Are Thoracic Aortic Aneurysm Patients at Increased Risk for Cardiovascular Diseases?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Parameters
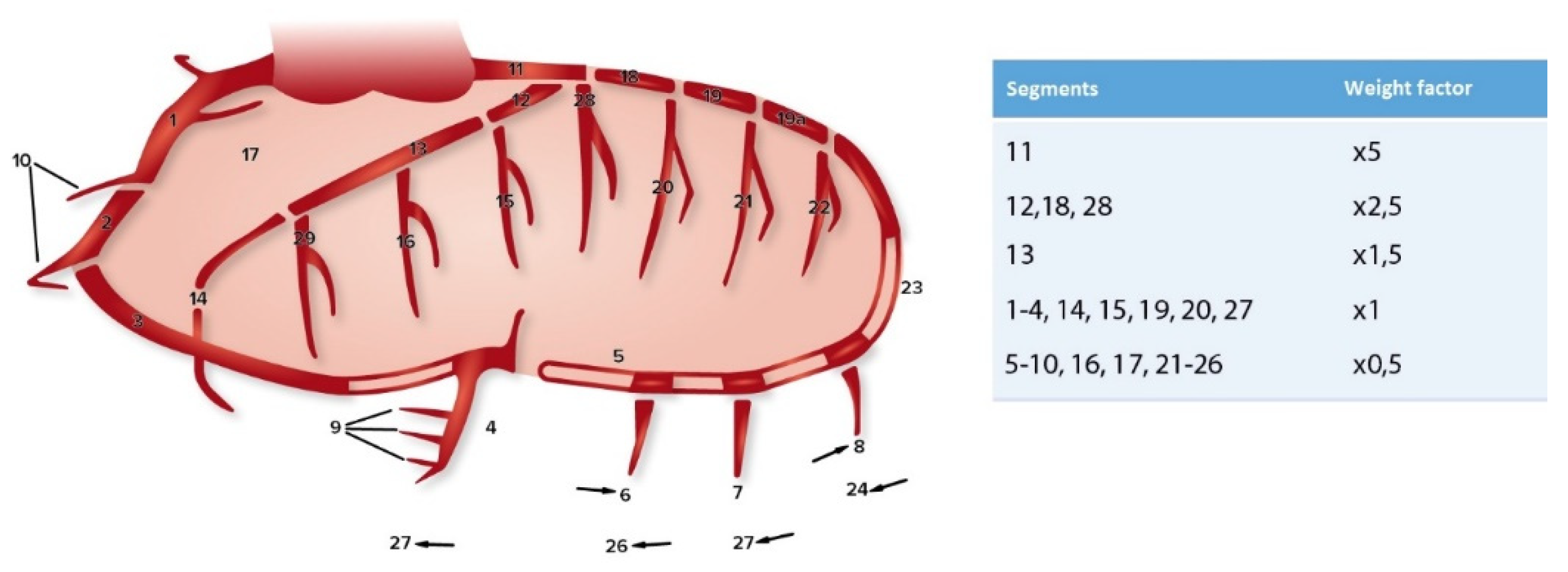
2.2. Coronary Sclerosis
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics Study Populations
3.2. Cardiovascular Risk profiles
3.3. Aortic Valve Morphology and Coronary Artery Disease Burden
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bulder, R.M.A.; Talvitie, M.; Bastiaannet, E.; Hamming, J.F.; Hultgren, R.; Lindeman, J.H. Long-term Prognosis After Elective Abdominal Aortic Aneurysm Repair is Poor in Women and Men: The Challenges Remain. Ann. Surg. 2020, 272, 773–778. [Google Scholar] [CrossRef]
- Kaasenbrood, L.; Boekholdt, S.M.; Van Der Graaf, Y.; Ray, K.K.; Peters, R.J.; Kastelein, J.J.; Amarenco, P.; LaRosa, J.C.; Cramer, M.J.M.; Westerink, J.; et al. Distribution of Estimated 10-Year Risk of Recurrent Vascular Events and Residual Risk in a Secondary Prevention Population. Circulation 2016, 134, 1419–1429. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar]
- Losenno, K.L.; Goodman, R.L.; Chu, M.W.A. Bicuspid aortic valve disease and ascending aortic aneurysms: Gaps in knowledge. Cardiol. Res. Pract. 2012, 2012, 145202. [Google Scholar] [CrossRef]
- Ward, C. Clinical significance of the bicuspid aortic valve. Heart 2000, 83, 81–85. [Google Scholar] [CrossRef]
- Dolmaci, O.B.; Legué, J.; Lindeman, J.H.; Driessen, A.H.; Klautz, R.J.; Van Brakel, T.J.; Siebelink, H.-M.J.; Mertens, B.J.A.; Poelmann, R.E.; Gittenberger-de Groot, A.C.; et al. Extent of Coronary Artery Disease in Patients With Stenotic Bicuspid Versus Tricuspid Aortic Valves. J. Am. Heart Assoc. 2021, 10, e020080. [Google Scholar] [CrossRef]
- Dolmaci, O.B.; Driessen, A.H.; Klautz, R.J.; Poelmann, R.; Lindeman, J.H.; Grewal, N. Comparative evaluation of coronary disease burden: Bicuspid valve disease is not atheroprotective. Open Heart 2021, 8, e001772. [Google Scholar] [CrossRef]
- NIVEL Zorgregistraties Eerste Lijn. Coronaire hartziekten 2019, B., Diabetes Mellitus 2019 and Cholesterol 2020. Available online: https://www.volksgezondheidenzorg.info (accessed on 23 March 2021).
- Hajar, R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views 2017, 18, 109–114. [Google Scholar] [CrossRef]
- Vlietstra, R.E.; Kronmal, R.A.; Frye, R.L.; Seth, A.K.; Tristani, F.E.; Killip, T., 3rd. Factors affecting the extent and severity of coronary artery disease in patients enrolled in the coronary artery surgery study. Arteriosclerosis 1982, 2, 208–215. [Google Scholar] [CrossRef]
- Emond, M.; Mock, M.B.; Davis, K.B.; Fisher, L.D.; Holmes, D.R.J.; Chaitman, B.R.; Kaiser, G.C.; Alderman, E.; Killip, T., 3rd. Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation 1994, 90, 2645–2657. [Google Scholar] [CrossRef]
- Scanlon, P.J.; Faxon, D.P.; Audet, A.M.; Carabello, B.; Dehmer, G.J.; Eagle, K.A.; Legako, R.D.; Leon, D.F.; Murray, J.A.; Nissen, S.E.; et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J. Am. Coll. Cardiol. 1999, 33, 1756–1824. [Google Scholar]
- Raaz, U.; Zöllner, A.M.; Schellinger, I.N.; Toh, R.; Nakagami, F.; Brandt, M.; Emrich, F.C.; Kayama, Y.; Eken, S.; Adam, M.; et al. Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation 2015, 131, 1783–1795. [Google Scholar] [CrossRef]
- Hoegh, A.; Lindholt, J.S. Basic Science Review: Vascular Distensibility as a Predictive Tool in the Management of Small Asymptomatic Abdominal Aortic Aneurysms. Vasc. Endovasc. Surg. 2009, 43, 333–338. [Google Scholar] [CrossRef]
- Perissiou, M.; Bailey, T.G.; Windsor, M.; Greaves, K.; Nam, M.C.; Russell, F.D.; O’Donnell, J.; Magee, R.; Jha, P.; Schulze, K.; et al. Aortic and Systemic Arterial Stiffness Responses to Acute Exercise in Patients With Small Abdominal Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 708–718. [Google Scholar] [CrossRef]
- Palombo, C.; Kozakova, M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vasc. Pharmacol. 2016, 77, 1–7. [Google Scholar] [CrossRef]
- Westerhof, N.; Lankhaar, J.W.; Westerhof, B.E. The arterial Windkessel. Med. Biol. Eng. Comput. 2009, 47, 131–141. [Google Scholar] [CrossRef]
- Achneck, H.; Modi, B.; Shaw, C.; Rizzo, J.; Albornoz, G.; Fusco, D.; Elefteriades, J. Ascending thoracic aneurysms are associated with decreased systemic atherosclerosis. Chest 2005, 128, 1580–1586. [Google Scholar] [CrossRef]
- Jackson, V.; Eriksson, M.J.; Caidahl, K.; Eriksson, P.; Franco-Cereceda, A. Ascending aortic dilatation is rarely associated with coronary artery disease regardless of aortic valve morphology. J. Thorac. Cardiovasc. Surg. 2014, 148, 2973–2980. [Google Scholar] [CrossRef]
- Agmon, Y.; Khandheria, B.K.; Meissner, I.; Schwartz, G.L.; Sicks, J.D.; Fought, A.J.; O’Fallon, W.M.; Wiebers, D.O.; Tajik, A.J. Is aortic dilatation an atherosclerosis-related process? Clinical, laboratory, and transesophageal echocardiographic correlates of thoracic aortic dimensions in the population with implications for thoracic aortic aneurysm formation. J. Am. Coll. Cardiol. 2003, 42, 1076–1083. [Google Scholar] [CrossRef]
- Islamoğlu, F.; Atay, Y.; Can, L.; Kara, E.; Ozbaran, M.; Yüksel, M.; Büket, S. Diagnosis and treatment of concomitant aortic and coronary disease: A retrospective study and brief review. Tex. Heart Inst. J. 1999, 26, 182–188. [Google Scholar] [PubMed]
- Creswell, L.L.; Kouchoukos, N.T.; Cox, J.L.; Rosenbloom, M. Coronary artery disease in patients with type A aortic dissection. Ann. Thorac. Surg. 1995, 59, 585–590. [Google Scholar] [CrossRef]
- Patel, K.; Zafar, M.A.; Ziganshin, B.A.; Elefteriades, J.A. Diabetes Mellitus: Is It Protective against Aneurysm? A Narrative Review. Cardiology 2018, 141, 107–122. [Google Scholar] [CrossRef]
- Raffort, J.; Larey, F.; Clément, M.; Hassen-Khodja, R.; Chinetti, G.; Mallat, Z. Diabetes and aortic aneurysm: Current state of the art. Cardiovasc. Res. 2018, 114, 1702–1713. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, R.T.; Wee, I.J.; Syn, N.L.; Choong, A.M. The association between diabetes and thoracic aortic aneurysms. J. Vasc. Surg. 2019, 69, 263–268. [Google Scholar] [CrossRef]
- Prakash, S.K.; Pedroza, C.; Khalil, Y.A.; Milewicz, D.M. Diabetes and Reduced Risk for Thoracic Aortic Aneurysms and Dissections: A Nationwide Case-Control Study. J. Am. Heart Assoc. 2012, 1, e000323. [Google Scholar] [CrossRef]
- Pannu, H.; Tran-Fadulu, V.; Papke, C.L.; Scherer, S.; Liu, Y.; Presley, C. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum. Mol. Genet. 2007, 16, 2453–2462. [Google Scholar] [CrossRef]

| TAA | Non-TAA | |||
|---|---|---|---|---|
| Characteristic | n = 70 | n = 169 | OR (95% CI) | p-Value |
| Male | 52 (74.3) | 123 (72.8) | 0.93 (0.49–1.75) | 0.873 |
| Age at surgery | 64 (54–73) | 62 (51–70) | 1.02 (0.99–1.04) | 0.112 |
| Body Mass Index | 26.1 ± 4 | 26.2 ± 4.2 | 0.99 (0.93–1.06) | 0.838 |
| Smoking status Never Former Currently | 66/70 * 32 (45.7) 19 (27.1) 15 (21.4) | 159/169 * 82 (51.6) 34 (20.1) 43 (25.4) | 0.89 (0.51–1.56) 1.48 (0.77–2.83) 0.80 (0.41–1.56) | 0.776 0.236 0.619 |
| Family history of CAD | 66/70 * 7 (10.6) | 154/169 * 23 (13.6) | 0.68 (0.28–1.66) | 0.521 |
| Diabetes | 1 (1.4) | 16 (9.5) | 0.14 (0.02–1.07) | 0.027 |
| Hypertension | 43 (61.4) | 109 (64.5) | 0.86 (0.49–1.53) | 0.658 |
| Hypercholesterolemia | 15 (21.4) | 44 (26) | 0.77 (0.40–1.50) | 0.511 |
| Preoperative creatinine (μmol/L) | 84 (69–98) | 83 (72–97) | 1.00 (0.99–1.01) | 0.910 |
| Previous MI | 3 (4.3) | 15 (8.9) | 0.46 (0.13–1.64) | 0.288 |
| Previous PCI | 2 (2.9) | 9 (5.3) | 0.52 (0.11–2.48) | 0.516 |
| Previous cardiac surgery | 1 (1.4) | 10 (5.9) | 0.23 (0.03–1.84) | 0.183 |
| TAA | Non-TAA | |||
|---|---|---|---|---|
| Surgery Type | n = 70 | n = 169 | OR (95% CI) | p-Value |
| Single AVR | 1 (1.4) | 34 (20.1) | 0.06 (0.01–0.43) | <0.001 |
| AVP | 13 (18.6) | 15 (8.9) | 2.34 (1.05–5.22) | 0.046 |
| Concomitant CABG | 11 (15.7) | 35 (20.7) | 0.71 (0.34–1.50) | 0.471 |
| Aortic procedures Root Ascending (Hemi)arch | 58 (82.9) 65 (92.9) 14 (20) | 56 (33.1) 18 (10.7) 3 (1.8) | 9.75 (4.85–19.3) 109 (38–306) 13.51 (3.83–50) | <0.001 <0.001 <0.001 |
| Other concomitant procedures | ||||
| Rhythm surgery | 9 (12.9) | 25 (14.8) | 0.85 (0.38–1.93) | 0.839 |
| MVP | 8 (11.4) | 33 (19.5) | 0.53 (0.23–1.22) | 0.186 |
| MVR | 1 (1.4) | 10 (5.9) | 0.23 (0.03–1.84) | 0.183 |
| TVP | 6 (8.6) | 30 (17.8) | 0.43 (0.17–1.10) | 0.077 |
| General Population | BAV | OR | 95% CI | p-Value | |
| Hypertension | 16% | 57.5% | 7.1 | 4.49–11.21 | <0.001 |
| Hypercholesterolemia | 9.1% | 20.7% | 2.61 | 1.49–4.57 | 0.002 |
| Diabetes mellitus | 6.6% | 2.3% | 0.33 | 0.08–1.38 | 0.162 |
| CAD | 3% | 2.3% | 0.76 | 0.18–3.24 | 1.000 |
| General Population | TAV | OR | 95% CI | p-Value | |
| Hypertension | 40.9% | 67.1% | 2.95 | 2.05–4.23 | <0.001 |
| Hypercholesterolemia | 22.7% | 27% | 1.26 | 0.85–1.85 | 0.257 |
| Diabetes mellitus | 16.9% | 9.9% | 0.54 | 0.31–0.94 | 0.032 |
| CAD | 11.4% | 10.5% | 0.91 | 0.53–1.59 | 0.891 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolmaci, O.B.; El Mathari, S.; Driessen, A.H.G.; Klautz, R.J.M.; Poelmann, R.E.; Lindeman, J.H.N.; Grewal, N. Are Thoracic Aortic Aneurysm Patients at Increased Risk for Cardiovascular Diseases? J. Clin. Med. 2023, 12, 272. https://doi.org/10.3390/jcm12010272
Dolmaci OB, El Mathari S, Driessen AHG, Klautz RJM, Poelmann RE, Lindeman JHN, Grewal N. Are Thoracic Aortic Aneurysm Patients at Increased Risk for Cardiovascular Diseases? Journal of Clinical Medicine. 2023; 12(1):272. https://doi.org/10.3390/jcm12010272
Chicago/Turabian StyleDolmaci, Onur B., Sulayman El Mathari, Antoine H. G. Driessen, Robert J. M. Klautz, Robert E. Poelmann, Jan H. N. Lindeman, and Nimrat Grewal. 2023. "Are Thoracic Aortic Aneurysm Patients at Increased Risk for Cardiovascular Diseases?" Journal of Clinical Medicine 12, no. 1: 272. https://doi.org/10.3390/jcm12010272
APA StyleDolmaci, O. B., El Mathari, S., Driessen, A. H. G., Klautz, R. J. M., Poelmann, R. E., Lindeman, J. H. N., & Grewal, N. (2023). Are Thoracic Aortic Aneurysm Patients at Increased Risk for Cardiovascular Diseases? Journal of Clinical Medicine, 12(1), 272. https://doi.org/10.3390/jcm12010272









