Revisiting the Role of Local Cryotherapy for Acne Treatment: A Review and Update
Abstract
1. Introduction
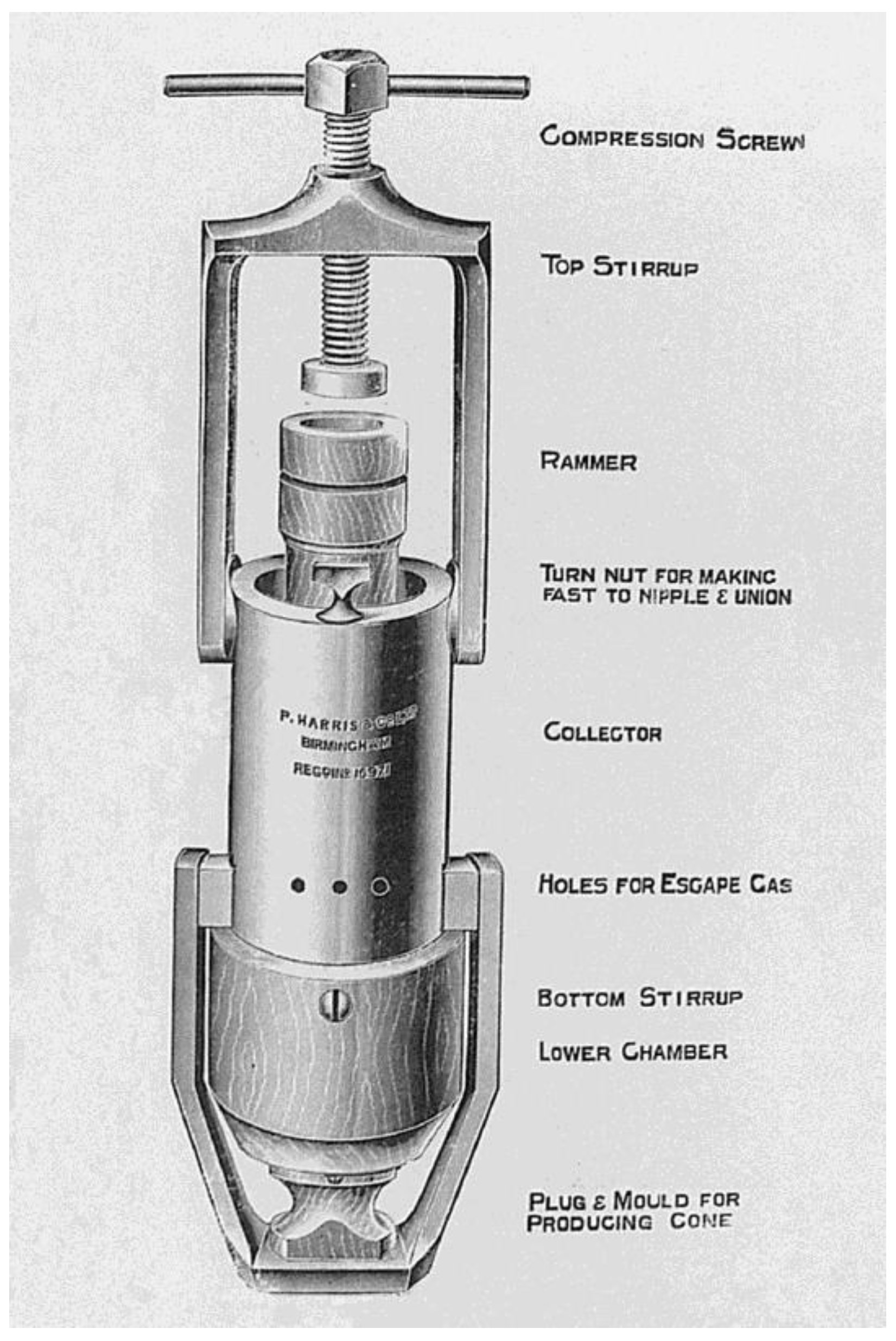
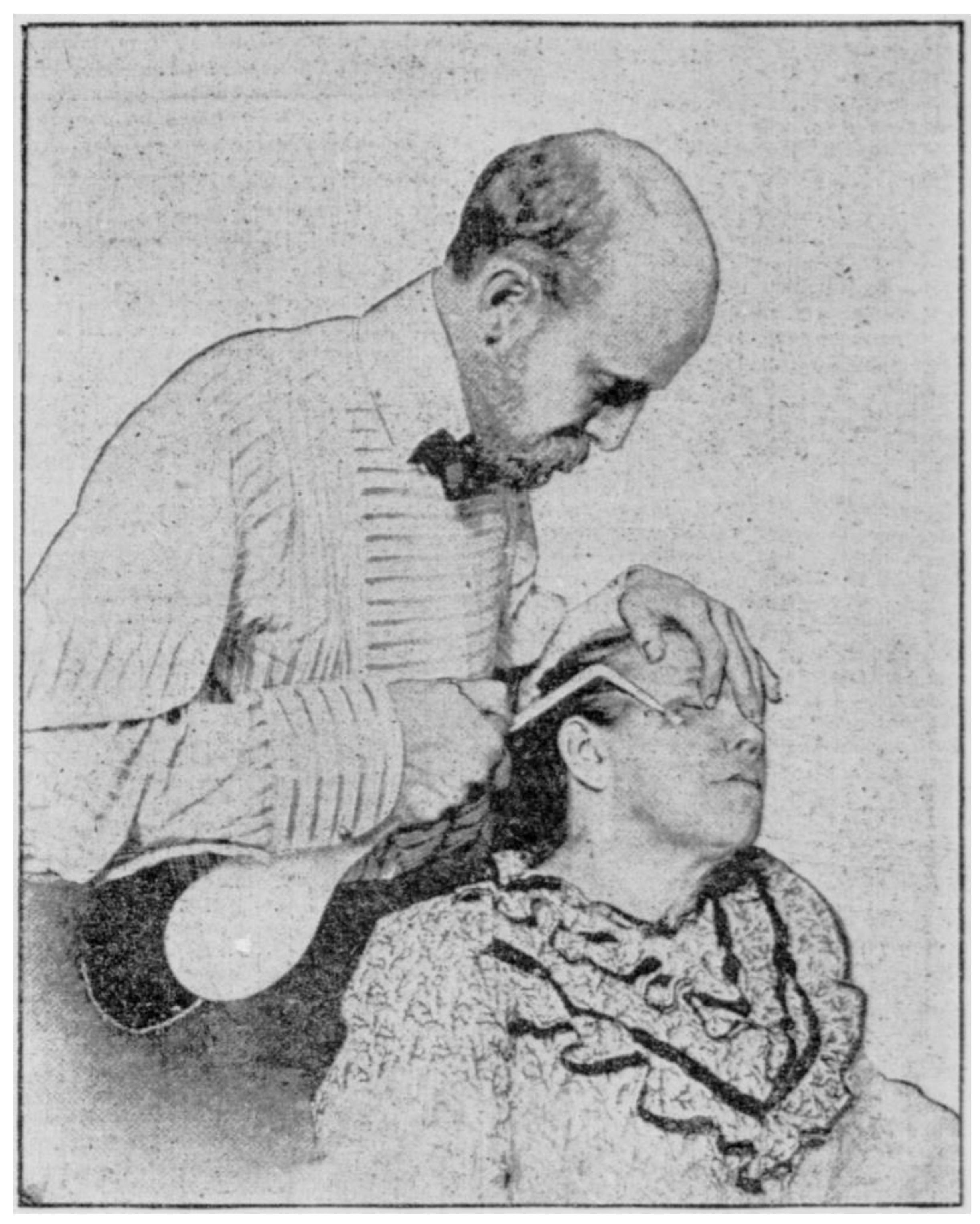
2. Brief History and Early Reports of Acne Cryotherapy
3. Cryotherapy for Acne
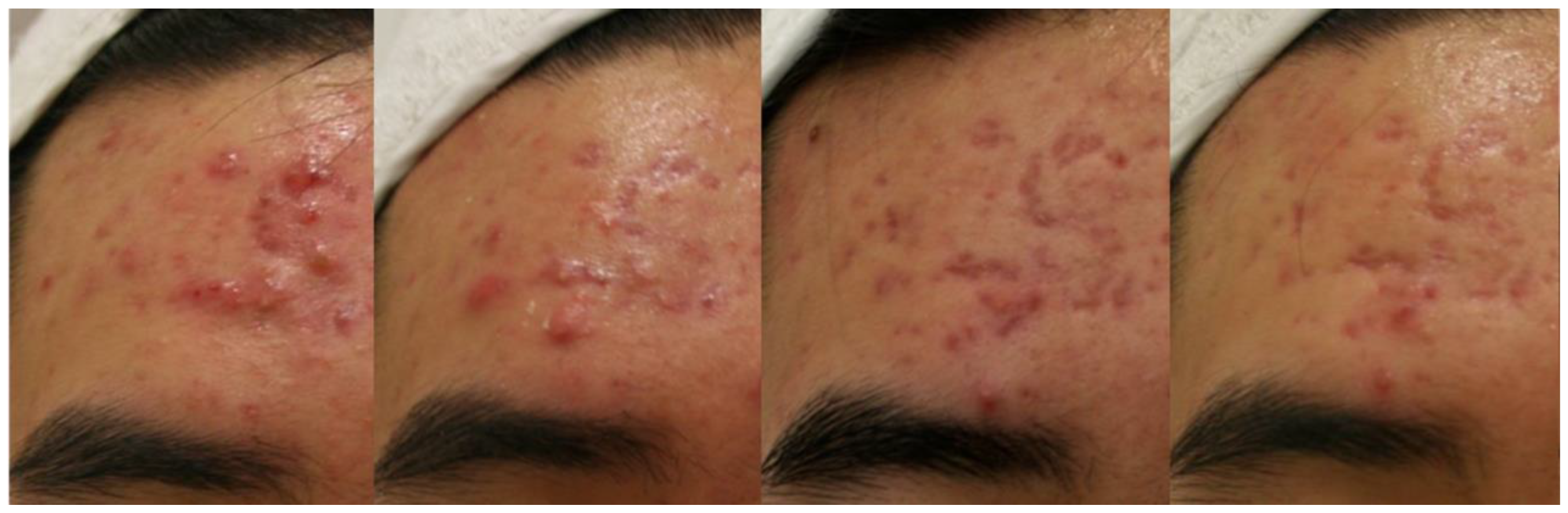
3.1. Cystic Acne
3.2. Acne Keloids
3.3. Other Subtypes of Acne
| Author, Year | n | Types of Acne | Refrigerant | Delivery Method | Control | Findings |
|---|---|---|---|---|---|---|
| Dobes and Keil, 1940 [8] | 115 | Papulopustular Nodulocystic Acne scars | Solid carbon dioxide slush | Contact method | Successful results in 70% of papulopustular acne patients Hard nodules respond better than soft cysts Unsatisfactory results in acne scars | |
| Goette, 1973 [13] | 25 | Comedopapular Papulopustular | Liquid nitrogen | Cotton tip | Topical medications | Cryotherapy-treated pustular acne lesions involuted more rapidly than lesions treated by topical medications |
| Leyden et al., 1974 [18] | 25 | Nodulocystic | Carbon dioxide Nitrous oxide Freon | Cryoprobe | Marked flattening or complete resolution of nodulo-cystic lesions in 7–10 days | |
| Layton et al., 1994 [25] | 11 | Acne keloids | Liquid nitrogen | Spray | Intralesional triamcinolone injection | Cryosurgery proved a significantly better treatment for these more vascular keloids than triamcinolone |
| Röhrs et al., 1997 [24] | 16 | Acne keloids | Nitrous oxide Liquid nitrogen | Contact method | Excellent and good results with flattening of the lesions to the skin level or slightly persisting hypertrophy were obtained in 73% of the lesions |
3.4. Acne-Induced Hyperpigmentation
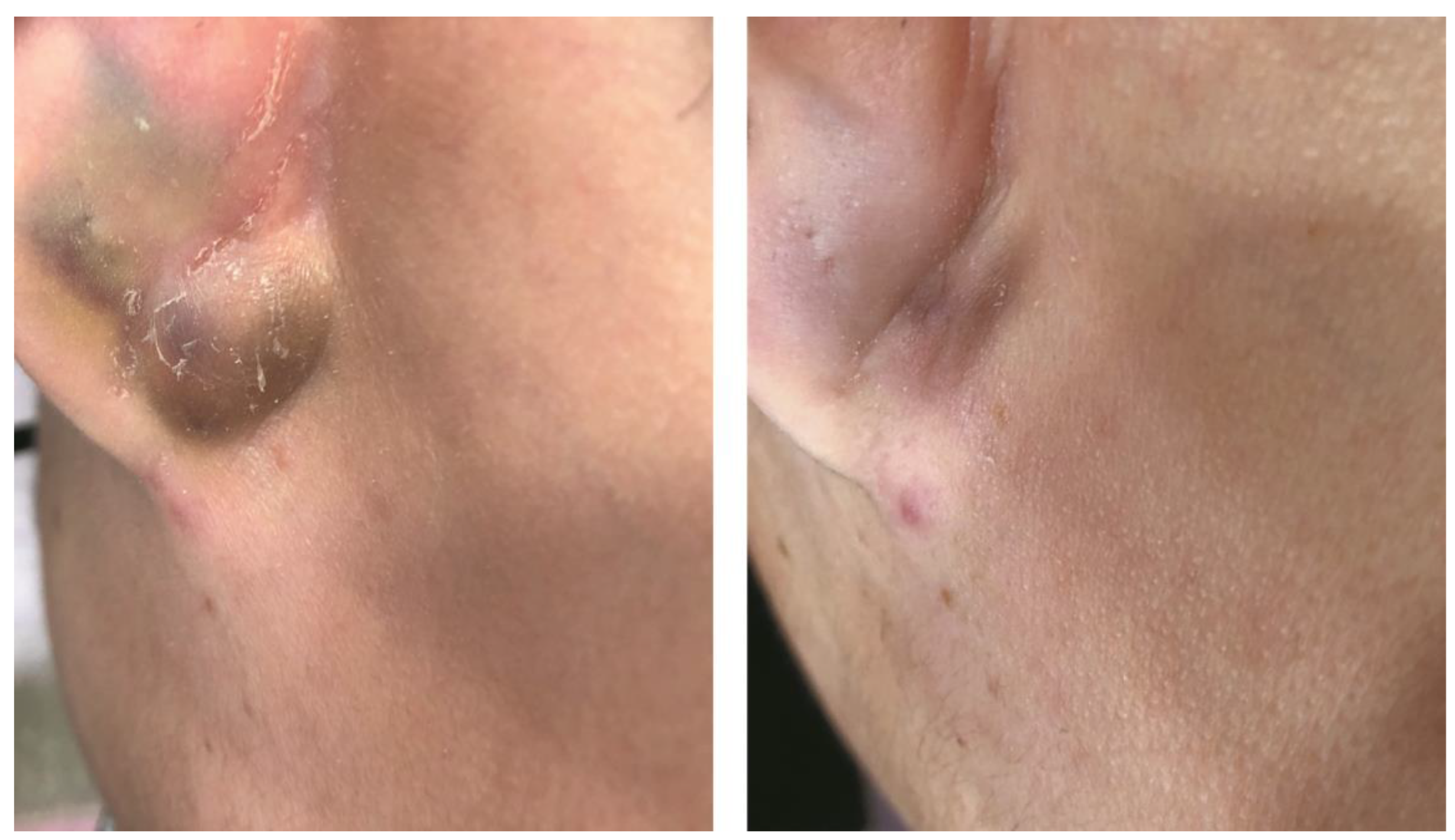
3.5. Hidradenitis Suppurativa
3.6. Other Inflammatory Dermatoses
4. Mechanism of Action
4.1. Histologic Studies
4.2. Possible Mechanisms of Action
4.3. Effects on the Sebaceous Gland
5. Adjunctive Uses of Cryotherapy in Acne Treatment
5.1. Cryotherapy Combined with Intralesional Injections
5.2. Intralesional Injections in the Treatment of Acne: The Pain Issue
5.3. Local Skin Cooling to Reduce Pain during Intralesional Injection
6. Side Effects
6.1. Pain and Discomfort
6.2. Pigmentary Alterations
6.3. Scarring
6.4. Rare Side Effects and Contraindications
7. Acne Cryotherapy: What Is the Optimal Temperature?
7.1. Therapeutic Temperature Range for Acne
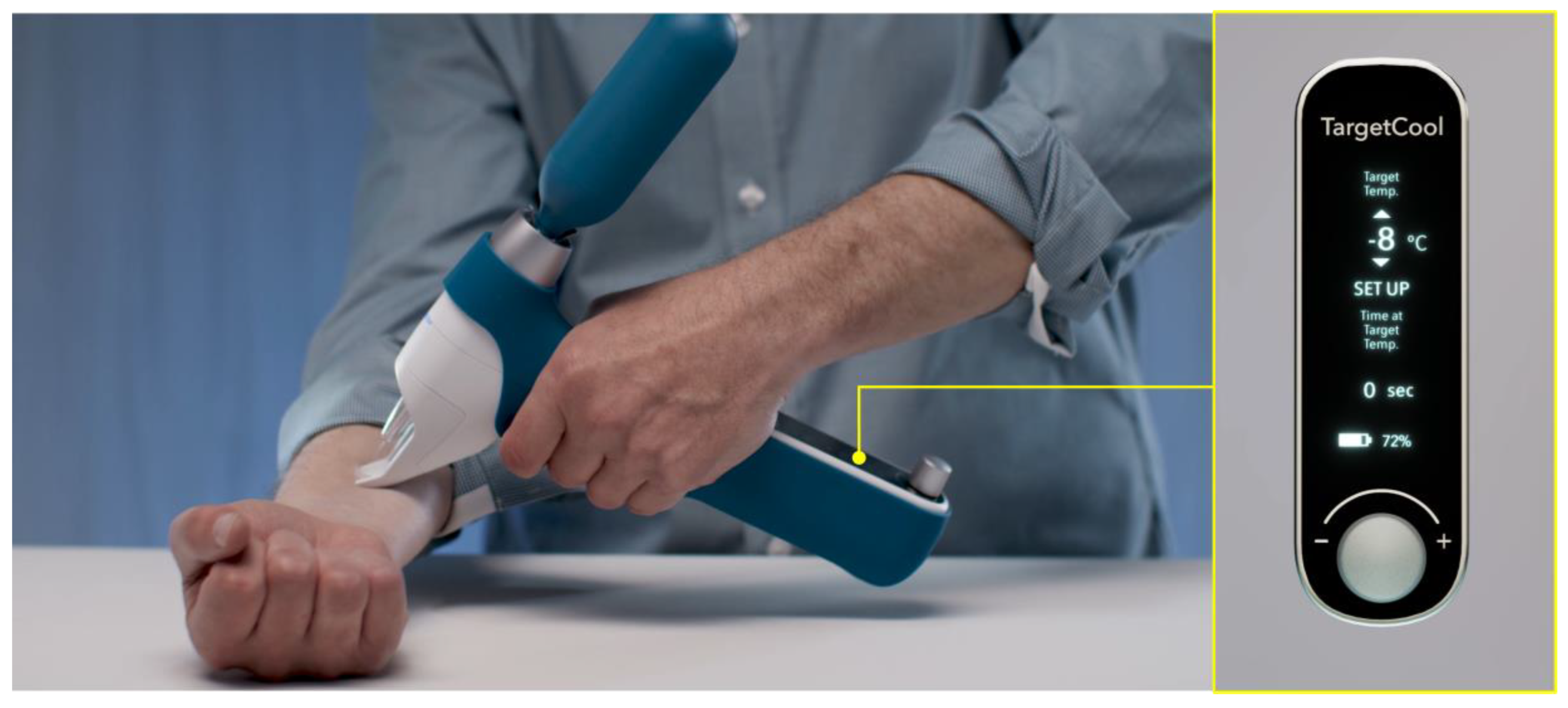
7.2. Monitoring and Control of Target Temperature during Cryotherapy
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taub, A.F. Procedural treatments for acne vulgaris. Dermatol. Surg. 2007, 33, 1005–1026. [Google Scholar] [CrossRef] [PubMed]
- Arnott, J. Practical illustrations of the remedial efficacy of a very low or anaesthetic temperature. I. In cancer. Lancet 1850, 56, 257–259. [Google Scholar] [CrossRef]
- Cooper, S.M.; Dawber, R.P.R. The history of cryosurgery. J. R. Soc. Med. 2001, 94, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.M.I. The story behind cryosurgery. J. Surg. Dermatol. 2017, 2, 191–193. [Google Scholar] [CrossRef]
- Crain, C.; Gibbons, J.A.; Marion, T. The father of modern cryotherapy. SKIN J. Cutan Med. 2019, 3, 283. [Google Scholar] [CrossRef]
- Hall-Edwards, J.F. Carbon Dioxide Snow: Its Therapeutic Uses, Methods of Collection and Application; Simpkin, Marshall, Hamilton, Kent: London, UK, 1913. [Google Scholar]
- Karp, F.L.; Nieman, H.A.; Lerner, C. Cryotherapy for acne and its scars. Arch. Derm. Syphilol. 1939, 39, 995. [Google Scholar] [CrossRef]
- Dobes, W.L.; Keil, H. Treatment of acne vulgaris by cryotherapy (slush method). Arch. Derm. Syphilol. 1940, 42, 547–558. [Google Scholar] [CrossRef]
- Kaminsky, A. Less common methods to treat acne. Dermatology 2003, 206, 68–73. [Google Scholar] [CrossRef]
- Pasquali, P. A short history of cryosurgery. In Cryosurgery; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–17. [Google Scholar] [CrossRef]
- Allington, H.V. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950, 72, 153–155. [Google Scholar]
- Graham, G.F. Cryotherapy against acne vulgaris yields “good to excellent results”. Dermatol. Pract. 1972, 5, 13–15. [Google Scholar]
- Goette, D.K. Liquid nitrogen in the treatment of acne vulgaris: A comparative study. S. Med. J. 1973, 66, 1131–1132. [Google Scholar] [CrossRef] [PubMed]
- Zacarian, S.A. Cryosurgery in dermatologic disorders and in the treatment of skin cancer. J. Cryosurg. 1968, 1, 70–75. [Google Scholar]
- Plewig, G.; Kligman, A.M. Physical therapy. In Acne and Rosacea, 3rd ed.; Plewig, G., Kligman, A.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 697–705. [Google Scholar] [CrossRef]
- Usatine, R.P.; Stulberg, D.L.; Colver, G.B. Cutaneous Cryosurgery, 4th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Pasquali, P. Cryosurgery. In Surgery of the Skin: Procedural Dermatology, 2nd ed.; Robinson, J.K., Hanke, C.W., Siegel, D.M., Fratila, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 153–166. [Google Scholar]
- Leyden, J.J.; Mills, O.H.; Kligman, A.M. Cryoprobe treatment of acne conglobata. Br. J. Dermatol. 1974, 90, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S.; Gross, E.R. Solid carbon dioxide therapy for cystic acne. Arch. Dermatol. 1949, 59, 664. [Google Scholar] [CrossRef]
- Cunliffe, W.J. Treatment of acne. In Acne; Cunliffe, W.J., Ed.; Martin Dunitz: London, UK, 1989; pp. 252–287. [Google Scholar]
- Thapa, D.P.; Jha, A.K.; Shrestha, S.; Joshi, S. Cryosurgery in a dermatology setup: A hospital based study. Our Dermatol. Online 2018, 9, 137–139. [Google Scholar] [CrossRef]
- Committee on Guidelines of Care, Task Force on Cryosurgery. Guidelines of care for cryosurgery. American Academy of Dermatology Committee on Guidelines of Care. J. Am. Acad. Dermatol. 1994, 31, 648–653. [Google Scholar]
- Zouboulis, C.C. Principles of cutaneous cryosurgery: An update. Dermatology 1999, 198, 111–117. [Google Scholar] [CrossRef]
- Röhrs, H.; Orfanos, C.E.; Zouboulis, C.C. Cryosurgical treatment of acne keloids. J. Investig. Dermatol. 1997, 108, 396. [Google Scholar]
- Layton, A.M.; Yip, J.; Cunliffe, W.J. A comparison of intralesional triamcinolone and cryosurgery in the treatment of acne keloids. Br. J. Dermatol. 1994, 130, 498–501. [Google Scholar] [CrossRef]
- Binic, I. Keloids and hypertrophic scars. In European Handbook of Dermatological Treatments, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 455–464. [Google Scholar] [CrossRef]
- Dilworth, G.R. Acne surgery. Can. Fam. Physician 1983, 29, 955–958. [Google Scholar]
- Brody, H.J. Solid carbon dioxide: Usage in slush or block form as therapeutic agent in dermatology. In Dermatological Cryosurgery and Cryotherapy; Springer: London, UK, 2016; pp. 201–206. [Google Scholar] [CrossRef]
- Jansen, T.; Lindner, A.; Plewig, G. Draining sinus in acne and rosacea: A clinical, histopathologic and experimental study. Hautarzt 1995, 46, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Lee, S.; Lee, E.H.; Ha, G.U.; Kim, G.H.; Lee, W.J. Effect of a precision cryotherapy device with temperature adjustability on pigmentation. Indian J. Dermatol. 2022, 67, 204. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.F.; Tuchayi, S.M. Acne. In Dermatological Cryosurgery and Cryotherapy; Springer: London, UK, 2016; pp. 319–323. [Google Scholar] [CrossRef]
- Gage, A.A.; Meenaghan, M.A.; Natiella, J.R.; Greene, G.W. Sensitivity of pigmented mucosa and skin to freezing injury. Cryobiology 1979, 16, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Burge, S.M.; Bristol, M.; Millard, P.R.; Dawber, R.P.R. Pigment changes in human skin after cryotherapy. Cryobiology 1986, 23, 422–432. [Google Scholar] [CrossRef]
- Munavalli, G.S.; Boey, G.; Bowes, L.E.; Kilmer, S.L.; LaTowsky, B.C.; Zelickson, B.D. Multi-center study of a novel, intelligent device delivering controlled, epidermal freezing for the treatment of benign pigmented lesions. Lasers Surg. Med. 2019, 51, S27. [Google Scholar] [CrossRef]
- Elbuluk, N.; Grimes, P.; Chien, A.; Hamzavi, I.; Alexis, A.; Taylor, S.; Gonzalez, N.; Weiss, J.; Desai, S.R.; Kang, S. The pathogenesis and management of acne-induced post-inflammatory hyperpigmentation. Am. J. Clin. Dermatol. 2021, 22, 829–836. [Google Scholar] [CrossRef]
- Stoll, D.M.; Fields, J.P.; King, L.E. Treatment of prurigo nodularis: Use of cryosurgery and intralesional steroids plus lidocaine. J. Dermatol. Surg. Oncol. 1983, 9, 922–924. [Google Scholar] [CrossRef]
- Lee, E.H.; Lee, H.J.; Park, K.D.; Lee, W.J. Effect of a new cryotherapy device on an itchy sensation in patients with mild atopic dermatitis. J. Cosmet. Dermatol. 2021, 20, 2906–2910. [Google Scholar] [CrossRef]
- Pink, A.; Anzengruber, F.; Navarini, A.A. Acne and hidradenitis suppurativa. Br. J. Dermatol. 2018, 178, 619–631. [Google Scholar] [CrossRef]
- Bong, J.L.; Shalders, K.; Saihan, E. Treatment of persistent painful nodules of hidradenitis suppurativa with cryotherapy. Clin. Exp. Dermatol. 2003, 28, 241–244. [Google Scholar] [CrossRef][Green Version]
- Pagliarello, C.; Fabrizi, G.; Feliciani, C.; di Nuzzo, S. Cryoinsufflation for Hurley stage II hidradenitis suppurativa: A useful treatment option when systemic therapies should be avoided. JAMA Dermatol. 2014, 150, 765. [Google Scholar] [CrossRef] [PubMed]
- Daveluy, S. Cryoinsufflation in the presurgical assessment of hidradenitis suppurativa. J. Am. Acad. Dermatol. 2020, 82, e127. [Google Scholar] [CrossRef] [PubMed]
- Dzidek and Piotrowska. The use of cryotherapy in cosmetology and the influence of cryogenic temperatures on selected skin parameters: A review of the literature. Cosmetics 2022, 9, 100. [Google Scholar] [CrossRef]
- Nouri, K.; Chartier, T.K.; Eaglstein, W.H.; Fla, M.; Taylor, J.R. Cryotherapy for psoriasis. Arch. Dermatol. 1997, 133, 1608. [Google Scholar] [CrossRef]
- de Souza, R.C.A.; Cunha, J.M.; Ferreira, S.H.; Cunha, F.Q.; Lima, H.C. Different inflammatory mediators induce inflammation and pain after application of liquid nitrogen to the skin. Cryobiology 2006, 53, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Har-Shai, Y.; Amar, M.; Sabo, E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast. Reconstr. Surg. 2003, 111, 1841–1852. [Google Scholar] [CrossRef]
- Dzubow, L.M. Histologic and temperature alterations induced by skin refrigerants. J. Am. Acad. Dermatol. 1985, 12, 796–810. [Google Scholar] [CrossRef]
- Rosenthal, J.M.; Mann, R.E.; Cohen, S.R. Identical twins presenting with pyogenic granuloma-like vascular proliferations during isotretinoin therapy. JAAD Case Rep. 2020, 6, 378–380. [Google Scholar] [CrossRef]
- Chao, B.H.; He, X.; Bischof, J.C. Pre-treatment inflammation induced by TNF-α augments cryosurgical injury on human prostate cancer. Cryobiology 2004, 49, 10–27. [Google Scholar] [CrossRef]
- Lalrengpuii, J.; Raza, K.; Mishra, A.; Shukla, R. Retinoid nanoparticles: Approachable gateway for acne treatment. Health Sci. Rev. 2022, 4, 100042. [Google Scholar] [CrossRef]
- Kotova, T.G.; Tsybusov, S.N.; Kochenov, V.I.; Tcyganov, M.I. Application of cryogenic methods in skin diseases of different etiology. In Dermatologic Surgery and Procedures; InTech: London, UK, 2018. [Google Scholar] [CrossRef][Green Version]
- Kim, J.H.; Uh, J.A.; Lee, J.H.; Lee, S.K.; Kim, M.S.; Lee, U.H. A study of clinical, histopathologic, and immunohistochemical changes after cryotherapy in actinic keratosis. J. Am. Acad. Dermatol. 2022, 87, AB133. [Google Scholar] [CrossRef]
- Knaggs, H.E.; Holland, D.B.; Morris, C.; Wood, E.J.; Cunliffe, W.J. Quantification of cellular proliferation in acne using the monoclonal antibody Ki-67. J. Investig. Dermatol. 1994, 102, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.A.L.; Panagiotopoulos, A.; Pasquali, P. Cryosurgery for common benign lesions. In Cryosurgery; Springer: Berlin/Heidelberg, Germany, 2015; pp. 93–105. [Google Scholar] [CrossRef]
- Guillot, X.; Tordi, N.; Laheurte, C.; Pazart, L.; Prati, C.; Saas, P.; Wendling, D. Local ice cryotherapy decreases synovial interleukin 6, interleukin 1β, vascular endothelial growth factor, prostaglandin-E2, and nuclear factor kappa B p65 in human knee arthritis: A controlled study. Arthritis Res. Ther. 2019, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.; Kim, J.; Kim, J.; Song, S.; Lee, W.; Lee, J.H. Tissue-remodelling M2 macrophages cecruits matrix metalloproteinase-9 for cryotherapy-induced fibrotic resolution during keloid treatment. Acta Derm. Venereol. 2020, 100, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tikuisis, P.; Ducharme, M.B.; Moroz, D.; Jacobs, I. Physiological responses of exercised-fatigued individuals exposed to wet-cold conditions. J. Appl. Physiol. 1999, 86, 1319–1328. [Google Scholar] [CrossRef]
- Garibyan, L.; Cornelissen, L.; Sipprell, W.; Pruessner, J.; Elmariah, S.; Luo, T.; Lerner, E.A.; Jung, Y.; Evans, C.; Zurakowski, D.; et al. Transient alterations of cutaneous sensory nerve function by noninvasive cryolipolysis. J. Investig. Dermatol. 2015, 135, 2623–2631. [Google Scholar] [CrossRef]
- Burge, S.M.; Dawber, R.P.R. Hair follicle destruction and regeneration in guinea pig skin after cutaneous freeze injury. Cryobiology 1990, 27, 153–163. [Google Scholar] [CrossRef]
- Ma, J.; Yu, X.; Lv, J.; Lin, D.; Lin, J.; Bai, Y.; Wang, Y.; Li, X.; Dong, J. Cryotherapy mediates histopathological and microstructural changes during the treatment of skin and subcutaneous tumors in dogs. Cryobiology 2021, 98, 164–171. [Google Scholar] [CrossRef]
- Ray Jalian, H.; Tam, J.; Vuong, L.N.; Fisher, J.; Garibyan, L.; Mihm, M.C.; Zurakowski, D.; Evans, C.L.; Anderson, R.R. Selective cryolysis of sebaceous glands. J. Investig. Dermatol. 2015, 135, 2173–2180. [Google Scholar] [CrossRef]
- Anna, M.; Magdalena, S.K. Evaluation of the influence of whole-body cryotherapy on selected skin parameters in healthy individuals: Pilot study. Cryobiology 2021, 100, 77–80. [Google Scholar] [CrossRef]
- Jung, Y.; Tam, J.; Ray Jalian, H.; Rox Anderson, R.; Evans, C.L. Longitudinal, 3D in vivo imaging of sebaceous glands by coherent anti-stokes Raman scattering microscopy: Normal function and response to cryotherapy. J. Investig. Dermatol. 2015, 135, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wheeland, R.G.; Wiley, M.D. Q-tip cryosurgery for the treatment of senile sebaceous hyperplasia. J. Dermatol. Surg. Oncol. 1987, 13, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Pakula, A.; Garden, J. Sebaceous hyperplasia and basal cell carcinoma in a renal transplant patient receiving cyclosporine. J. Am. Acad. Dermatol. 1992, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Hussein, L.; Perrett, C.M. Treatment of sebaceous gland hyperplasia: A review of the literature. J. Dermatolog. Treat. 2021, 32, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Ataş, H.; Gönül, M. Evaluation of the efficacy of cryosurgery in patients with sebaceous hyperplasia of the face. J. Cutan Med. Surg. 2017, 21, 202–206. [Google Scholar] [CrossRef]
- el Darouti, M.A.; al Rubaie, S.M. Cutaneous leishmaniasis: Treatment with combined cryotherapy and intralesional stibogluconate injection. Int. J. Dermatol. 1990, 29, 56–59. [Google Scholar] [CrossRef]
- Dowlati, B.; Firooz, A.; Dowlati, Y. Granuloma faciale: Successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int. J. Dermatol. 1997, 36, 548–551. [Google Scholar] [CrossRef]
- Ibrahim, H.; el Taieb, M.; Nada, E.; Kamal, E.; Hegazy, E. Combined intralesional injection of tuberculin purified protein derivative plus cryotherapy versus each alone in the treatment of multiple common warts. Dermatol. Ther. 2022, 35, e15350. [Google Scholar] [CrossRef]
- Emad, M.; Omidvari, S.; Dastgheib, L.; Mortazavi, A.; Ghaem, H. Surgical excision and immediate postoperative radiotherapy versus cryotherapy and intralesional steroids in the management of keloids: A prospective clinical trial. Med. Princ. Pract. 2010, 19, 402–405. [Google Scholar] [CrossRef]
- Rusciani, A.; Ricci, F.; Curinga, G. Acne scar treatment. In European Handbook of Dermatological Treatments, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1073–1080. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Sugeng, M.W.; Goon, A.; Chan, Y.; Goh, C. A comparison of the combined effect of cryotherapy and corticosteroid injections versus corticosteroids and cryotherapy alone on keloids: A controlled study. J. Dermatolog. Treat. 2001, 12, 87–90. [Google Scholar] [CrossRef]
- Boutli-Kasapidou, F.; Tsakiri, A.; Anagnostou, E.; Mourellou, O. Hypertrophic and keloidal scars: An approach to polytherapy. Int. J. Dermatol. 2005, 44, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Guo, Y. Granulomatous reaction to intralesional Kenalog (triamcinolone) injection in acne: A case report. Am. J. Dermatopathol. 2019, 41, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.M.; Rasmussen, J.E. Intralesional corticosteroids in the treatment of nodulocystic acne. Arch. Dermatol. 1983, 119, 480–481. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, T.; Taliercio, M.; Nia, J.K.; Hashim, P.W.; Zeichner, J.A. Dermatologist use of intralesional triamcinolone in the treatment of acne. J. Clin. Aesthet. Dermatol. 2020, 13, 41–43. [Google Scholar]
- Leeming, J.A. Intralesional triamcinolone in the treatment of cystic acne. S. Afr. Med. J. 1965, 39, 567–571. [Google Scholar]
- Young, K.D. Pediatric procedural pain. Ann. Emerg. Med. 2005, 45, 160–171. [Google Scholar] [CrossRef]
- Lim, K. Painless steroid injections for hypertrophic scars and keloids. Br. J. Plast. Surg. 2004, 57, 475–477. [Google Scholar] [CrossRef]
- Humphrey, G.B.; Boon, C.M.J.; van Linden van den Heuvell, G.F.E.C.; van de Wiel, H.B.M. The occurrence of high levels of acute behavioral distress in children and adolescents undergoing routine venipunctures. Pediatrics 1992, 90, 87–91. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Bhat, S.A. Blood injury and injection phobia: The neglected one. Behav. Neurol. 2014, 2014, 471340. [Google Scholar] [CrossRef]
- Freeman, D.; Lambe, S.; Yu, L.M.; Freeman, J.; Chadwick, A.; Vaccari, C.; Waite, F.; Rosebrock, L.; Petit, A.; Vanderslott, S.; et al. Injection fears and COVID-19 vaccine hesitancy. Psychol. Med. 2021, 1–11. [Google Scholar] [CrossRef]
- Zakria, D.; Patrinely, J.R.; Dewan, A.K.; Albers, S.E.; Wheless, L.E.; Simmons, A.N.; Drolet, B.C. Intralesional corticosteroid injections are less painful without local anesthetic: A double-blind, randomized controlled trial. J. Dermatolog. Treat. 2022, 33, 2034–2037. [Google Scholar] [CrossRef] [PubMed]
- Strazar, R.; Lalonde, D. Minimizing injection pain in local anesthesia. CMAJ 2012, 184, 2016. [Google Scholar] [CrossRef] [PubMed]
- Chuang, G.S.; Rogers, G.S.; Zeltser, R. Poiseuille’s law and large-bore needles: Insights into the delivery of corticosteroid injections in the treatment of keloids. J. Am. Acad. Dermatol. 2008, 59, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Verbov, J. The place of intralesional steroid therapy in dermatology. Br. J. Dermatol. 1976, 94, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L. Reactions following intralesional and sublesional injections of corticosteroids. JAMA 1962, 182, 613–616. [Google Scholar] [CrossRef]
- Nduka, C.; van Dam, H.; Davis, K.; Shibu, M. Painless steroid injections for hypertrophic scars and keloids. Br. J. Plast. Surg. 2003, 56, 842. [Google Scholar] [CrossRef]
- Alsantali, A. A comparative trial of ice application versus EMLA cream in alleviation of pain during botulinum toxin injections for palmar hyperhidrosis. Clin. Cosmet. Investig. Dermatol. 2018, 11, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Kanlayanaphotporn, R.; Janwantanakul, P. Comparison of skin surface temperature during the application of various cryotherapy modalities. Arch. Phys. Med. Rehabil. 2005, 86, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Trescot, A.M. Cryoanalgesia in interventional pain management. Pain Physician 2003, 6, 345–360. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, R.W.; Eun, S.H.; Jung, H.M.; Kim, G.M.; Bae, J.M. Liquid nitrogen spray cooling for reducing injection pain: A pilot study. Ann. Dermatol. 2022, 34, 144. [Google Scholar] [CrossRef]
- Plotkin, S. Clinical comparison of preinjection anesthetics. J. Am. Podiatr. Med. Assoc. 1998, 88, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Frankel, S.; Welsh, E.; Halem, M. Cryoanalgesia with dichlorotetrafluoroethane lessens the pain of botulinum toxin injections for the treatment of palmar hyperhidrosis. Dermatol. Surg. 2003, 29, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Irkoren, S.; Ozkan, H.S.; Karaca, H. A clinical comparison of EMLA cream and ethyl chloride spray application for pain relief of forehead botulinum toxin injection. Ann. Plast. Surg. 2015, 75, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.F. Cryosurgical treatment of acne. Cutis 1975, 16, 509–513. [Google Scholar]
- Jung, S.; Yoo, K.H.; Na, S.; Kim, J. Efficacy of a new cryotherapy device on pain relief during the laser tattoo removal. Med. Lasers 2022, 11, 173–177. [Google Scholar] [CrossRef]
- Chuang, G.S.; Farinelli, W.; Anderson, R.R. Selective cryolysis of melanocytes: Critical temperature and exposure time to induce selective pigmentary loss in Yucatan pig skin. Lasers Surg Med. 2021, 53, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.H.; Graham, G.F. A complication of cryosurgery in a patient with cryofibrinogenemia. J. Dermatol. Surg. Oncol. 1978, 4, 743–744. [Google Scholar] [CrossRef]
- Chao, D.L.; Rinella, N.T.; Khanani, A.M.; Wykoff, C.C.; Kim, G.H. Cooling anesthesia for intravitreal injection: Results of the prospective open-label, dose-ranging COOL-1 trial. Clin. Ophthalmol. 2021, 15, 4659–4666. [Google Scholar] [CrossRef]
- Ernst, E.; Fialka, V. Ice freezes pain? A review of the clinical effectiveness of analgesic cold therapy. J. Pain Symptom Manag. 1994, 9, 56–59. [Google Scholar] [CrossRef]
- Zhou, L.; Kambin, P.; Casey, K.F.; Bonner, F.J.; O’Brien, E.; Shao, Z.; Ou, S. Mechanism research of cryoanalgesia. Neurol. Res. 1995, 17, 307–311. [Google Scholar] [CrossRef]
- De Caro, C.; Cristiano, C.; Avagliano, C.; Bertamino, A.; Ostacolo, C.; Campiglia, P.; Gómez-Monterrey, I.; La Rana, G.; Gualillo, O.; Calignano, A.; et al. Characterization of new TRPM8 modulators in pain perception. Int. J. Mol. Sci. 2019, 20, 5544. [Google Scholar] [CrossRef] [PubMed]
- Leal-Silva, H.; Carmona-Hernández, E.; Grijalva-Vázquez, M.; Padilla-Ascencio, B. Crioterapia en acné inflamatorio. Dermatol. Rev. Mex. 2018, 62, 461–468. [Google Scholar]
- Cunliffe, W.J. Acne. In European Handbook of Dermatological Treatments, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 3–9. [Google Scholar] [CrossRef]
- Krunic, A.L.; Marini, L.G. Cryosurgery. In European Handbook of Dermatological Treatments, 3rd ed.; Katsambas, A.D., Lotti, T.M., Dessinioti, C., Massimiliano D’Erme, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1139–1149. [Google Scholar] [CrossRef]
- Abramovits, W. Tissue temperature monitors. In Dermatological Cryosurgery and Cryotherapy; Abramovits, W., Graham, G., Har-Shai, Y., Strumia, R., Eds.; Springer: London, UK, 2016; pp. 135–136. [Google Scholar] [CrossRef]
- Aguilar, G.; Majaron, B.; Verkruysse, W.; Zhou, Y.; Nelson, J.S.; Lavernia, E.J. Theoretical and experimental analysis of droplet diameter, temperature, and evaporation rate evolution in cryogenic sprays. Int. J. Heat Mass Transf. 2001, 44, 3201–3211. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rho, N.-K. Revisiting the Role of Local Cryotherapy for Acne Treatment: A Review and Update. J. Clin. Med. 2023, 12, 26. https://doi.org/10.3390/jcm12010026
Rho N-K. Revisiting the Role of Local Cryotherapy for Acne Treatment: A Review and Update. Journal of Clinical Medicine. 2023; 12(1):26. https://doi.org/10.3390/jcm12010026
Chicago/Turabian StyleRho, Nark-Kyoung. 2023. "Revisiting the Role of Local Cryotherapy for Acne Treatment: A Review and Update" Journal of Clinical Medicine 12, no. 1: 26. https://doi.org/10.3390/jcm12010026
APA StyleRho, N.-K. (2023). Revisiting the Role of Local Cryotherapy for Acne Treatment: A Review and Update. Journal of Clinical Medicine, 12(1), 26. https://doi.org/10.3390/jcm12010026






