Asymmetric Intrastromal Corneal Ring Segments with Progressive Base Width and Thickness for Keratoconus: Evaluation of Efficacy and Analysis of Epithelial Remodeling
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Examination Protocol
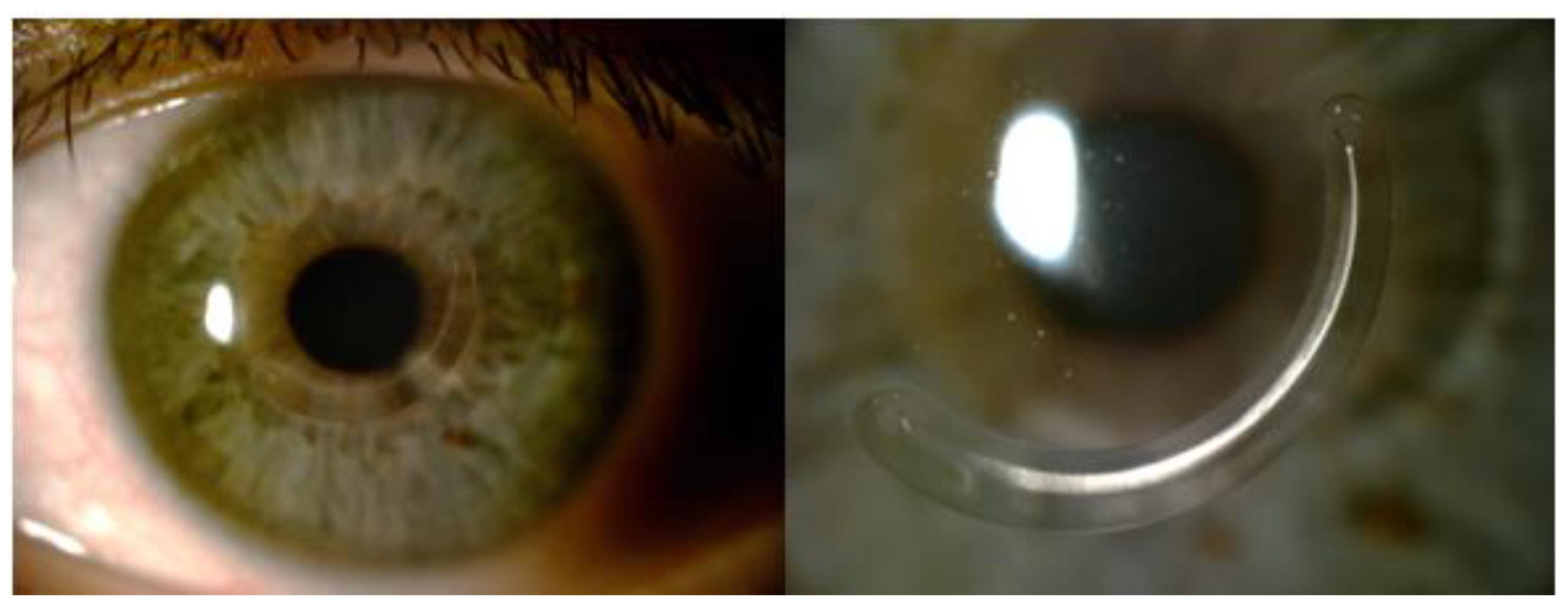
2.3. Surgical Technique
2.4. Intrastromal Corneal Ring Segments
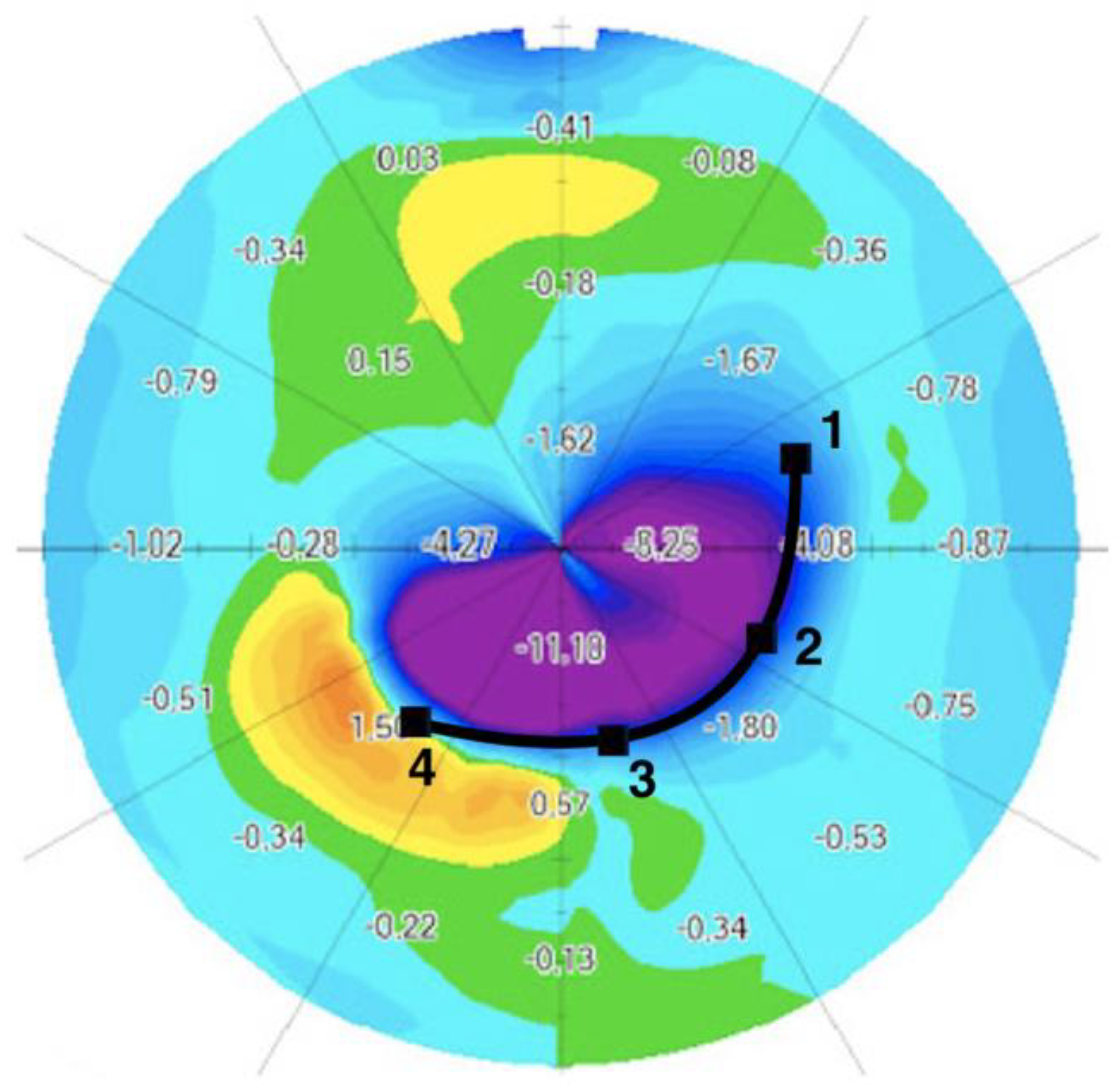
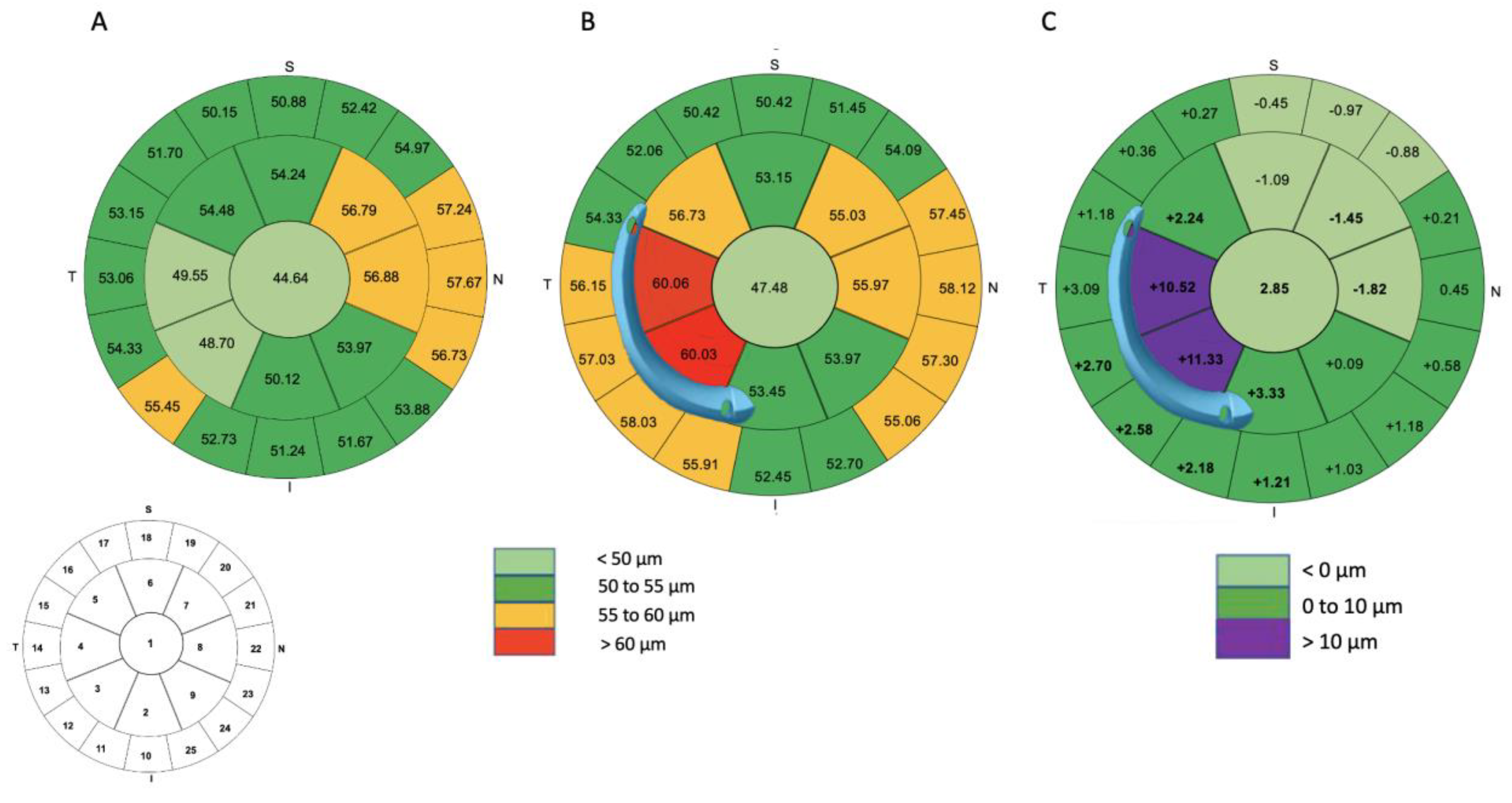
2.5. Differential Map
2.6. Corneal Aberrations
2.7. Data Analysis
2.8. Safety and Efficacy
2.9. Statistical Analysis
3. Results
3.1. Study Population
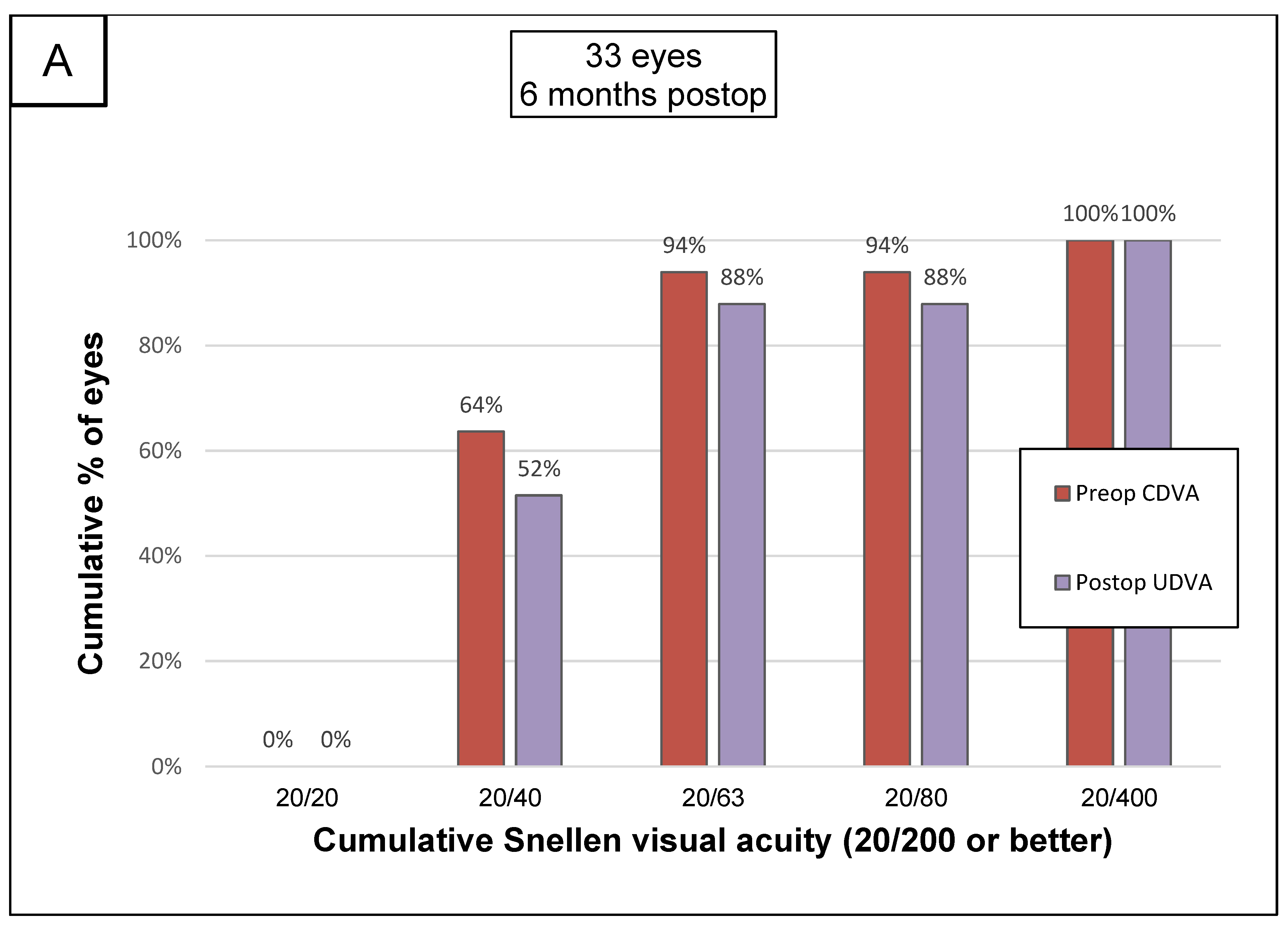
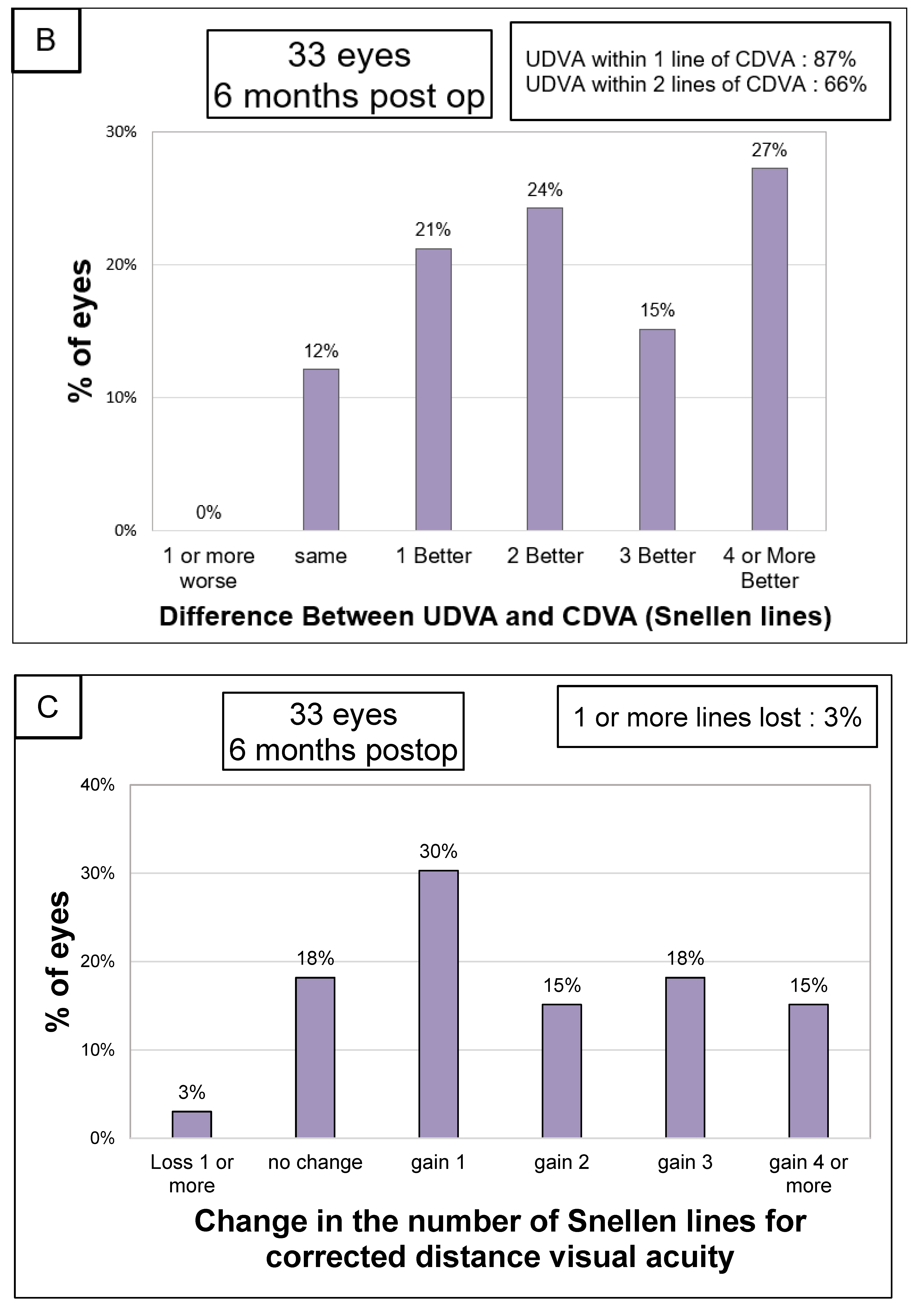
3.2. Visual Acuity and Refraction
3.3. Differential Map Analysis
3.4. Safety and Efficacity
3.5. Topographic Results
3.6. Pachymetric Analysis
3.7. Aberrometry
3.8. Correlation
4. Discussion
4.1. Clinical, Topographic and Aberrometric Parameters
4.2. Pachymetric Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef] [PubMed]
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef] [PubMed]
- Benoist d’Azy, C.; Pereira, B.; Chiambaretta, F.; Dutheil, F. Efficacy of Different Procedures of Intra-Corneal Ring Segment Implantation in Keratoconus: A Systematic Review and Meta-Analysis. Transl. Vis. Sci. Technol. 2019, 8, 38. [Google Scholar]
- Sakellaris, D.; Balidis, M.; Gorou, O.; Szentmary, N.; Alexoudis, A.; Grieshaber, M.C.; Sagri, D.; Scholl, H.; Gatzioufas, Z. Intracorneal Ring Segment Implantation in the Management of Keratoconus: An Evidence-Based Approach. Ophthalmol. Ther. 2019, 8, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Vega-Estrada, A.; Alio, J.L.; Brenner, L.F.; Javaloy, J.; Plaza Puche, A.B.; Barraquer, R.I.; Teus, M.A.; Murta, J.; Henriques, J.; Uceda-Montanes, A. Outcome analysis of intracorneal ring segments for the treatment of keratoconus based on visual, refractive, and aberrometric impairment. Am. J. Ophthalmol. 2013, 155, 575–584.e1. [Google Scholar] [CrossRef] [PubMed]
- Vega-Estrada, A.; Chorro, E.; Sewelam, A.; Alio, J.L. Clinical Outcomes of a New Asymmetric Intracorneal Ring Segment for the Treatment of Keratoconus. Cornea 2019, 38, 1228–1232. [Google Scholar] [CrossRef]
- Park, S.E.; Tseng, M.; Lee, J.K. Effectiveness of intracorneal ring segments for keratoconus. Curr. Opin. Ophthalmol. 2019, 30, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Piñero, D.P.; Alio, J.L.; Teus, M.A.; Barraquer, R.I.; Uceda-Montañés, A. Modeling the intracorneal ring segment effect in keratoconus using refractive, keratometric, and corneal aberrometric data. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5583–5591. [Google Scholar] [CrossRef]
- Barugel, R.; David, C.; Kallel, S.; Borderie, M.; Cuyaubère, R.; Goemaere, I.; Borderie, V.; Bouheraoua, N. Comparative Study of Asymmetric Versus Non-asymmetric Intrastromal Corneal Ring Segments for the Management of Keratoconus. J. Refract. Surg. 2021, 37, 552–561. [Google Scholar] [CrossRef]
- Fernández-Vega Cueto, L.; Lisa, C.; Poo-López, A.; Madrid-Costa, D.; Merayo-Lloves, J.; Alfonso, J.F. Intrastromal Corneal Ring Segment Implantation in 409 Paracentral Keratoconic Eyes. Cornea 2016, 35, 1421–1426. [Google Scholar] [CrossRef]
- Esaka, Y.; Kojima, T.; Dogru, M.; Hasegawa, A.; Tamaoki, A.; Uno, Y.; Nishida, T.; Nakamura, T.; Hara, S.; Ichikawa, K. Prediction of Best-Corrected Visual Acuity with Swept-Source Optical Coherence Tomography Parameters in Keratoconus. Cornea 2019, 38, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, H.; Piñero, D.P.; Álvarez de Toledo, J.; Barraquer, R.I.; García de Oteyza, G. Clinical Outcomes of Femtosecond Laser-Assisted Implantation of Asymmetric ICRS in Keratoconus with No Coincidence of Topographic and Comatic Axes. J. Refract. Surg. 2021, 37, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Sardiña, R.C.; Arango, A.; Alfonso, J.F.; Álvarez de Toledo, J.; Piñero, D.P. Prospective clinical evaluation of the effectiveness of asymmetric intracorneal ring with variable thickness and width for the management of keratoconus. J. Cataract Refract. Surg. 2021, 47, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.M.; Marques, J.H.; Neves, M.M.; Gomes, M.; Oliveira, L. Asymmetric Thickness Intracorneal Ring Segments for Keratoconus. Clin. Ophthalmol. 2020, 14, 4415–4421. [Google Scholar] [CrossRef] [PubMed]
- Prisant, O.; Pottier, E.; Guedj, T.; Hoang Xuan, T. Clinical Outcomes of an Asymmetric Model of Intrastromal Corneal Ring Segments for the Correction of Keratoconus. Cornea 2020, 39, 155–160. [Google Scholar] [CrossRef]
- Coskunseven, E.; Jankov, M.R.; Grentzelos, M.A.; Plaka, A.D.; Limnopoulou, A.N.; Kymionis, G.D. Topography-guided transepithelial PRK after intracorneal ring segments implantation and corneal collagen CXL in a three-step procedure for keratoconus. J. Refract. Surg. 2013, 29, 54–58. [Google Scholar] [CrossRef]
- Lee, H.; Kang, D.S.Y.; Ha, B.J.; Choi, J.Y.; Kim, E.K.; Seo, K.Y.; Kim, T. Visual rehabilitation in moderate keratoconus: Combined corneal wavefront-guided transepithelial photorefractive keratectomy and high-fluence accelerated corneal collagen cross-linking after intracorneal ring segment implantation. BMC Ophthalmol. 2017, 17, 270. [Google Scholar] [CrossRef]
- Zeraid, F.M.; Jawkhab, A.A.; Al-Tuwairqi, W.S.; Osuagwu, U.L. Visual rehabilitation in low-moderate keratoconus: Intracorneal ring segment implantation followed by same-day topography-guided photorefractive keratectomy and collagen cross linking. Int. J. Ophthalmol. 2014, 7, 800–806. [Google Scholar]
- Kymionis, G.D.; Grentzelos, M.A.; Kankariya, V.P.; Liakopoulos, D.A.; Karavitaki, A.E.; Portaliou, D.M.; Tsoulnaras, K.I.; Pallikaris, I.G. Long-term results of combined transepithelial phototherapeutic keratectomy and corneal collagen crosslinking for keratoconus: Cretan protocol. J. Cataract Refract. Surg. 2014, 40, 1439–1445. [Google Scholar] [CrossRef]
- Grentzelos, M.A.; Kounis, G.A.; Diakonis, V.F.; Siganos, C.S.; Tsilimbaris, M.K.; Pallikaris, I.G.; Kymionis, G.D. Combined transepithelial phototherapeutic keratectomy and conventional photorefractive keratectomy followed simultaneously by corneal crosslinking for keratoconus: Cretan protocol plus. J. Cataract Refract. Surg. 2017, 43, 1257–1262. [Google Scholar] [CrossRef]
- Kanellopoulos, A.J.; Asimellis, G. Keratoconus management: Long-term stability of topography-guided normalization combined with high-fluence CXL stabilization (the Athens Protocol). J. Refract. Surg. 2014, 30, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Srivannaboon, S.; Holland, S.P. Epithelial and stromal changes induced by intacs examined by three-dimensional very high-frequency digital ultrasound. J. Refract. Surg. 2001, 17, 310–318. [Google Scholar] [CrossRef] [PubMed]
- David, C.; Reinstein, D.Z.; Archer, T.J.; Kallel, S.; Vida, R.S.; Goemaere, I.; Cuyaubère, R.; Borderie, M.; Laroche, L.; Borderie, V.; et al. Postoperative Corneal Epithelial Remodeling After Intracorneal Ring Segment Procedures for Keratoconus: An Optical Coherence Tomography Study. J. Refract. Surg. 2021, 37, 404–413. [Google Scholar] [CrossRef]
- Savini, G.; Schiano-Lomoriello, D.; Hoffer, K.J. Repeatability of automatic measurements by a new anterior segment optical coherence tomographer combined with Placido topography and agreement with 2 Scheimpflug cameras. J. Cataract Refract. Surg. 2018, 44, 471–478. [Google Scholar] [CrossRef]
- Vega-Estrada, A.; Mimouni, M.; Espla, E.; Alió Del Barrio, J.; Alio, J.L. Corneal Epithelial Thickness Intrasubject Repeatability and its Relation With Visual Limitation in Keratoconus. Am. J. Ophthalmol. 2019, 200, 255–262. [Google Scholar] [CrossRef]
- Alpins, N.A. A new method of analyzing vectors for changes in astigmatism. J. Cataract Refract. Surg. 1993, 19, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Alpins, N.A. Vector analysis of astigmatism changes by flattening, steepening, and torque. J. Cataract Refract. Surg. 1997, 23, 1503–1514. [Google Scholar] [CrossRef]
- Hersh, P.S.; Issa, R.; Greenstein, S.A. Corneal crosslinking and intracorneal ring segments for keratoconus: A randomized study of concurrent versus sequential surgery. J. Cataract Refract. Surg. 2019, 45, 830–839. [Google Scholar] [CrossRef]
- Serpe, C.C.; Mello, G.R.; Seven, I.; Dupps, W.J.J.; Santhiago, M.R. Results of intrastromal corneal ring segment implanted alone or combined with same-day corneal crosslinking and their correlation with preoperative corneal biomechanical strain from finite element analysis. J. Cataract Refract. Surg. 2021, 47, 916–926. [Google Scholar]
- Greenstein, S.A.; Chung, D.; Rosato, L.; Gelles, J.D.; Hersh, P.S. Corneal higher-order aberrations after crosslinking and intrastromal corneal ring segments for keratoconus. J. Cataract Refract. Surg. 2020, 46, 979–985. [Google Scholar] [CrossRef]
- Alió, J.L.; Shabayek, M.H. Corneal higher order aberrations: A method to grade keratoconus. J. Refract. Surg. 2006, 22, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, J.F.; Fernández-Vega Cueto, L.; Baamonde, B.; Merayo-Lloves, J.; Madrid-Costa, D.; Montés-Micó, R. Inferior intrastromal corneal ring segments in paracentral keratoconus with no coincident topographic and coma axis. J. Refract. Surg. 2013, 29, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Shabayek, M.H.; Alió, J.L. Intrastromal corneal ring segment implantation by femtosecond laser for keratoconus correction. Ophthalmology 2007, 114, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Vega-Estrada, A.; Alio, J.L. Keratoconus Corneal Posterior Surface Characterization According to the Degree of Visual Limitation. Cornea 2019, 38, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J. Refract. Surg. 2009, 25, 604–610. [Google Scholar]
- Vinciguerra, P.; Roberts, C.J.; Albé, E.; Romano, M.R.; Mahmoud, A.; Trazza, S.; Vinciguerra, R. Corneal curvature gradient map: A new corneal topography map to predict the corneal healing process. J. Refract. Surg. 2014, 30, 202–207. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M. Rate of change of curvature of the corneal stromal surface drives epithelial compensatory changes and remodeling. J. Refract Surg. 2014, 30, 799–802. [Google Scholar] [CrossRef]
- Franco, J.; White, C.A.; Kruh, J.N. Analysis of Compensatory Corneal Epithelial Thickness Changes in Keratoconus Using Corneal Tomography. Cornea 2020, 39, 298–302. [Google Scholar] [CrossRef]
- Zhou, W.; Reinstein, D.Z.; Archer, T.J.; Nitter, T.; Feng, Y.; Mule, G.; Stojanovic, A. The Impact of Epithelial Remodeling on Surgical Techniques Used in Topography-guided Surface Ablation in Irregular Corneas. J. Refract. Surg. 2022, 38, 529–537. [Google Scholar] [CrossRef]
- Kremer, I.; Aizenman, I.; Lichter, H.; Shayer, S.; Levinger, S. Simultaneous wavefront-guided photorefractive keratectomy and corneal collagen crosslinking after intrastromal corneal ring segment implantation for keratoconus. J. Cataract Refract. Surg. 2012, 38, 1802–1807. [Google Scholar] [CrossRef]
- Nguyen, N.; Gelles, J.D.; Greenstein, S.A.; Hersh, P.S. Incidence and associations of intracorneal ring segment explantation. J. Cataract Refract. Surg. 2019, 45, 153–158. [Google Scholar] [CrossRef] [PubMed]
| Parameters | |
|---|---|
| Age (years) | 32.42 ± 10.53 [18 to 60] |
| Sex | |
| Female | 17 |
| Male | 16 |
| Keratoconus type | |
| Duck | 33 |
| Amsler–Krumeich stage | |
| I | 20 |
| II | 11 |
| III | 1 |
| IV | 1 |
| UDVA (LogMAR) | 0.75 ± 0.38 [0.10 to 1.40] |
| UDVA (Snellen) | 20/100 [20/500 to 20/25] |
| CDVA (LogMAR) | 0.30 ±0.18 [0.0 to 7.00] |
| CDVA (Snellen) | 20/40 [20/100 to 20/25] |
| K mean at 3 mm (D) | 47.95 ±3.94 [40.32 to 60.67] |
| Sphere (D) | −3.64 ± 3.36 [−11.75 to 2.25] |
| Cylinder (D) | −3.73 ± 2.16 [−8.75 to −0.75] |
| Spherical equivalent (D) | −5.51 ± 3.68 [−15.13 to 1.25] |
| Minimum corneal thickness (μm) | 437 ± 39.38 [366 to 570] |
| Symmetry index front | 10.31 ± 3.14 [3.14 to 15.69] |
| Parameters | Preoperative | M6 | Δ | p-Value * |
|---|---|---|---|---|
| UDVA (LogMAR) | 0.75 ± 0.38 [0.10 to 1.40] | 0.37 ± 0.24 [0.00 to 1.00] | −0.38 ± 0.29 [−1.10 to 0.00] | <0.001 |
| UDVA (Snellen) | 20/100 [20/500 to 20/25] | 20/50 [20/200 to 20/20] | ||
| CDVA (LogMar) | 0.32 ± 0.19 [0.0 to 7.00] | 0.12 ± 0.12 [0.10 to 0.40] | −0.20 ± 0.19 [−0.40 to 0.10] | <0.001 |
| CDVA (Snellen) | 20/40 [20/100 to 20/20] | 20/25 [20/40 to 20/20] | ||
| Sphere (D) | −3.64 ± 3.36 [−11.75 to 2.25] | −0.87 ± 2.63 [−7.00 to 4.75] | 2.77 ± 3.01 [−3.50 to 11.50] | <0.001 |
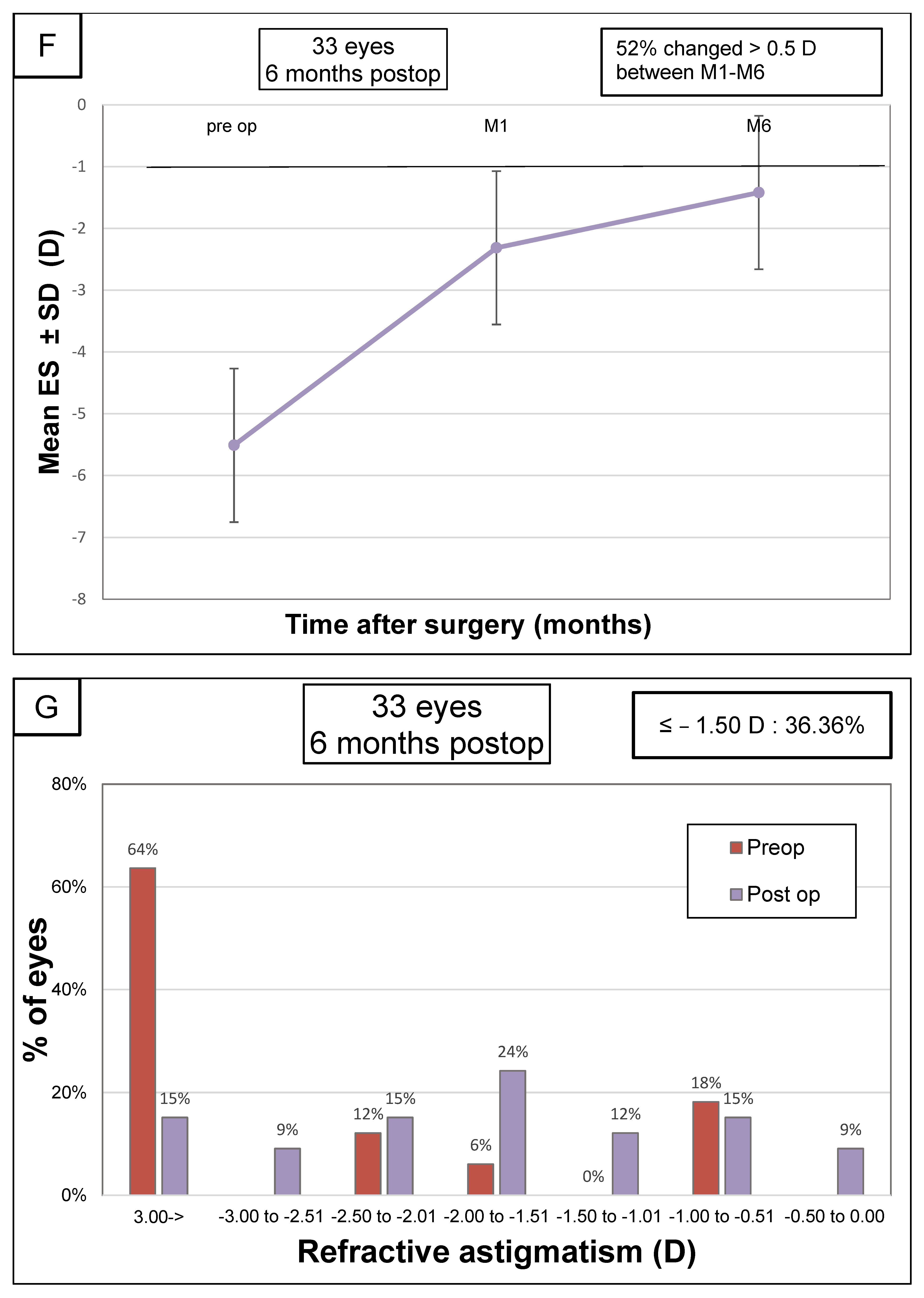
| Refractive cylinder (D) | −3.73 ± 2.16 [−8.75 to −0.75] | −1.99 ± 1.10 [−5.00 to −0.25] | 1.74 ± 2.23 [−4.00 to 6.25] | <0.001 |
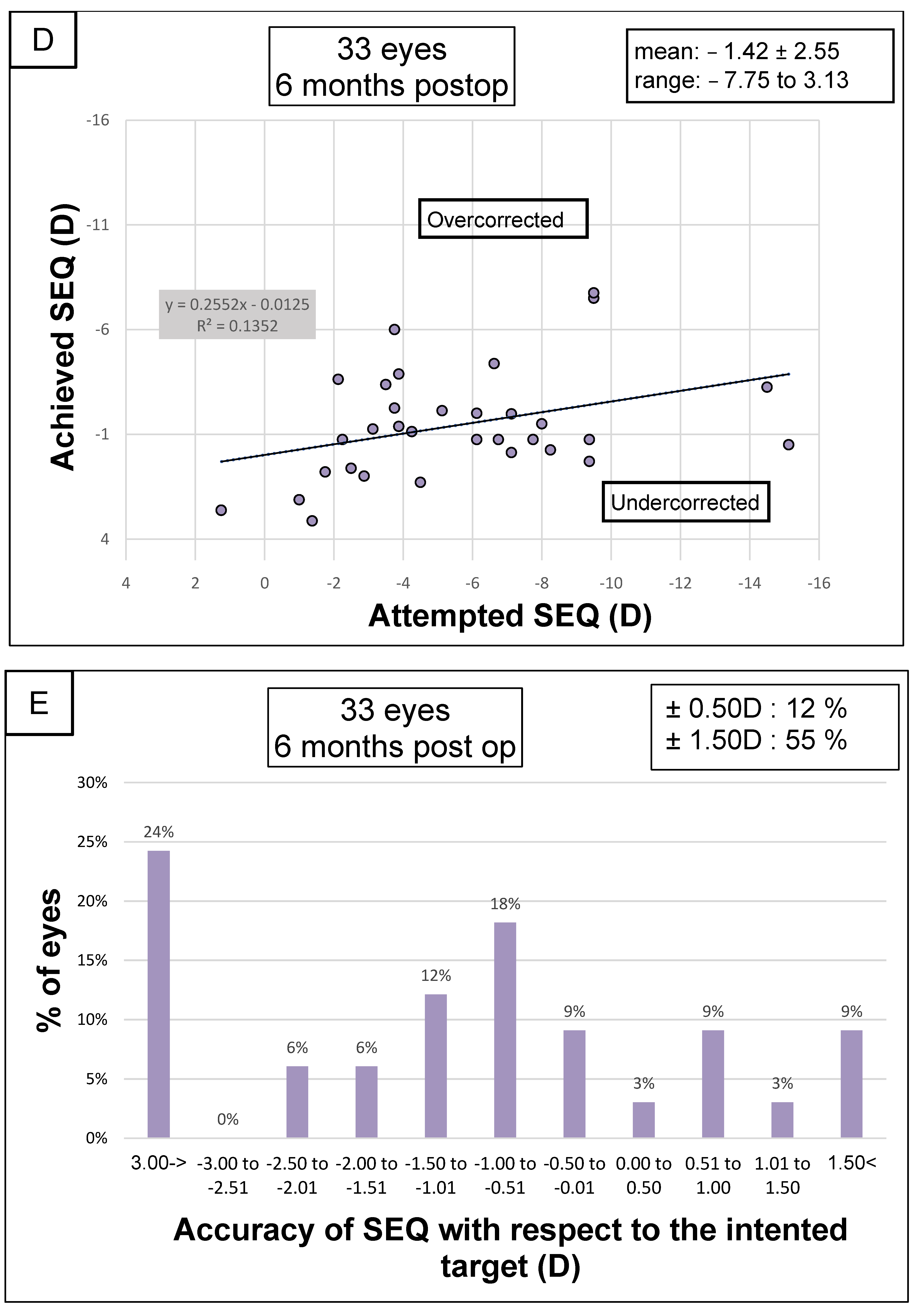
| Spherical equivalent (D) | −5.51 ± 3.68 [−15.13 to 1.25] | −1.42 ± 2.55 [−7.75 to 3.13] | 4.09 ± 3.62 [−2.25 to 14.63] | <0.001 |
| Preoperative | M6 | Δ | p-Value * | |
|---|---|---|---|---|
| Central 3 mm | ||||
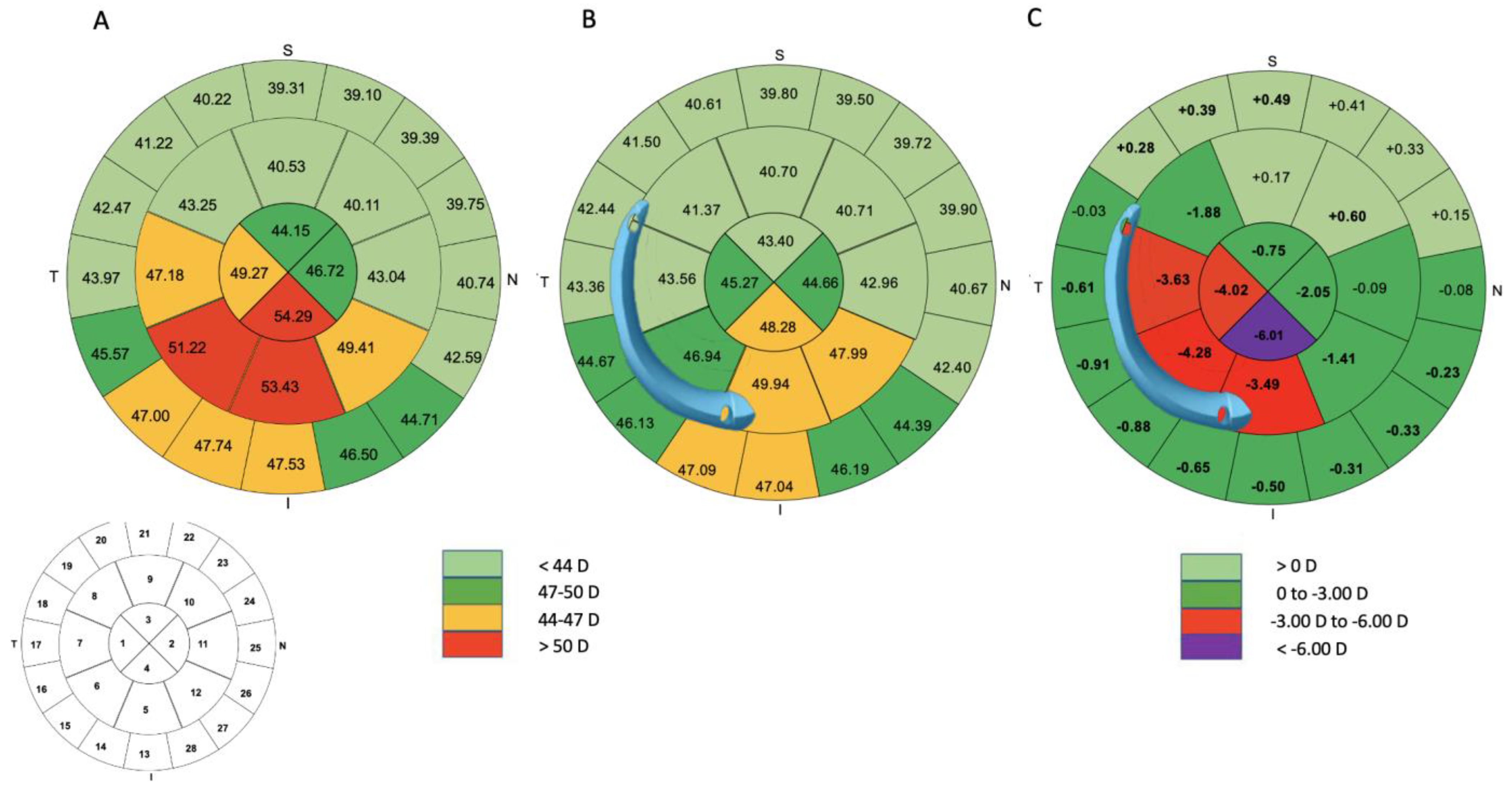
| Zone 1 (D) | 49.29 ± 4.43 [42.41 to 63.70] | 45.27 ± 3.50 [39.50 to 54.27] | −4.02 ± 3.03 [−9.68 to 1.58] | <0.001 |
| Zone 2 (D) | 46.72 ± 4.35 [38.22 to 57.64] | 44.66 ± 3.44 [38.97 to 54.01] | −2.05 ± 2.73 [−8.41 to 4.10] | <0.001 |
| Zone 3 (D) | 44.15 ± 5.18 [32.57 to 56.10] | 43.40 ± 4.55 [32.76 to 53.76] | −0.75 ± 1.83 [−6.24 to 4.59] | 0.01 |
| Zone 4 (D) | 54.29 ± 5.84 [45.44 to 70.83] | 48.28 ± 7.24 [36.99 to 69.07] | −6.01 ± 4.85 [−13.70 to 5.64] | <0.001 |
| Central 3 to 5 mm | ||||
| Zone 5 (D) | 53.43 ± 3.46 [45.97 to 64.06] | 49.94 ± 3.72 [38.39 to 55.70] | −3.49 ± 4.02 [−17.65 to 1.74] | <0.001 |
| Zone 6 (D) | 51.22 ± 3.81 [43.88 to 63.73] | 46.94 ± 3.45 [38.73 to 53.46] | −4.28 ± 2.57 [−10.27 to −1.00] | <0.001 |
| Zone 7 (D) | 47.18 ± 4.05 [40.80 to 58.43] | 43.56 ± 3.13 [38.31 to 50.00] | −3.63 ± 2.58 [−12.71 to 0.30] | <0.001 |
| Zone 8 (D) | 43.25 ± 3.46 [36.53 to 50.93] | 41.37 ± 3.11 [35.18 to 47.63] | −1.88 ± 1.38 [−4.93 to 0.92] | <0.001 |
| Zone 9 (D) | 40.53 ± 2.92 [33.79 to 46.73] | 40.70 ± 3.06 [33.92 to 46.56] | 0.17 ± 0.87 [−1.92 to 2.62] | 0.13 |
| Zone 10 (D) | 40.11 ± 2.73 [32.80 to 45.88] | 40.71 ± 2.85 [33.96 to 46.25] | 0.60 ± 0.62 [−0.54 to 2.04] | <0.001 |
| Zone 11 (D) | 43.04 ± 2.64 [36.28 to 47.20] | 42.96 ± 2.55 [36.98 to 46.82] | −0.09 ± 0.86 [−1.91 to 1.29] | 0.28 |
| Zone 12 (D) | 49.41 ± 2.94 [43.95 to 57.30] | 47.99 ± 3.15 [39.03 to 53.58] | −1.41 ± 2.66 [−10.32 to 2.50] | 0.002 |
| Central 5 to 8 mm | ||||
| Zone 13 (D) | 47.53 ± 2.49 [42.77 to 53.41] | 47.04 ± 2.52 [41.70 to 52.72] | −0.50 ± 0.75 [−3.20 to 0.97] | <0.001 |
| Zone 14 (D) | 47.74 ± 2.56 [43.12 to 53.44] | 47.09 ± 2.57 [42.07 to 52.26] | −0.65 ± 0.82 [−3.94 to 0.82] | <0.001 |
| Zone 15 (D) | 47.00 ± 2.65 [43.47 to 53.54] | 46.13 ± 2.65 [41.60 to 52.16] | −0.88 ± 0.79 [−3.83 to 0.01] | <0.001 |
| Zone 16 (D) | 45.57 ± 2.80 [40.84 to 53.31] | 44.67 ± 2.77 [40.47 to 51.48] | −0.91 ± 0.73 [−3.17 to 0.54] | <0.001 |
| Zone 17 (D) | 43.97 ± 2.87 [38.64 to 52.06] | 43.36 ± 2.87 [38.86 to 50.71] | −0.61 ± 0.86 [−2.33 to 1.90] | <0.001 |
| Zone 18 (D) | 42.47 ± 2.79 [37.44 to 49.51] | 42.44 ± 2.99 [37.21 to 50.77] | −0.03 ± 0.81 [−1.33 to 1.29] | 0.42 |
| Zone 19 (D) | 41.22 ± 2.70 [36.85 to 47.24] | 41.50 ± 2.75 [36.17 to 48.21] | 0.28 ± 0.67 [−0.98 to 1.34] | 0.01 |
| Zone 20 (D) | 40.22 ± 2.58 [35.60 to 46.30] | 40.61 ± 2.58 [35.44 to 45.60] | 0.39 ± 0.72 [−0.92 to 2.41] | 0.002 |
| Zone 21 (D) | 39.31 ± 2.74 [33.97 to 45.86] | 39.80 ± 2.75 [34.70 to 45.30] | 0.49 ± 1.01 [−2.65 to 2.16] | 0.004 |
| Zone 22 (D) | 39.10 ± 2.58 [34.09 to 45.89] | 39.50 ± 2.88 [32.00 to 45.39] | 0.41 ± 1.16 [−4.00 to 2.85] | 0.03 |
| Zone 23 (D) | 39.39 ± 2.58 [34.04 to 46.02] | 39.72 ± 2.84 [32.77 to 45.67] | 0.33 ± 1.11 [−4.53 to 2.48] | 0.05 |
| Zone 24 (D) | 39.75 ± 2.53 [34.69 to 45.89] | 39.90 ± 2.49 [34.79 to 45.46] | 0.15 ± 0.60 [−1.30 to 1.21] | 0.07 |
| Zone 25 (D) | 40.74 ± 2.29 [35.88 to 45.96] | 40.67 ± 2.30 [35.98 to 45.45] | −0.08 ± 0.62 [−1.53 to 1.08] | 0.26 |
| Zone 26 (D) | 42.59 ± 2.26 [37.97 to 46.12] | 42.40 ± 2.22 [37.39 to 46.16] | −0.23 ± 0.58 [−1.77 to 1.10] | 0.02 |
| Zone 27 (D) | 44.71 ± 2.26 [40.72 to 49.01] | 44.39 ± 2.66 [37.97 to 49.30] | −0.33 ± 0.99 [−4.81 to 1.26] | 0.03 |
| Zone 28 (D) | 46.50 ± 2.41 [42.75 to 51.53] | 46.19 ± 2.54 [40.95 to 51.19] | −0.31 ± 0.88 [−2.19 to 1.99] | 0.02 |
| Preoperative | M6 | Δ | p-Value * | |
|---|---|---|---|---|
| Anterior surface | ||||
| K1 (D) | 45.63 ± 3.73 [38.58 to 57.78] | 43.61 ± 3.52 [37.88 to 52.90] | −2.02 ± 2.17 [−5.97 to 1.57] | <0.001 |
| K2 (D) | 50.57 ± 4.48 [42.10 to 63.86] | 46.38 ± 3.89 [40.53 to 57.05] | −4.18 ± 2.01 [−8.15 to −0.79] | <0.001 |
| Km 3 mm (D) | 47.95 ± 3.94 [40.32 to 60.67] | 44.95 ± 3.59 [39.32 to 54.90] | −3.00 ± 1.97 [−6.42 to 0.22] | <0.001 |
| Km 5 mm (D) | 46.98 ± 3.25 [40.09 to 57.14] | 44.32 ± 2.89 [39.80 to 51.97] | −2.66 ± 1.49 [−5.17 to 0.02] | <0.001 |
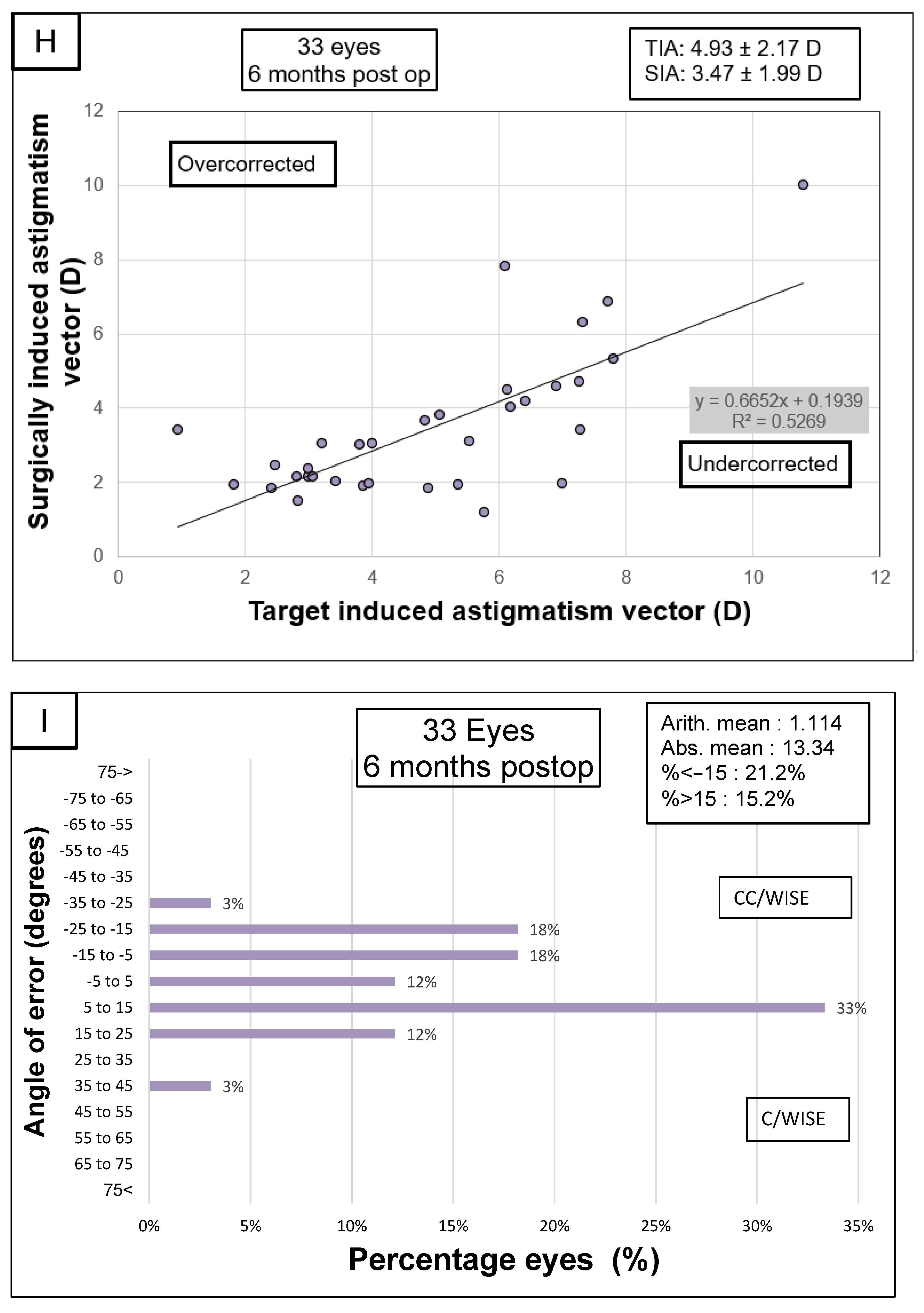
| Astigmatism 3 mm (D) | 4.93 ± 2.17 [0.93 to 10.79] | 2.74 ± 1.63 [0.98 to 8.21] | −2.19 ± 1.57 [−5.17 to 1.89] | <0.001 |
| Astigmatism 5 mm (D) | 3.64 ± 1.62 [1.17 to 7.53] | 2.22 ± 1.46 [0.45 to 7.32] | −1.43 ± 1.04 [−3.98 to 0.60] | <0.001 |
| Q-factor (8 mm) | −0.96 ± 0.46 [−1.78 to −0.18] | −0.26 ± 0.60 [−1.67 to 0.75] | 0.67 ± 0.53 [−1.03 to 1.69] | <0.001 |
| SIF 3 mm (D) | 10.31 ± 3.14 [3.14 to 15.69] | 6.42 ± 3.08 [0.71 to 12.94] | −3.89 ± 2.83 [−11.79 to 0.73] | <0.001 |
| Posterior surface | ||||
| K1 (D) | −6.94 ± 0.78 [−9.42 to −5.85] | −6.88 ± 0.71 [ −8.89 to −5.90] | 0.06 ± 0.32 [−0.48 to 0.83] | 0.15 |
| K2 (D) | −7.80 ± 0.90 [−10.47 to −6.45] | −7.39 ± 0.73 [−9.31 to −6.38] | 0.40 ± 0.35 [−0.12 to 1.16] | <0.001 |
| Km 3 mm (D) | −7.35 ± 0.80 [−9.91 to −6.16] | −7.14 ± 0.71 [−9.09 to −6.29] | 0.21 ± 0.28 [−0.17 to 0.83] | <0.001 |
| Km 5 mm (D) | −6.95 ± 0.61 [−8.85 to −6.07] | −6.77 ± 0.54 [−8.24 to −6.03] | 0.19 ± 0.18 [−0.09 to 0.61] | <0.001 |
| Astigmatism 3 mm (D) | −0.85 ± 0.39 [−2.32 to −0.16] | −0.53 ± 0.30 [−1.35 to −0.07] | 0.32 ± 0.32 [−0.69 to 0.97] | <0.001 |
| Astigmatism 5 mm (D) | −0.61 ± 0.28 [−1.59 to −0.12] | −0.39 ± 0.20 [−0.90 to −0.08] | 0.22 ± 0.21 [−0.17 to 0.69] | <0.001 |
| Q-factor (8 mm) | −1.26 ± 0.56 [−2.30 to −0.17] | −1.67 ± 0.71 [−3.88 to −0.56] | −0.41 ± 0.41 [−1.86 to 0.38] | <0.001 |
| Q-factor (5 mm) | −1.30 ± 1.24 [−3.65 to 2.52] | −2,58 ± 2.83 [−7.72 to 3.72] | −1.28 ± 2.86 [−6.73 to 3.53] | 0.007 |
| Preoperative | M6 | Δ | p-Value * | |
|---|---|---|---|---|
| Central 3 mm | ||||
| Zone 1 (μm) | 44.64 ± 4.26 [36.00 to 54.00] | 47.48 ± 6.85 [37.00 to 64.00] | 2.85 ± 5.93 [−8.00 to 18.00] | 0.004 |
| Central 3 to 5 mm | ||||
| Zone 2 (μm) | 50.12 ± 4.88 [39.00 to 62.00] | 53.45 ± 8.97 [39.00 to 83.00] | 3.33 ± 9.19 [−8.00 to 36.00] | 0.02 |
| Zone 3 (μm) | 48.70 ± 4.47 [38.00 to 56.00] | 60.03 ± 11.95 [44.00 to 91.00] | 11.33 ± 12.95 [−8.00 to 45.00] | <0.001 |
| Zone 4 (μm) | 49.55 ± 4.27 [39.00 to 59.00] | 60.06 ± 11.00 [45.00 to 90.00] | 10.52 ± 12.46 [−9.00 to 39.00] | <0.001 |
| Zone 5 (μm) | 54.48 ± 4.89 [41.00 to 64.00] | 56.73 ± 7.40 [40.00 to 73.00] | 2.24 ± 5.67 [−11.00 to 15.00] | 0.01 |
| Zone 6 (μm) | 54.24 ± 5.20 [46.00 to 70.00] | 53.15 ± 4.52 [46.00 to 63.00] | −1.09 ± 4.96 [−11.00 to 12.00] | 0.09 |
| Zone 7 (μm) | 56.48 ± 4.80 [48.00 to 71.00] | 55.03 ± 5.82 [47.00 to 72.00] | −1.45 ± 4.84 [−12.00 to 15.00] | 0.04 |
| Zone 8 (μm) | 56.79 ± 5.57 [50.00 to 74.00] | 55.97 ± 5.85 [47.00 to 74.00] | −1.82 ± 4.33 [−11.00 to 6.00] | 0.01 |
| Zone 9 (μm) | 53.88 ± 4.14 [46.00 to 63.00] | 53.97 ± 8.33 [38.00 to 82.00] | 0.09 ± 6.24 [−8.00 to 22.00] | 0.47 |
| Central 5 to 8 mm | ||||
| Zone 10 (μm) | 51.24 ± 5.26 [40.00 to 61.00] | 52.45 ± 6.21 [42.00 to 66.00] | 1.21 ± 5.73 [−6.00 to 20.00] | 0.11 |
| Zone 11 (μm) | 52.73 ± 5.75 [39.00 to 64.00] | 55.91 ± 6.26 [43.00 to 73.00] | 2.18 ± 6.28 [−7.00 to 25.00] | 0.03 |
| Zone 12 (μm) | 55.45 ± 5.36 [43.00 to 68.00] | 58.03 ± 5.37 [48.00 to 75.00] | 2.58 ± 5.63 [−9.00 to 23.00] | 0.006 |
| Zone 13 (μm) | 54.33 ± 6.09 [37.00 to 65.00] | 57.03 ± 5.06 [46.00 to 65.00] | 2.70 ± 6.41 [−7.00 to 27.00] | 0.01 |
| Zone 14 (μm) | 53.06 ± 5.34 [36.00 to 62.00] | 56.15 ± 5.12 [44.00 to 67.00] | 3.09 ± 6.83 [−7.00 to 29.00] | 0.007 |
| Zone 15 (μm) | 53.15 ± 4.26 [42.00 to 63.00] | 54.33 ± 5.32 [46.00 to 69.00] | 1.18 ± 5.68 [−7.00 to 21.00] | 0.12 |
| Zone 16 (μm) | 51.70 ± 4.77 [35.00 to 62.00] | 52.06 ± 4.23 [46.00 to 62.00] | 0.36 ± 4.76 [−9.00 to 13.00] | 0.33 |
| Zone 17 (μm) | 50.15 ± 3.89 [42.00 to 59.00] | 50.42 ± 3.30 [44.00 to 56.00] | 0.27 ± 3.49 [−7.00 to 8.00] | 0.33 |
| Zone 18 (μm) | 50.88 ± 4.22 [44.00 to 58.00] | 50.42 ± 3.31 [42.00 to 55.00] | −0.45 ± 3.80 [−10.00 to 5.00] | 0.25 |
| Zone 19 (μm) | 52.42 ± 3.60 [46.00 to 59.00] | 51.45 ± 3.00 [47.00 to 59.00] | −0.97 ± 3.39 [−8.00 to 5.00] | 0.05 |
| Zone 20 (μm) | 54.97 ± 3.39 [49.00 to 61.00] | 54.09 ± 3.34 [47.00 to 62.00] | −0.88 ± 3.40 [−8.00 to 7.00] | 0.07 |
| Zone 21 (μm) | 57.24 ± 3.59 [47.00 to 66.00] | 57.45 ± 4.23 [50.00 to 70.00] | 0.21 ± 3.67 [−9.00 to 10.00] | 0.37 |
| Zone 22 (μm) | 57.67 ± 4.50 [48.00 to 67.00] | 58.12 ± 4.51 [49.00 to 67.00] | 0.45 ± 4.67 [−7.00 to 15.00] | 0.29 |
| Zone 23 (μm) | 56.73 ± 4.10 [46.00 to 65.00] | 57.30 ± 5.01 [45.00 to 67.00] | 0.58 ± 4.17 [−11.00 to 8.00] | 0.22 |
| Zone 24 (μm) | 53.88 ± 4.94 [42.00 to 63.00] | 55.06 ± 5.71 [44.00 to 66.00] | 1.18 ± 4.67 [−5.00 to 15.00] | 0.08 |
| Zone 25 (μm) | 51.67 ± 4.79 [41.00 to 61.00] | 52.70 ± 6.57 [40.00 to 65.00] | 1.03 ± 4.93 [−7.00 to 16.00] | 0.12 |
| Preoperative | M6 | Δ | p-Value * | |
|---|---|---|---|---|
| Total cornea | ||||
| RMS Total Aberrations (μm) | 2.84 ± 1.22 [0.87 to 4.68] | 2.33 ± 0.99 [0.44 to 4.89] | −0.51 ± 0.68 [−2.34 to 0.94] | <0.001 |
| RMS Total HOA (μm) | 1.93 ± 0.89 [0.43 to 3.65] | 1.69 ± 0.78 [0.32 to 3.53] | −0.24 ± 0.69 [−2.25 to 1.48] | 0.02 |
| RMS Spherical Aberration (μm) | −0.10 ± 0.21 [−0.49 to 0.48] | −0.03 ± 0.57 [−1.02 to 1.54] | 0.07 ± 0.50 [−1.00 to 1.39] | 0.20 |
| RMS Coma (μm) | 1.62 ± 0.81 [0.37 to 3.30] | 0.99 ± 0.59 [0.23 to 2.42] | −0.63 ± 0.74 [−2.50 to 0.67] | <0.001 |
| RMS Astigmatism (μm) | 1.99 ± 1.03 [0.66 to 4.14] | 1.48 ± 0.87 [0.12 to 4.36] | −0.51 ± 0.61 [−1.68 to 0.81] | <0.001 |
| RMS Trefoil (μm) | 0.73 ± 0.43 [0.08 to 1.71] | 0.56 ± 0.40 [0.06 to 1.61] | −0.17 ± 0.51 [−1.25 to 0.66] | 0.03 |
| Anterior cornea | ||||
| RMS Spherical Aberration (μm) | −0.10 ± 0.26 [−0.57 to 0.65] | 0.00 ± 0.43 [−0.83 to 1.29] | 0.10 ± 0.34 [−0.80 to 1.01] | 0.05 |
| RMS Coma (μm) | 2.12 ± 1.03 [0.43 to 4.14] | 1.37 ± 0.85 [0.15 to 3.00] | −0.75 ± 0.73 [−2.35 to 0.77] | <0.001 |
| Coma Axis (°) | 88.88 ± 18.47 [57.00 to 151.00] | 92.12 ± 25.74 [51.00 to 171.00] | −3.24 ± 26.48 [−58.00 to 96.00] | 0.24 |
| RMS Astigmatism (μm) | 2.46 ± 1.21 [0.86 to 5.20] | 1.61 ± 1.02 [0.36 to 5.32] | −0.86 ± 0.71 [−2.22 to 0.40] | <0.001 |
| RMS Trefoil (μm) | 0.87 ± 0.51 [0.10 to 1.91] | 0.51 ± 0.38 [0.09 to 1.70] | −0.36 ± 0.55 [−1.30 to 0.58] | <0.001 |
| Posterior cornea | ||||
| RMS Spherical Aberration (μm) | 0.00 ± 0.09 [−0.21 to 0.23] | −0.07 ± 0.24 [−0.56 to 0.27] | −0.07 ± 0.27 [−0.60 to 0.37] | 0.07 |
| RMS Coma (μm) | 0.50 ± 0.24 [0.08 to 1.07] | 0.59 ± 0.39 [0.02 to 1.54] | 0.08 ± 0.29 [−0.45 to 0.73] | 0.06 |
| Coma Axis (°) | 267.73 ± 17.79 [228.00 to 308.00] | 267.55 ± 36.10 [223.00 to 358.00] | 0.08 ± 25.62 [−79.00 to 32.00] | 0.48 |
| RMS Astigmatism (μm) | 0.51 ± 0.26 [0.15 to 1.45] | 0.36 ± 0.23 [0.05 to 1.12] | −0.15 ± 0.29 [−0.82 to 0.33] | 0.003 |
| RMS Trefoil (μm) | 0.16 ± 0.10 [0.02 to 0.51] | 0.25 ± 0.14 [0.02 to 0.51] | 0.09 ± 0.13 [−0.17 to 0.39] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benlarbi, A.; Kallel, S.; David, C.; Barugel, R.; Hays, Q.; Goemaere, I.; Cuyaubere, R.; Borderie, M.; Borderie, V.; Bouheraoua, N. Asymmetric Intrastromal Corneal Ring Segments with Progressive Base Width and Thickness for Keratoconus: Evaluation of Efficacy and Analysis of Epithelial Remodeling. J. Clin. Med. 2023, 12, 1673. https://doi.org/10.3390/jcm12041673
Benlarbi A, Kallel S, David C, Barugel R, Hays Q, Goemaere I, Cuyaubere R, Borderie M, Borderie V, Bouheraoua N. Asymmetric Intrastromal Corneal Ring Segments with Progressive Base Width and Thickness for Keratoconus: Evaluation of Efficacy and Analysis of Epithelial Remodeling. Journal of Clinical Medicine. 2023; 12(4):1673. https://doi.org/10.3390/jcm12041673
Chicago/Turabian StyleBenlarbi, Abdelmajid, Sofiene Kallel, Clementine David, Raphael Barugel, Quentin Hays, Isabelle Goemaere, Roxane Cuyaubere, Marie Borderie, Vincent Borderie, and Nacim Bouheraoua. 2023. "Asymmetric Intrastromal Corneal Ring Segments with Progressive Base Width and Thickness for Keratoconus: Evaluation of Efficacy and Analysis of Epithelial Remodeling" Journal of Clinical Medicine 12, no. 4: 1673. https://doi.org/10.3390/jcm12041673
APA StyleBenlarbi, A., Kallel, S., David, C., Barugel, R., Hays, Q., Goemaere, I., Cuyaubere, R., Borderie, M., Borderie, V., & Bouheraoua, N. (2023). Asymmetric Intrastromal Corneal Ring Segments with Progressive Base Width and Thickness for Keratoconus: Evaluation of Efficacy and Analysis of Epithelial Remodeling. Journal of Clinical Medicine, 12(4), 1673. https://doi.org/10.3390/jcm12041673






