V.; Ruiz-Bell, E.; Faus-Matoses, I.; Ozcan, M.; Salvatore, S.; Faus-Llacer, V.J. An 8-year prospective clinical investigation on the survival rate of feldspathic veneers: Influence of occlusal splint in patients with bruxism. J. Dent. 2020, 99, 103352. [Google Scholar] [CrossRef]
- Fotiadou, C.; Manhart, J.; Diegritz, C.; Folwaczny, M.; Hickel, R.; Frasheri, I. Longevity of lithium disilicate indirect restorations in posterior teeth prepared by undergraduate students: A retrospective study up to 8.5 years. J. Dent. 2021, 105, 103569. [Google Scholar] [CrossRef]
- Moreira, A.; Freitas, F.; Marques, D.; Carames, J. Aesthetic Rehabilitation of a Patient with Bruxism Using Ceramic Veneers and Overlays Combined with Four-Point Monolithic Zirconia Crowns for Occlusal Stabilization: A 4-Year Follow-Up. Case Rep. Dent. 2019, 2019, 1640563. [Google Scholar] [CrossRef] [PubMed]
- Schmitter, M.; Boemicke, W.; Stober, T. Bruxism in prospective studies of veneered zirconia restorations-a systematic review. Int. J. Prosthodont. 2014, 27, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Simeone, P.; Gracis, S. Eleven-Year Retrospective Survival Study of 275 Veneered Lithium Disilicate Single Crowns. Int. J. Periodontics Restor. Dent. 2015, 35, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Koenig, V.; Wulfman, C.; Bekaert, S.; Dupont, N.; Le Goff, S.; Eldafrawy, M.; Vanheusden, A.; Mainjot, A. Clinical behavior of second-generation zirconia monolithic posterior restorations: Two-year results of a prospective study with Ex vivo analyses including patients with clinical signs of bruxism. J. Dent. 2019, 91, 103229. [Google Scholar] [CrossRef]
- Gardell, E.; Larsson, C.; von Steyern, P.V. Translucent Zirconium Dioxide and Lithium Disilicate: A 3-Year Follow-up of a Prospective, Practice-Based Randomized Controlled Trial on Posterior Monolithic Crowns. Int. J. Prosthodont. 2021, 34, 163–172. [Google Scholar] [CrossRef]
- Rauch, A.; Reich, S.; Dalchau, L.; Schierz, O. Clinical survival of chair-side generated monolithic lithium disilicate crowns:10-year results. Clin. Oral Investig. 2018, 22, 1763–1769. [Google Scholar] [CrossRef]
- Sulaiman, T.A.; Abdulmajeed, A.A.; Delgado, A.; Donovan, T.E. Fracture rate of 188695 lithium disilicate and zirconia ceramic restorations after up to 7.5 years of clinical service: A dental laboratory survey. J. Prosthet. Dent. 2020, 123, 807–810. [Google Scholar] [CrossRef]
- Schmitz, J.H.; Beani, M. Effect of different cement types on monolithic lithium disilicate complete crowns with feather-edge preparation design in the posterior region. J. Prosthet. Dent. 2016, 115, 678–683. [Google Scholar] [CrossRef]
- Schmitz, J.H.; Cortellini, D.; Granata, S.; Valenti, M. Monolithic lithium disilicate complete single crowns with feather-edge preparation design in the posterior region: A multicentric retrospective study up to 12 years. Quintessence Int. 2017, 48, 601–608. [Google Scholar] [CrossRef]
- Seydler, B.; Schmitter, M. Clinical performance of two different CAD/CAM-fabricated ceramic crowns: 2-Year results. J. Prosthet. Dent. 2015, 114, 212–216. [Google Scholar] [CrossRef]
- Malament, K.A.; Margvelashvili-Malament, M.; Natto, Z.S.; Thompson, V.; Rekow, D.; Att, W. Comparison of 16.9-year survival of pressed acid etched e.max lithium disilicate glass-ceramic complete and partial coverage restorations in posterior teeth: Performance and outcomes as a function of tooth position, age, sex, and thickness of ceramic material. J. Prosthet. Dent. 2021, 126, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Leitao, C.; Fernandes, G.V.O.; Azevedo, L.P.P.; Araujo, F.M.; Donato, H.; Correia, A.R.M. Clinical performance of monolithic CAD/CAM tooth-supported zirconia restorations: Systematic review and meta-analysis. J. Prosthodont. Res. 2022, 66, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Comba, A.; Ferrero, G.; Italia, E.; Michelotto Tempesta, R.; Paolone, G.; Mazzoni, A.; Breschi, L.; Scotti, N. External gap progression after cyclic fatigue of adhesive overlays and crowns made with high translucency zirconia or lithium silicate. J. Esthet. Restor. Dent. 2022, 34, 557–564. [Google Scholar] [CrossRef]
- Maroulakos, G.; Thompson, G.A.; Kontogiorgos, E.D. Effect of cement type on the clinical performance and complications of zirconia and lithium disilicate tooth-supported crowns: A systematic review. Report of the Committee on Research in Fixed Prosthodontics of the American Academy of Fixed Prosthodontics. J. Prosthet. Dent. 2019, 121, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Waldecker, M.; Behnisch, R.; Rammelsberg, P.; Bömicke, W. Five-year clinical performance of monolithic and partially veneered zirconia single crowns-a prospective observational study. J. Prosthodont. Res. 2022, 66, 339–345. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef]
- Lavigne, G.J.; Guitard, F.; Rompre, P.H.; Montplaisir, J.Y. Variability in sleep bruxism activity over time. J. Sleep Res. 2001, 10, 237–244. [Google Scholar] [CrossRef]
| Characteristic | Outcome | LiDi-SB n = 29 | LiDi-no SB n = 24 | Z-SB n = 23 | Z-no SB n = 27 |
|---|---|---|---|---|---|
| Sex, n (%) | Man | 10 (34.5) | 10 (41.7) | 15 (65.2) | 8 (29.6) |
| Woman | 19 (65.5) | 14 (58.3) | 8 (34.8) | 19 (70.4) | |
| Participant age at SC cementation, mean (SD) | 48.1 (12.21) | 56.2 (12.33) | 48.7 (10.49) | 62.0 (8.44) | |
| SB categories, n (%) | Moderate SB | 15 (51.7) | 0 (0) | 7 (30.4) | 0 (0) |
| Severe SB | 14 (48.3) | 0 (0) | 16 (69.6) | 0 (0) | |
| No SB | 0 (0) | 24 (100.0) | 0 (0) | 27 (100.0) | |
| Tooth, n (%) | 1st molar | 21 (72.4) | 16 (66.7) | 20 (87.0) | 16 (59.3) |
| 2nd molar | 8 (27.6) | 8 (33.3) | 3 (13.0) | 11 (40.7) | |
| Jaw, n (%) | Maxilla | 8 (27.6) | 8 (33.3) | 9 (39.1) | 7 (25.9) |
| Mandible | 21 (72.4) | 16 (66.7) | 14 (60.9) | 20 (74.1) | |
| Endodontic status, n (%) | Vital | 22 (75.9) | 19 (79.2) | 15 (65.2) | 21 (77.8) |
| Endodontically treated | 7 (24.1) | 5 (20.8) | 8 (34.8) | 6 (22.2) | |
| PPD (mm, deepest of 6 measurements per tooth), mean (SD) | 3.2 (0.62) | 3.0 (0.62) | 2.8 (0.58) | 3.0 (0.55) | |
| PPD antagonist (mm, deepest of 6 measurements per tooth), mean (SD) | 3.1 (0.86) | 3.1 (0.93) | 3.1 (0.60) | 3.3 (1.12) | |
| Tooth mobility (0–3) 1, n (%) | 0 | 29 (100.0) | 23 (95.8) | 23 (100.0) | 27 (100.0) |
| 1 | 0 (0) | 1 (4.2) | 0 (0) | 0 (0) | |
| Tooth mobility antagonist (0–3) 1, n (%) | 0 | 29 (100.0) | 24 (100.0) | 23 (100.0) | 26 (100.0) |
| Missing teeth 2, n (%) | 0 | 23 (79.3) | 22 (91.7) | 20 (87.0) | 19 (70.4) |
| 1 | 4 (13.8) | 2 (8.3) | 3 (13.0) | 5 (18.5) | |
| 2 | 2 (6.9) | 0 (0) | 0 (0) | 3 (11.1) | |
| Occlusal guidance | Canine | 9 (31.0) | 6 (25.0) | 6 (26.1) | 6 (23.1) |
| Group | 20 (69.0) | 18 (75.0) | 17 (73.9) | 20 (76.9) | |
| Minimum occlusal SC thickness (mm), mean (SD) | 1.4 (0.17) | 1.4 (0.14) | 1.3 (0.13) | 1.4 (0.15) | |
| Minimum axial wall thickness (mm), mean (SD) | 1.2 (0.13) | 1.2 (0.14) | 1.2 (0.15) | 1.2 (0.17) | |
| Occlusal adjustments prior to SC cementation, n (%) | Yes | 22 (75.9) | 22 (91.7) | 19 (82.6) | 22 (84.6) |
| No | 7 (24.1) | 2 (8.3) | 4 (17.4) | 4 (15.4) | |
| Occlusal adjustments after SC cementation, n (%) | No | 23 (79.3) | 20 (83.3) | 23 (100.0) | 23 (85.2) |
| Yes, one | 6 (20.7) | 4 (16.7) | 0 (0) | 4 (14.8) | |
| Yes, multiple | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Event | LiDi-SB n = 29 | LiDi-no SB n = 24 | Z-SB n = 23 | Z-no SB n = 27 |
|---|---|---|---|---|
| Technical complications/ceramic defects, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Symptomatic irreversible pulpitis, n (%) | 1 (3.4) | 1 (4.2) | 1 (4.3) | 0 (0) |
| Vertical root fracture, n (%) | 0 (0) | 0 (0) | 1 (4.3) 1 | 0 (0) |
| Intact SC removed, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (3.7) 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitter, M.; Bömicke, W.; Behnisch, R.; Lorenzo Bermejo, J.; Waldecker, M.; Rammelsberg, P.; Ohlmann, B. Ceramic Crowns and Sleep Bruxism: First Results from a Randomized Trial. J. Clin. Med. 2023, 12, 273. https://doi.org/10.3390/jcm12010273
Schmitter M, Bömicke W, Behnisch R, Lorenzo Bermejo J, Waldecker M, Rammelsberg P, Ohlmann B. Ceramic Crowns and Sleep Bruxism: First Results from a Randomized Trial. Journal of Clinical Medicine. 2023; 12(1):273. https://doi.org/10.3390/jcm12010273
Chicago/Turabian StyleSchmitter, Marc, Wolfgang Bömicke, Rouven Behnisch, Justo Lorenzo Bermejo, Moritz Waldecker, Peter Rammelsberg, and Brigitte Ohlmann. 2023. "Ceramic Crowns and Sleep Bruxism: First Results from a Randomized Trial" Journal of Clinical Medicine 12, no. 1: 273. https://doi.org/10.3390/jcm12010273
APA StyleSchmitter, M., Bömicke, W., Behnisch, R., Lorenzo Bermejo, J., Waldecker, M., Rammelsberg, P., & Ohlmann, B. (2023). Ceramic Crowns and Sleep Bruxism: First Results from a Randomized Trial. Journal of Clinical Medicine, 12(1), 273. https://doi.org/10.3390/jcm12010273








