Using the First-Eye Back-Calculated Effective Lens Position to Improve Refractive Outcome of the Second Eye
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Characteristics:
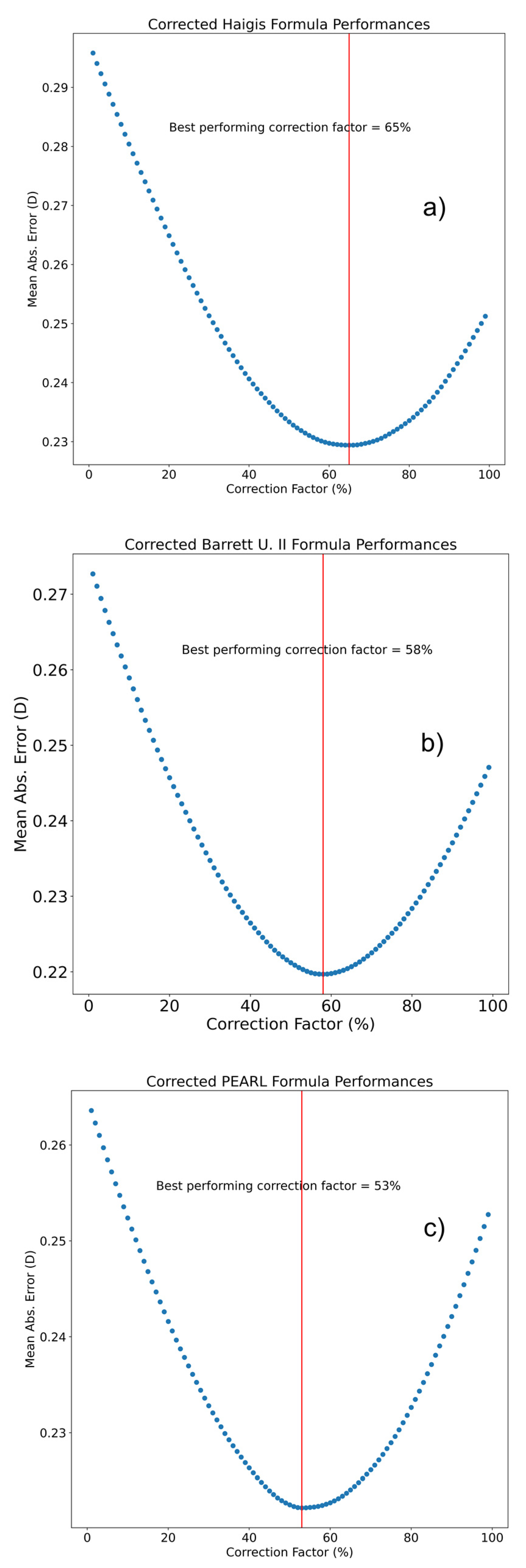
3.2. IOL Constant Optimization and Formula Design
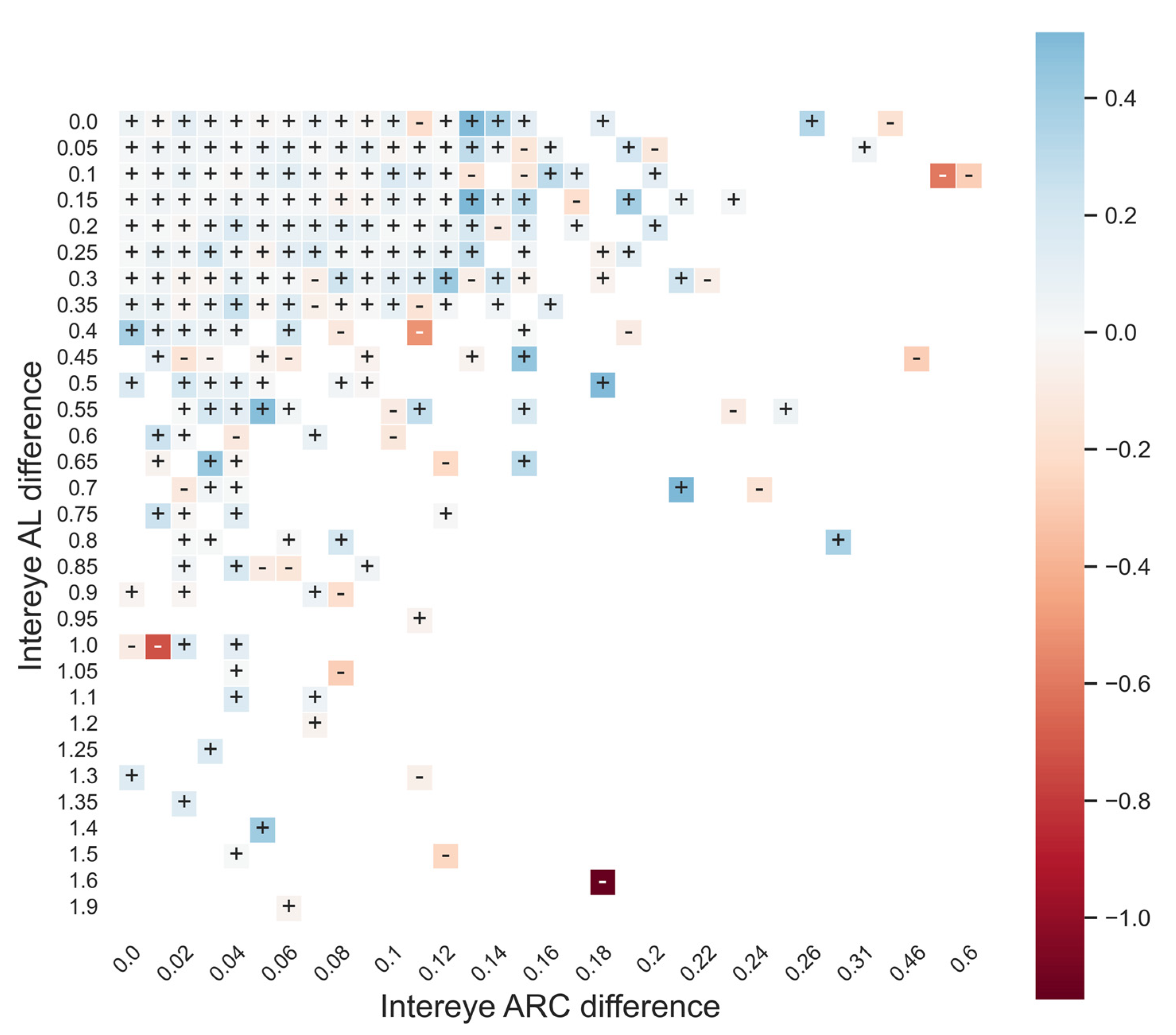
3.3. Second-Eye Enhancement Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, S.H.; Zein, M. Vision Restoration: Cataract Surgery and Surgical Correction of Myopia, Hyperopia, and Presbyopia. Med. Clin. N. Am. 2021, 105, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Melles, R.B.; Holladay, J.T.; Chang, W.J. Accuracy of Intraocular Lens Calculation Formulas. Ophthalmology 2018, 125, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.V.; Hodge, C.; Sutton, G.; Lawless, M. Contributors to the Vision Eye Institute IOL outcomes registry Comparison of Hill-radial basis function, Barrett Universal and current third generation formulas for the calculation of intraocular lens power during cataract surgery. Clin. Experiment. Ophthalmol. 2018, 46, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Jivrajka, R.V.; Shammas, M.C.; Shammas, H.J. Improving the second-eye refractive error in patients undergoing bilateral sequential cataract surgery. Ophthalmology 2012, 119, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Covert, D.J.; Henry, C.R.; Koenig, S.B. Intraocular lens power selection in the second eye of patients undergoing bilateral, sequential cataract extraction. Ophthalmology 2010, 117, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.M.J.; Barrett, G.D. Using the first-eye prediction error in cataract surgery to refine the refractive outcome of the second eye. J. Cataract Refract. Surg. 2019, 45, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, J.; Irwig, L.; Macaskill, P.; Hennessy, M.P. Intraocular lens power in bilateral cataract surgery: Whether adjusting for error of predicted refraction in the first eye improves prediction in the second eye. J. Cataract Refract. Surg. 2006, 32, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Aristodemou, P.; Knox Cartwright, N.E.; Sparrow, J.M.; Johnston, R.L. First eye prediction error improves second eye refractive outcome results in 2129 patients after bilateral sequential cataract surgery. Ophthalmology 2011, 118, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Muthappan, V.; Paskowitz, D.; Kazimierczak, A.; Jun, A.S.; Ladas, J.; Kuo, I.C. Measurement and use of postoperative anterior chamber depth of fellow eye in refractive outcomes. J. Cataract Refract. Surg. 2015, 41, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Klein, É.; Malecaze, F.; Bart, V.; Barnoud, A.; Lévy, P.; Gauthier, L.; Fournié, P. New polynomial regression formula to improve second-eye refractive outcomes in sequential bilateral cataract surgery. J. Cataract Refract. Surg. 2022, 48, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Holladay, J.T.; Wilcox, R.R.; Koch, D.D.; Wang, L. Review and recommendations for univariate statistical analysis of spherical equivalent prediction error for IOL power calculations. J. Cataract Refract. Surg. 2021, 47, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.C.; Hütz, W.W. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J. Cataract Refract. Surg. 2010, 36, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Haigis, W.; Lege, B.; Miller, N.; Schneider, B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2000, 238, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Debellemanière, G.; Dubois, M.; Gauvin, M.; Wallerstein, A.; Brenner, L.F.; Rampat, R.; Saad, A.; Gatinel, D. The PEARL-DGS Formula: The Development of an Open-source Machine Learning-based Thick IOL Calculation Formula. Am. J. Ophthalmol. 2021, 232, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, M.; Feng, R.; Liang, F.; Liu, X.; He, C.; Fan, S. Comparison of Formula-Specific Factors and Artificial Intelligence Formulas with Axial Length Adjustments in Bilateral Cataract Patients with Long Axial Length. Ophthalmol. Ther. 2022, 11, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Wendelstein, J.A.; Reifeltshammer, S.A.; Hoffmann, P.C.; Fischinger, I.; Mariacher, S.; Bolz, M.; Langenbucher, A.; Hirnschall, N. Project Hyperopic Power Prediction II: The Effects of Second Eye Refinement Methods on Prediction Error in Hyperopic Eyes. Curr. Eye Res. 2022, 47, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.; Løgstrup, N.; Olesen, H.; Corydon, L. Using the surgical result in the first eye to calculate intraocular lens power for the second eye. J. Cataract Refract. Surg. 1993, 19, 36–39. [Google Scholar] [CrossRef] [PubMed]
- ISO 11979-2:2014; Ophthalmic Implants—Intraocular Lenses—Part 2: Optical Properties and Test Methods. ISO: Geneva, Switzerland, 2014. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/05/56/55682.html (accessed on 11 November 2022).
- Zudans, J.V.; Desai, N.R.; Trattler, W.B. Comparison of prediction error: Labeled versus unlabeled intraocular lens manufacturing tolerance. J. Cataract Refract. Surg. 2012, 38, 394–402. [Google Scholar] [CrossRef] [PubMed]

| Training Set (n = 878) | Test Set (n = 1500) | |
|---|---|---|
| Female sex, n (%) | 474 (54.0) | 802 (53.5) |
| Right eye, n (%) | 439 (50.0) | 726 (48.4) |
| Age (y) | 57.1 ± 6.1 | 57.2 ± 6.2 |
| IOL power (D) | 22.29 ± 3.0 | 22.27 ± 2.9 |
| AL (mm) | 23.4 ± 1.08 | 23.37 ± 1.07 |
| Mean keratometry (D) | 43.21 ± 1.37 | 43.37 ± 1.48 |
| Anterior chamber depth (mm) | 3.18 ± 0.33 | 3.17 ± 0.33 |
| Lens thickness (mm) | 4.4 ± 0.31 | 4.41 ± 0.32 |
| Central corneal thickness (mm) | 0.549 ± 0.033 | 0.55 ± 0.033 |
| Corneal diameter (mm) | 12.25 ± 0.4 | 12.23 ± 0.41 |
| Postoperative SE (D) | −0.127 ± 0.36 | −0.158 ± 0.39 |
| Sd | ME | MAE | Δ MAE/Base Formula (Wilcoxon p-Value) | Δ MAE CF/LP (Wilcoxon p-Value) | Med AE | Min | Max | |
|---|---|---|---|---|---|---|---|---|
| Haigis—Triple Optimized | 0.394 | 0.031 | 0.302 | - | - | 0.244 | −1.557 | 2.358 |
| Haigis—LP | 0.313 | 0.01 | 0.236 | −0.066 (<0.001) | - | 0.186 | −1.819 | 1.502 |
| Haigis—CF | 0.308 | 0.01 | 0.231 | −0.071 (<0.001) | −0.005 (<0.001) | 0.185 | −1.564 | 1.544 |
| Pearl | 0.348 | 0.015 | 0.262 | - | - | 0.208 | −1.58 | 2.132 |
| Pearl—LP | 0.294 | 0.028 | 0.222 | −0.04 (<0.001) | - | 0.173 | −1.296 | 1.628 |
| Pearl—CF | 0.292 | 0.007 | 0.219 | −0.43 (<0.001) | −0.003 (>0.05) | 0.174 | −1.269 | 1.574 |
| Barrett U II | 0.378 | 0.006 | 0.284 | - | - | 0.225 | −1.745 | 2.235 |
| Barrett U II—CF | 0.302 | −0.001 | 0.226 | −0.058 (<0.001) | - | 0.18 | −1.36 | 1.896 |
| PEARL-LP | PEARL-CF | Haigis-LP | Haigis-CF | Barrett U II-CF | |
|---|---|---|---|---|---|
| Highly Beneficial | 1.67 | 0.87 | 3 | 3.33 | 2.07 |
| Beneficial | 27.13 | 27.73 | 32.13 | 33.27 | 31.93 |
| Neutral | 55.2 | 58.33 | 49.53 | 47.73 | 51 |
| Detrimental | 15.47 | 12.73 | 14.6 | 14.87 | 14.4 |
| Highly Detrimental | 0.53 | 0.33 | 0.73 | 0.8 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechleb, N.; Debellemanière, G.; Gauvin, M.; Wallerstein, A.; Saad, A.; Gatinel, D. Using the First-Eye Back-Calculated Effective Lens Position to Improve Refractive Outcome of the Second Eye. J. Clin. Med. 2023, 12, 184. https://doi.org/10.3390/jcm12010184
Mechleb N, Debellemanière G, Gauvin M, Wallerstein A, Saad A, Gatinel D. Using the First-Eye Back-Calculated Effective Lens Position to Improve Refractive Outcome of the Second Eye. Journal of Clinical Medicine. 2023; 12(1):184. https://doi.org/10.3390/jcm12010184
Chicago/Turabian StyleMechleb, Nicole, Guillaume Debellemanière, Mathieu Gauvin, Avi Wallerstein, Alain Saad, and Damien Gatinel. 2023. "Using the First-Eye Back-Calculated Effective Lens Position to Improve Refractive Outcome of the Second Eye" Journal of Clinical Medicine 12, no. 1: 184. https://doi.org/10.3390/jcm12010184
APA StyleMechleb, N., Debellemanière, G., Gauvin, M., Wallerstein, A., Saad, A., & Gatinel, D. (2023). Using the First-Eye Back-Calculated Effective Lens Position to Improve Refractive Outcome of the Second Eye. Journal of Clinical Medicine, 12(1), 184. https://doi.org/10.3390/jcm12010184








