Recent Clinical and Preclinical Advances in External Stimuli-Responsive Therapies for Head and Neck Squamous Cell Carcinoma
Abstract
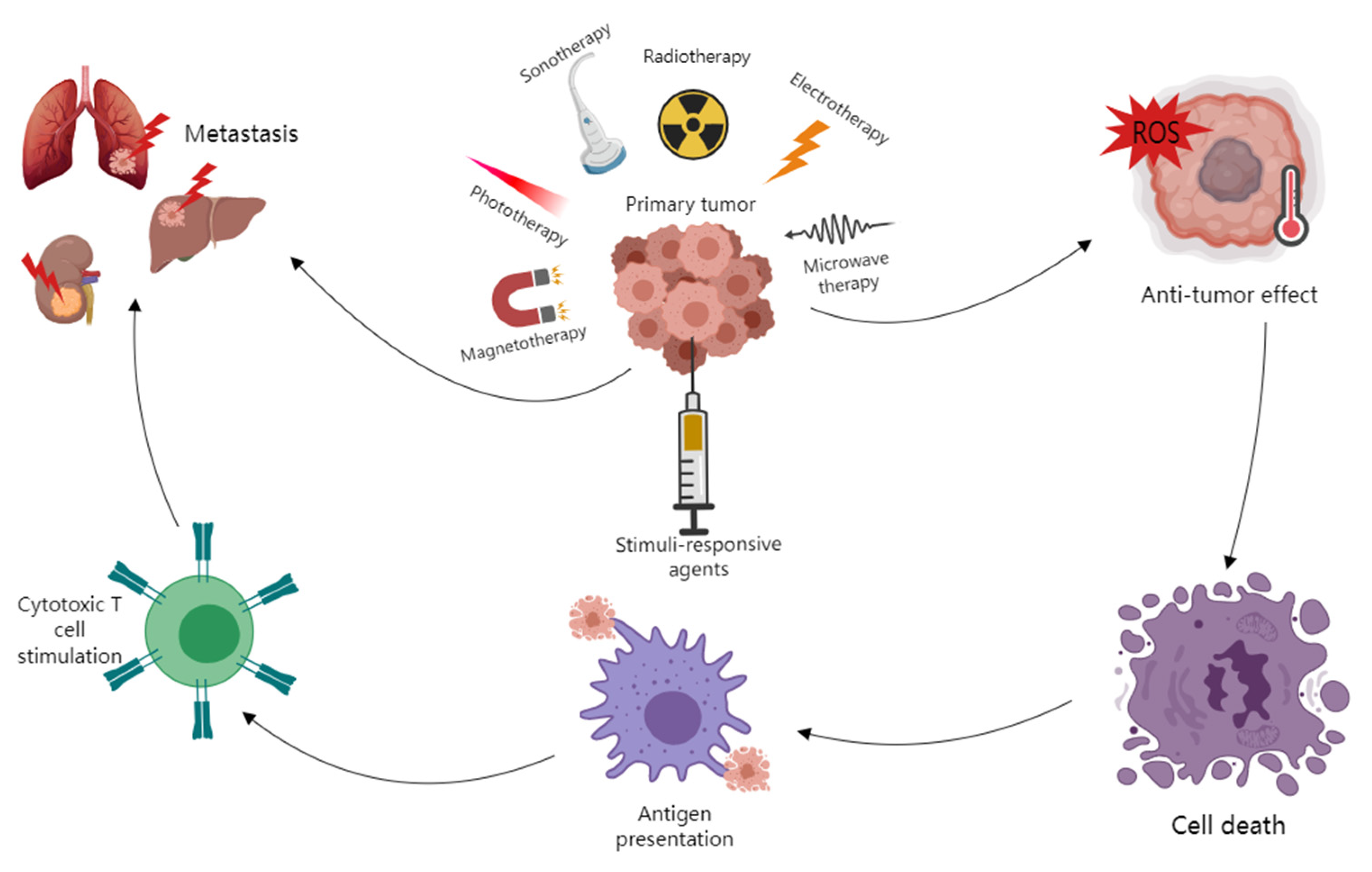
1. Introduction
2. Photodynamic Therapy (PDT)
2.1. Introduction to Photodynamic Therapy
2.2. Introduction to Sensitizers
2.3. Clinical Development of Photodynamic Therapy
2.4. Preclinical Development of Photodynamic Therapy
3. Photothermal Therapy (PTT)
3.1. Introduction to Photothermal Therapy
3.2. The Current Clinical Development of Photothermal Therapy
3.3. Introduction to Sensitizers
3.4. Preclinical Development of Photothermal Therapy
4. Sonodynamic Therapy (SDT)
4.1. Introduction to Sonodynamic Therapy
4.2. Introduction to Sensitizers
4.3. The Current Clinical Development of Sonodynamic Therapy
4.4. Preclinical Development of Sonodynamic Therapy
5. Radiodynamic Therapy (RDT)
5.1. Introduction to Radiodynamic Therapy
5.2. Introduction to Sensitizers
5.3. Preclinical Development of Sonodynamic Therapy
6. Microwave Dynamic and Microwave Thermodynamic Therapy
6.1. Introduction to Microwave Therapy
6.2. Introduction to Sensitizers and the Preclinical Development of Microwave-Based Therapies
7. Magnetothermal and Magnetodynamic Therapy
7.1. Introduction to Magnetic Therapy
7.2. Introduction to Sensitizers and Preclinical Development of Magnetic Therapies
8. Electrodynamic Therapy (EDT)
8.1. Introduction to Electrodynamic Therapy and Sensitizer
8.2. Preclinical Development of Electrodynamic Therapy
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, J.; Bari, S.; Kirtane, K.; Chung, C.H. Recent Advances and Future Directions in Clinical Management of Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 338. [Google Scholar] [CrossRef]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B., 3rd; Ajani, J.A.; Catalano, R.B.; Engelking, C.; Kornblau, S.M.; Martenson, J.A., Jr.; McCallum, R.; Mitchell, E.P.; O’Dorisio, T.M.; Vokes, E.E.; et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J. Clin. Oncol. 2004, 22, 2918–2926. [Google Scholar] [CrossRef]
- Regelink, G.; Brouwer, J.; de Bree, R.; Pruim, J.; van der Laan, B.F.; Vaalburg, W.; Hoekstra, O.S.; Comans, E.F.; Vissink, A.; Leemans, C.R.; et al. Detection of unknown primary tumours and distant metastases in patients with cervical metastases: Value of FDG-PET versus conventional modalities. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Jansma, J.; Vissink, A.; Spijkervet, F.K.; Roodenburg, J.L.; Panders, A.K.; Vermey, A.; Szabó, B.G.; Gravenmade, E.J. Protocol for the prevention and treatment of oral sequelae resulting from head and neck radiation therapy. Cancer 1992, 70, 2171–2180. [Google Scholar] [CrossRef]
- Saâda-Bouzid, E.; Defaucheux, C.; Karabajakian, A.; Coloma, V.P.; Servois, V.; Paoletti, X.; Even, C.; Fayette, J.; Guigay, J.; Loirat, D.; et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1605–1611. [Google Scholar] [CrossRef]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Ibarra, A.M.C.; Cecatto, R.B.; Motta, L.J.; Dos Santos Franco, A.L.; de Fátima Teixeira da Silva, D.; Nunes, F.D.; Hamblin, M.R.; Rodrigues, M. Photodynamic therapy for squamous cell carcinoma of the head and neck: Narrative review focusing on photosensitizers. Lasers Med. Sci. 2022, 37, 1441–1470. [Google Scholar] [CrossRef]
- Poonia, M.; Ramalingam, K.; Goyal, S.; Sidhu, S.K. Nanotechnology in oral cancer: A comprehensive review. J. Oral. Maxillofac. Pathol. 2017, 21, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Sun, Q.; Bai, R.; Zhang, Y.; Zhuang, Z.; Zhang, X.; Xin, T.; Chen, S.; Han, B. Progress of Nanomaterials-Based Photothermal Therapy for Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Abdel-kader, M.H. CHAPTER 1 The Journey of PDT Throughout History: PDT from Pharos to Present. In Photodynamic Medicine: From Bench to Clinic; The Royal Society of Chemistry: London, UK, 2016; pp. 1–21. [Google Scholar]
- Fan, K.F.; Hopper, C.; Speight, P.M.; Buonaccorsi, G.A.; Bown, S.G. Photodynamic therapy using mTHPC for malignant disease in the oral cavity. Int. J. Cancer 1997, 73, 25–32. [Google Scholar] [CrossRef]
- Lambert, A.; Nees, L.; Nuyts, S.; Clement, P.; Meulemans, J.; Delaere, P.; Vander Poorten, V. Photodynamic Therapy as an Alternative Therapeutic Tool in Functionally Inoperable Oral and Oropharyngeal Carcinoma: A Single Tertiary Center Retrospective Cohort Analysis. Front. Oncol. 2021, 11, 626394. [Google Scholar] [CrossRef] [PubMed]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef]
- Hosokawa, S.; Takebayashi, S.; Takahashi, G.; Okamura, J.; Mineta, H. Photodynamic therapy in patients with head and neck squamous cell carcinoma. Lasers Surg. Med. 2018, 50, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, S.; Takahashi, G.; Sugiyama, K.-I.; Takebayashi, S.; Okamura, J.; Takizawa, Y.; Mineta, H. Porfimer sodium-mediated photodynamic therapy in patients with head and neck squamous cell carcinoma. Photodiagnosis Photodyn. Ther. 2019, 29, 101627. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.; van Doeveren, T.; Tan, I.; Dilci, A.; van Veen, R.; Karakullukcu, B. The use of photodynamic therapy as adjuvant therapy to surgery in recurrent malignant tumors of the paranasal sinuses. Photodiagnosis Photodyn. Ther. 2015, 12, 414–421. [Google Scholar] [CrossRef]
- Yan, J.; Wang, P.; Li, L.; Zhang, L.; Zhang, G.; Tang, Y.; Wang, X. Surgery sequential with 5-Aminolevulinic acid photodynamic therapy for lip squamous cell carcinoma: Two cases reports. Photodiagnosis Photodyn. Ther. 2020, 32, 102043. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Meng, J.; Wen, N. The use of topical ALA-photodynamic therapy combined with induction chemotherapy for locally advanced oral squamous cell carcinoma. Am. J. Otolaryngol. 2021, 42, 103112. [Google Scholar] [CrossRef]
- Rigual, N.; Shafirstein, G.; Cooper, M.T.; Baumann, H.; Bellnier, D.A.; Sunar, U.; Tracy, E.C.; Rohrbach, D.J.; Wilding, G.; Tan, W.; et al. Photodynamic Therapy with 3-(1′-Hexyloxyethyl) Pyropheophorbide a for Cancer of the Oral Cavity. Clin. Cancer Res. 2013, 19, 6605–6613. [Google Scholar] [CrossRef] [PubMed]
- Shafirstein, G.; Rigual, N.R.; Arshad, H.; Cooper, M.T.; Bellnier, D.A.; Wilding, G.; Tan, W.; Merzianu, M.; Henderson, B.W. Photodynamic therapy with 3-(1′-hexyloxyethyl) pyropheophorbide-a for early-stage cancer of the larynx: Phase Ib study. Head Neck 2015, 38, E377–E383. [Google Scholar] [CrossRef]
- Ikeda, H.; Ohba, S.; Egashira, K.; Asahina, I. The effect of photodynamic therapy with talaporfin sodium, a second-generation photosensitizer, on oral squamous cell carcinoma: A series of eight cases. Photodiagnosis Photodyn. Ther. 2018, 21, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Kasai, H.; Horimatsu, T.; Yoshimura, K.; Teramukai, S.; Morita, S.; Tada, H.; Yamamoto, Y.; Kataoka, H.; Kakushima, N.; et al. A multicenter phase II study of salvage photodynamic therapy using talaporfin sodium (ME2906) and a diode laser (PNL6405EPG) for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Oncotarget 2016, 8, 22135–22144. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; de Bruijn, H.S.; Ten Hagen, T.L.; van Dam, G.M.; Roodenburg, J.L.N.; Berg, K.; Witjes, M.J.H.; Robinson, D.J. Targeted Photodynamic Therapy of Human Head and Neck Squamous Cell Carcinoma with Anti-epidermal Growth Factor Receptor Antibody Cetuximab and Photosensitizer IR700DX in the Mouse Skin-fold Window Chamber Model. Photochem. Photobiol. 2020, 96, 708–717. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, Y.; Dong, Z.; Chen, X.; Wang, Y.; Li, T.; Li, J.; Zang, S.; He, X.; Chen, D.; et al. Cellular Hypoxia Mitigation by Dandelion-like Nanoparticles for Synergistic Photodynamic Therapy of Oral Squamous Cell Carcinoma. ACS Appl. Mater. Interfaces 2022, 14, 44039–44053. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wang, X.; Li, F.; Wang, S.; Zhao, J.; Wang, J.; Liu, J.; Lyu, C.; Ye, P.; Tan, H.; et al. Engineered biomimetic nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against glioblastoma. Nat. Commun. 2022, 13, 4214. [Google Scholar] [CrossRef]
- Castro, C.I.; Briceno, J.C. Perfluorocarbon-Based Oxygen Carriers: Review of Products and Trials. Artif. Organs 2010, 34, 622–634. [Google Scholar] [CrossRef]
- Ma, Z.; Jia, X.; Bai, J.; Ruan, Y.; Wang, C.; Li, J.; Zhang, M.; Jiang, X. MnO2Gatekeeper: An Intelligent and O2-Evolving Shell for Preventing Premature Release of High Cargo Payload Core, Overcoming Tumor Hypoxia, and Acidic H2O2-Sensitive MRI. Adv. Funct. Mater. 2016, 27, 1604258. [Google Scholar] [CrossRef]
- Xu, T.; Ma, Y.; Yuan, Q.; Hu, H.; Hu, X.; Qian, Z.; Rolle, J.K.; Gu, Y.; Li, S. Enhanced Ferroptosis by Oxygen-Boosted Phototherapy Based on a 2-in-1 Nanoplatform of Ferrous Hemoglobin for Tumor Synergistic Therapy. ACS Nano 2020, 14, 3414–3425. [Google Scholar] [CrossRef]
- Israel, L.L.; Braubach, O.; Galstyan, A.; Chiechi, A.; Shatalova, E.S.; Grodzinski, Z.; Ding, H.; Black, K.L.; Ljubimova, J.Y.; Holler, E. A Combination of Tri-Leucine and Angiopep-2 Drives a Polyanionic Polymalic Acid Nanodrug Platform across the Blood–Brain Barrier. ACS Nano 2019, 13, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Chen, X.; Chen, E.; Zhang, J.; Huang, D.; Yang, D.; Ding, Y.; Qian, H.; Feijen, J.; Chen, W. Folated pH-degradable nanogels for the simultaneous delivery of docetaxel and an IDO1-inhibitor in enhancing cancer chemo-immunotherapy. Biomater. Sci. 2019, 7, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Zou, Z.; Sha, H.; Su, S.; Qian, H.; Meng, F.; Chen, F.; Du, S.; Zhou, S.; Chen, H.; et al. iRGD synergizes with PD-1 knockout immunotherapy by enhancing lymphocyte infiltration in gastric cancer. Nat. Commun. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Tang, C.; Xu, W.; Ran, J.; Wei, Z.; Wang, Y.; Zou, H.; Cheng, W.; Cai, Y.; Han, W. Hypoxia-Targeting Multifunctional Nanoparticles for Sensitized Chemotherapy and Phototherapy in Head and Neck Squamous Cell Carcinoma. Int. J. Nanomed. 2020, 15, 347–361. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Brace, C. Thermal Tumor Ablation in Clinical Use. IEEE Pulse 2011, 2, 28–38. [Google Scholar] [CrossRef]
- Jia, J.; Liu, G.; Xu, W.; Tian, X.; Li, S.; Han, F.; Feng, Y.; Dong, X.; Chen, H. Fine-Tuning the Homometallic Interface of Au-on-Au Nanorods and Their Photothermal Therapy in the NIR-II Window. Angew. Chem. Int. Ed. 2020, 59, 14443–14448. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Wang, J.; Zhang, P.; Huang, X.; Zhang, Z.; Guo, D.-S.; Yang, X. Ag@S-nitrosothiol core–shell nanoparticles for chemo and photothermal synergistic tumor targeted therapy. J. Mater. Chem. B 2020, 8, 5483–5490. [Google Scholar] [CrossRef]
- Tang, S.; Chen, M.; Zheng, N. Sub-10-nm Pd Nanosheets with Renal Clearance for Efficient Near-Infrared Photothermal Cancer Therapy. Small 2014, 10, 3139–3144. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Wan, H.-Y.; Jia, H.; Liu, L.; Wang, J. Porous Pt Nanoparticles with High Near-Infrared Photothermal Conversion Efficiencies for Photothermal Therapy. Adv. Healthc. Mater. 2016, 5, 3165–3172. [Google Scholar] [CrossRef]
- Liu, Y.; Shipton, M.K.; Ryan, J.; Kaufman, E.D.; Franzen, A.S.; Feldheim, D.L. Synthesis, Stability, and Cellular Internalization of Gold Nanoparticles Containing Mixed Peptide−Poly(ethylene glycol) Monolayers. Anal. Chem. 2007, 79, 2221–2229. [Google Scholar] [CrossRef]
- Liao, Y.-T.; Liu, C.-H.; Chin, Y.; Chen, S.-Y.; Liu, S.H.; Hsu, Y.-C.; Wu, K.C.-W. Biocompatible and multifunctional gold nanorods for effective photothermal therapy of oral squamous cell carcinoma. J. Mater. Chem. B 2019, 7, 4451–4460. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, L.; Wang, G.; Yang, K.; Chen, M.; Tian, R.; Ma, Q.; Zhu, L. Hybrid graphene/Au activatable theranostic agent for multimodalities imaging guided enhanced photothermal therapy. Biomaterials 2016, 79, 36–45. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, F.; Tian, R.; Zhang, L.; Fu, G.; Yang, L.; Zhu, L. Nanotubes-Embedded Indocyanine Green–Hyaluronic Acid Nanoparticles for Photoacoustic-Imaging-Guided Phototherapy. ACS Appl. Mater. Interfaces 2016, 8, 5608–5617. [Google Scholar] [CrossRef]
- Shen, S.; Kong, F.; Guo, X.; Wu, L.; Shen, H.; Xie, M.; Wang, X.; Jin, Y.; Ge, Y. CMCTS stabilized Fe3O4 particles with extremely low toxicity as highly efficient near-infrared photothermal agents for in vivo tumor ablation. Nanoscale 2013, 5, 8056–8066. [Google Scholar] [CrossRef]
- Maor, I.; Asadi, S.; Korganbayev, S.; Dahis, D.; Shamay, Y.; Schena, E.; Azhari, H.; Saccomandi, P.; Weitz, I.S. Laser-induced thermal response and controlled release of copper oxide nanoparticles from multifunctional polymeric nanocarriers. Sci. Technol. Adv. Mater. 2021, 22, 218–233. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Wang, F.; Yang, M.; Xie, L.; Zeng, X. Biosafety, Nontoxic Nanoparticles for VL–NIR Photothermal Therapy against Oral Squamous Cell Carcinoma. ACS Omega 2021, 6, 11240–11247. [Google Scholar] [CrossRef]
- Ren, S.; Cheng, X.; Chen, M.; Liu, C.; Zhao, P.; Huang, W.; He, J.; Zhou, Z.; Miao, L. Hypotoxic and Rapidly Metabolic PEG-PCL-C3-ICG Nanoparticles for Fluorescence-Guided Photothermal/Photodynamic Therapy against OSCC. ACS Appl. Mater. Interfaces 2017, 9, 31509–31518. [Google Scholar] [CrossRef]
- Bu, L.-L.; Wang, H.-Q.; Pan, Y.; Chen, L.; Wu, H.; Wu, X.; Zhao, C.; Rao, L.; Liu, B.; Sun, Z.-J. Gelatinase-sensitive nanoparticles loaded with photosensitizer and STAT3 inhibitor for cancer photothermal therapy and immunotherapy. J. Nanobiotechnol. 2021, 19, 379. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, J.; Jin, L.; Hong, L.; Wang, F.; Mao, Z.; Wu, M. Cancer cell membrane-coated gold nanorods for photothermal therapy and radiotherapy on oral squamous cancer. J. Mater. Chem. B 2020, 8, 7253–7263. [Google Scholar] [CrossRef]
- Melancon, M.P.; Lu, W.; Zhong, M.; Zhou, M.; Liang, G.; Elliott, A.M.; Hazle, J.D.; Myers, J.N.; Li, C.; Stafford, R.J. Targeted multifunctional gold-based nanoshells for magnetic resonance-guided laser ablation of head and neck cancer. Biomaterials 2011, 32, 7600–7608. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, T.S.; Meidanchi, A.; Shokri, A.; Shakeri-Zadeh, A. Fe3O4@Au/reduced graphene oxide nanostructures: Combinatorial effects of radiotherapy and photothermal therapy on oral squamous carcinoma KB cell line. Ceram. Int. 2020, 46, 28676–28685. [Google Scholar] [CrossRef]
- Park, S.-W.; Jang, B.; Kim, H.; Lee, J.; Park, J.Y.; Kang, S.-O.; Choa, Y.-H. Highly Water-Dispersible Graphene Nanosheets From Electrochemical Exfoliation of Graphite. Front. Chem. 2021, 9, 699231. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, W.; Huang, Q.; Li, C.; Chen, W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine 2010, 5, 1161–1171. [Google Scholar] [CrossRef]
- Zuo, J.; Huo, M.; Wang, L.; Li, J.; Chen, Y.; Xiong, P. Photonic hyperthermal and sonodynamic nanotherapy targeting oral squamous cell carcinoma. J. Mater. Chem. B 2020, 8, 9084–9093. [Google Scholar] [CrossRef]
- Huang, X.; Deng, G.; Han, Y.; Yang, G.; Zou, R.; Zhang, Z.; Sun, S.; Hu, J. Right Cu2−x S@MnS Core–Shell Nanoparticles as a Photo/H2O2-Responsive Platform for Effective Cancer Theranostics. Adv. Sci. 2019, 6, 1901461. [Google Scholar] [CrossRef]
- Qian, M.; Cheng, Z.; Luo, G.; Galluzzi, M.; Shen, Y.; Li, Z.; Yang, H.; Yu, X. Molybdenum Diphosphide Nanorods with Laser-Potentiated Peroxidase Catalytic/Mild-Photothermal Therapy of Oral Cancer. Adv. Sci. 2021, 9, 2101527. [Google Scholar] [CrossRef]
- Novotny, J.A.; Peterson, C.A. Molybdenum. Adv. Nutr. Int. Rev. J. 2018, 9, 272–273. [Google Scholar] [CrossRef]
- Ren, W.; Qiu, L.-H.; Gao, Z.; Li, P.; Zhao, X.; Hu, C.-C. Preparation of multifunctional nanoparticles targeting tongue cancer and in vitro study. Hua Xi Kou Qiang Yi Xue Za Zhi 2018, 36, 240–246. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, D.; Wang, L.; Sun, Y.; Li, J.J.; Hill, B.; Yang, F.; Li, Y.; Lam, K.S. Unique Photochemo-Immuno-Nanoplatform against Orthotopic Xenograft Oral Cancer and Metastatic Syngeneic Breast Cancer. Nano Lett. 2018, 18, 7092–7103. [Google Scholar] [CrossRef]
- Xiong, J.; Feng, J.; Qiu, L.; Gao, Z.; Li, P.; Pang, L.; Zhang, Z. SDF-1-loaded PLGA nanoparticles for the targeted photoacoustic imaging and photothermal therapy of metastatic lymph nodes in tongue squamous cell carcinoma. Int. J. Pharm. 2018, 554, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, D.; Pan, J.; Xia, C.; Fan, L.; Pu, Y.; Zhang, Q.; Ni, Y.H.; Wang, J.; Hu, Q. A near infrared light-triggered human serum albumin drug delivery system with coordination bonding of indocyanine green and cisplatin for targeting photochemistry therapy against oral squamous cell cancer. Biomater. Sci. 2019, 7, 5270–5282. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, W.; Jeon, S.; Choi, Y.; Cho, Y. Electroactive Polypyrrole Nanowire Arrays: Synergistic Effect of Cancer Treatment by On-Demand Drug Release and Photothermal Therapy. Langmuir 2015, 31, 4264–4269. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Jiang, L.; Hao, L.; Lu, J.; Liu, Z.; Lei, Z.; Li, Y.; Hua, C.; Li, W.; Li, X. A novel theranostic nanoplatform for imaging-guided chemo-photothermal therapy in oral squamous cell carcinoma. J. Mater. Chem. B 2021, 9, 6006–6016. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hao, Y.; Li, W.; Xiao, Y.; Zhou, T.; Hu, D.; Liu, Q.; Zhou, X.; Qian, Z. Near-Infrared Responsive Doxorubicin Loaded Hollow Mesoporous Prussian Blue Nanoparticles Combined with Dissolvable Hyaluronic Acid Microneedle System for Human Oral Squamous Cell Carcinoma Therapy. J. Biomed. Nanotechnol. 2020, 16, 721–738. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhen, W.; Wang, Y.; Liu, J.; Jin, L.; Zhang, T.; Zhang, S.; Zhao, Y.; Song, S.; Li, C.; et al. One-Dimensional Fe2P Acts as a Fenton Agent in Response to NIR II Light and Ultrasound for Deep Tumor Synergetic Theranostics. Angew. Chem. Int. Ed. 2019, 58, 2407–2412. [Google Scholar] [CrossRef]
- Yumita, N.; Nishigaki, R.; Umemura, K.; Umemura, S.-I. Hematoporphyrin as a Sensitizer of Cell-damaging Effect of Ultrasound. Jpn. J. Cancer Res. 1989, 80, 219–222. [Google Scholar] [CrossRef]
- Wan, G.-Y.; Liu, Y.; Chen, B.-W.; Liu, Y.-Y.; Wang, Y.; Zhang, N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol. Med. 2016, 13, 325–338. [Google Scholar] [CrossRef]
- Hoogenboom, M.; Eikelenboom, D.; den Brok, M.H.; Heerschap, A.; Fütterer, J.J.; Adema, G.J. Mechanical High-Intensity Focused Ultrasound Destruction of Soft Tissue: Working Mechanisms and Physiologic Effects. Ultrasound Med. Biol. 2015, 41, 1500–1517. [Google Scholar] [CrossRef]
- Li, J.-H.; Yue, W.; Huang, Z.; Chen, Z.-Q.; Zhan, Q.; Ren, F.-B.; Liu, J.-Y.; Fu, S.-B. Calcium overload induces C6 rat glioma cell apoptosis in sonodynamic therapy. Int. J. Radiat. Biol. 2011, 87, 1061–1066. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, Q.; Wang, Y.; Wang, X.; Wang, P.; Zhang, L.; Su, S. Comparison of Accumulation, Subcellular Location, and Sonodynamic Cytotoxicity between Hematoporphyrin and Protoporphyrin IX in L1210 Cells. Chemotherapy 2010, 56, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, P.; Wang, X.; Su, X.; Liu, Q. Involvement of Mitochondrial and Reactive Oxygen Species in the Sonodynamic Toxicity of Chlorin e6 in Human Leukemia K562 Cells. Ultrasound Med. Biol. 2014, 40, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Yamamoto, M.; Yoshino, S.; Umemura, S.-I.; Sasaki, K.; Fukushima, T. Sonodynamic therapy consisting of focused ultrasound and a photosensitizer causes a selective antitumor effect in a rat intracranial glioma model. Anticancer Res. 2009, 29, 943–950. [Google Scholar] [PubMed]
- Sakusabe, N.; Okada, K.; Sato, K.; Kamada, S.; Yoshida, Y.; Suzuki, T. Enhanced Sonodynamic Antitumor Effect of Ultrasound in the Presence of Nonsteroidal Anti-inflammatory Drugs. Jpn. J. Cancer Res. 1999, 90, 1146–1151. [Google Scholar] [CrossRef]
- Okada, K.; Itoi, E.; Miyakoshi, N.; Nakajima, M.; Suzuki, T.; Nishida, J. Enhanced Antitumor Effect of Ultrasound in the Presence of Piroxicam in a Mouse Air Pouch Model. Jpn. J. Cancer Res. 2002, 93, 216–222. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, X.; Zhu, X.; Lv, F.; Zhong, Z.; Zhang, F.; Guo, W.; Cao, W.; Yang, L.; Tian, Y. Apoptosis of THP-1 Derived Macrophages Induced by Sonodynamic Therapy Using a New Sonosensitizer Hydroxyl Acetylated Curcumin. PLoS ONE 2014, 9, e93133. [Google Scholar] [CrossRef]
- Kim, T.I.; Jeong, K.H.; Shin, M.K. Verrucous epidermal nevus (VEN) successfully treated with indocyanine green (ICG) photodynamic therapy (PDT). JAAD Case Rep. 2015, 1, 312–314. [Google Scholar] [CrossRef]
- Wang, X.; Leung, A.W.; Jiang, Y.; Yu, H.; Li, X.; Xu, C. Hypocrellin B-mediated sonodynamic action induces apoptosis of hepatocellular carcinoma cells. Ultrasonics 2012, 52, 543–546. [Google Scholar] [CrossRef]
- Lawrence, J.E.; Steele, C.J.; Rovin, R.A.; Belton, R.J., Jr.; Winn, R.J. Dexamethasone alone and in combination with desipramine, phenytoin, valproic acid or levetiracetam interferes with 5-ALA-mediated PpIX production and cellular retention in glioblastoma cells. J. Neuro-Oncol. 2015, 127, 15–21. [Google Scholar] [CrossRef]
- Ninomiya, K.; Fukuda, A.; Ogino, C.; Shimizu, N. Targeted sonocatalytic cancer cell injury using avidin-conjugated titanium dioxide nanoparticles. Ultrason. Sonochem. 2014, 21, 1624–1628. [Google Scholar] [CrossRef]
- Osminkina, L.A.; Sivakov, V.A.; Mysov, G.A.; Georgobiani, V.A.; Natashina, U.; Talkenberg, F.; Solovyev, V.V.; Kudryavtsev, A.A.; Timoshenko, V. Nanoparticles prepared from porous silicon nanowires for bio-imaging and sonodynamic therapy. Nanoscale Res. Lett. 2014, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, W.; Xu, Z.; Luo, Y.; Mitchell, D.; Moss, R.W. Sonodynamic and Photodynamic Therapy in Advanced Breast Carcinoma: A Report of 3 Cases. Integr. Cancer Ther. 2009, 8, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.N.; Fulle, R.; Lewis, T. Activated Cancer Therapy Using Light and Ultrasound—A Case Series of Sonodynamic Photodynamic Therapy in 115 Patients Over a 4 Year Period. Curr. Drug Ther. 2009, 4, 179–193. [Google Scholar] [CrossRef]
- Lv, Y.; Zheng, J.; Zhou, Q.; Jia, L.; Wang, C.; Liu, N.; Zhao, H.; Ji, H.; Li, B.; Cao, W. Antiproliferative and Apoptosis-inducing Effect of exo-Protoporphyrin IX based Sonodynamic Therapy on Human Oral Squamous Cell Carcinoma. Sci. Rep. 2017, 7, 40967. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Cui, H.; Zhang, R.; Zheng, J.; Cao, W. Apoptosis of SAS cells induced by sonodynamic therapy using 5-aminolevulinic acid sonosensitizer. Anticancer Res. 2011, 31, 39–45. [Google Scholar] [PubMed]
- Lv, Y.; Fang, M.; Zheng, J.; Yang, B.; Li, H.; Xiuzigao, Z.; Song, W.; Chen, Y.; Cao, W. Low-intensity Ultrasound Combined with 5-aminolevulinic Acid Administration in the Treatment of Human Tongue Squamous Carcinoma. Cell. Physiol. Biochem. 2012, 30, 321–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, L.; Hu, Z.; Cao, W.; Zhuang, D. Hematoporphyrin monomethyl ether-mediated sonodynamic therapy induces A-253 cell apoptosis. Oncol. Lett. 2020, 19, 3223–3228. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Etemad-Moghadam, S.; Alaeddini, M.; Bahador, A. Modulation of the triggered apoptosis by nano emodin transfersome-mediated sonodynamic therapy on head and neck squamous cell carcinoma cell lines. Photodiagnosis Photodyn. Ther. 2021, 34, 102253. [Google Scholar] [CrossRef]
- Sun, S.; Wang, D.; Yin, R.; Zhang, P.; Jiang, R.; Xiao, C. A Two-In-One Nanoprodrug for Photoacoustic Imaging-Guided Enhanced Sonodynamic Therapy. Small 2022, 18, e2202558. [Google Scholar] [CrossRef]
- Miyoshi, N.; Kundu, S.K.; Tuziuti, T.; Yasui, K.; Shimada, I.; Ito, Y. Combination of Sonodynamic and Photodynamic Therapy against Cancer Would Be Effective through Using a Regulated Size of Nanoparticles. Nanosci. Nanoeng. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Su, X.; Wang, P.; Zhang, K.; Feng, X.; Liu, Q. Combination of Protoporphyrin IX-mediated Sonodynamic Treatment with Doxorubicin Synergistically Induced Apoptotic Cell Death of a Multidrug-Resistant Leukemia K562/DOX Cell Line. Ultrasound Med. Biol. 2015, 41, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Sazgarnia, A.; Shanei, A.; Meibodi, N.T.; Eshghi, H.; Nassir, H. A Novel Nanosonosensitizer for Sonodynamic Therapy: In vivo study on a colon tumor model. J. Ultrasound Med. 2011, 30, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Nomikou, N.; Fowley, C.; Byrne, N.M.; McCaughan, B.; McHale, A.P.; Callan, J.F. Microbubble–sonosensitiser conjugates as therapeutics in sonodynamic therapy. Chem. Commun. 2012, 48, 8332–8334. [Google Scholar] [CrossRef] [PubMed]
- Belanova, A.; Chmykhalo, V.; Beseda, D.; Belousova, M.; Butova, V.; Soldatov, A.; Makarenko, Y.; Zolotukhin, P. A mini-review of X-ray photodynamic therapy (XPDT) nonoagent constituents’ safety and relevant design considerations. Photochem. Photobiol. Sci. 2020, 19, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Abliz, E.; Collins, J.E.; Bell, H.; Tata, D.B. Novel applications of diagnostic X-rays in activating a clinical photodynamic drug: Photofrin II through X-ray induced visible luminescence from "rare-earth" formulated particles. J. X-Ray Sci. Technol. 2011, 19, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lu, N.; Shen, Z.; Tang, W.; Shen, B.; Cui, Z.; Shan, L.; Yang, Z.; Wang, Z.; Jacobson, O.; et al. Generic synthesis of small-sized hollow mesoporous organosilica nanoparticles for oxygen-independent X-ray-activated synergistic therapy. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Jenh, Y.-J.; Wu, S.-K.; Chen, Y.-S.; Hanagata, N.; Lin, F.-H. Non-invasive Photodynamic Therapy in Brain Cancer by Use of Tb3+-Doped LaF3 Nanoparticles in Combination with Photosensitizer Through X-ray Irradiation: A Proof-of-Concept Study. Nanoscale Res. Lett. 2017, 12, 1–6. [Google Scholar] [CrossRef]
- Wang, G.D.; Nguyen, H.T.; Chen, H.; Cox, P.B.; Wang, L.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J. X-Ray Induced Photodynamic Therapy: A Combination of Radiotherapy and Photodynamic Therapy. Theranostics 2016, 6, 2295–2305. [Google Scholar] [CrossRef]
- Song, L.; Li, P.-P.; Yang, W.; Lin, X.-H.; Liang, H.; Chen, X.-F.; Liu, G.; Li, J.; Yang, H.-H. Low-Dose X-ray Activation of W(VI)-Doped Persistent Luminescence Nanoparticles for Deep-Tissue Photodynamic Therapy. Adv. Funct. Mater. 2018, 28, 1707496. [Google Scholar] [CrossRef]
- Tew, L.S.; Cai, M.-T.; Lo, L.-W.; Khung, Y.L.; Chen, N.-T. Pollen-Structured Gold Nanoclusters for X-ray Induced Photodynamic Therapy. Materials 2018, 11, 1170. [Google Scholar] [CrossRef]
- Yang, C.-C.; Tsai, M.-H.; Li, K.-Y.; Hou, C.-H.; Lin, F.-H. Carbon-Doped TiO2 Activated by X-ray Irradiation for the Generation of Reactive Oxygen Species to Enhance Photodynamic Therapy in Tumor Treatment. Int. J. Mol. Sci. 2019, 20, 2072. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Ni, K.; Veroneau, S.S.; Song, Y.; Lin, W. Nanoscale Metal–Organic Layers for Radiotherapy–Radiodynamic Therapy. J. Am. Chem. Soc. 2018, 140, 16971–16975. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yao, M.; Ma, L.; Hossu, M.; Han, X.; Juzenas, P.; Chen, W. X-ray-induced nanoparticle-based photodynamic therapy of cancer. Nanomedicine 2014, 9, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Xu, Z.; Culbert, A.; Luo, T.; Guo, N.; Yang, K.; Pearson, E.; Ben Preusser, B.; Wu, T.; La Riviere, P.; et al. Synergistic checkpoint-blockade and radiotherapy–radiodynamic therapy via an immunomodulatory nanoscale metal–organic framework. Nat. Biomed. Eng. 2022, 6, 144–156. [Google Scholar] [CrossRef]
- Sun, W.; Shi, T.; Luo, L.; Chen, X.; Lv, P.; Lv, Y.; Zhuang, Y.; Zhu, J.; Liu, G.; Chen, X.; et al. Monodisperse and Uniform Mesoporous Silicate Nanosensitizers Achieve Low-Dose X-Ray-Induced Deep-Penetrating Photodynamic Therapy. Adv. Mater. 2019, 31, e1808024. [Google Scholar] [CrossRef]
- Wu, Q.; Li, M.; Tan, L.; Yu, J.; Chen, Z.; Su, L.; Ren, X.; Fu, C.; Ren, J.; Li, L.; et al. A tumor treatment strategy based on biodegradable BSA@ZIF-8 for simultaneously ablating tumors and inhibiting infection. Nanoscale Horizons 2018, 3, 606–615. [Google Scholar] [CrossRef]
- Chen, X.; Fu, C.; Wang, Y.; Wu, Q.; Meng, X.; Xu, K. Mitochondria-targeting nanoparticles for enhanced microwave ablation of cancer. Nanoscale 2018, 10, 15677–15685. [Google Scholar] [CrossRef]
- Hu, H.; Feng, W.; Qian, X.; Yu, L.; Chen, Y.; Li, Y. Emerging Nanomedicine-Enabled/Enhanced Nanodynamic Therapies beyond Traditional Photodynamics. Adv. Mater. 2021, 33, e2005062. [Google Scholar] [CrossRef]
- Wu, Q.; Xia, N.; Long, D.; Tan, L.; Rao, W.; Yu, J.; Fu, C.; Ren, X.; Li, H.; Gou, L.; et al. Dual-Functional Supernanoparticles with Microwave Dynamic Therapy and Microwave Thermal Therapy. Nano Lett. 2019, 19, 5277–5286. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Z.; Pan, Z.; Hao, Y.; Wang, C.; Dong, Z.; Li, Q.; Han, Y.; Tian, L.; Feng, L.; et al. Metallo-alginate hydrogel can potentiate microwave tumor ablation for synergistic cancer treatment. Sci. Adv. 2022, 8, eabo5285. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, B.; Li, Y.; Ma, X.; Jiao, W.; Shi, K.; Zhang, T.; Chen, S.; He, Y.; Liang, X.-J.; et al. Graphene Oxide-Grafted Magnetic Nanorings Mediated Magnetothermodynamic Therapy Favoring Reactive Oxygen Species-Related Immune Response for Enhanced Antitumor Efficacy. ACS Nano 2020, 14, 1936–1950. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sahoo, S.K. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today 2013, 19, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Seegenschmiedt, M.H.; Sauer, R.; Fietkau, R.; Iro, H.; Chalal, J.A.; Brady, L.W. Interstitial thermal radiation therapy: Five-year experience with head and neck tumors. Radiology 1992, 184, 795–804. [Google Scholar] [CrossRef]
- Su, Z.; Liu, D.; Chen, L.; Zhang, J.; Ru, L.; Chen, Z.; Gao, Z.; Wang, X. CD44-Targeted Magnetic Nanoparticles Kill Head And Neck Squamous Cell Carcinoma Stem Cells In An Alternating Magnetic Field. Int. J. Nanomed. 2019, 14, 7549–7560. [Google Scholar] [CrossRef]
- Legge, C.J.; Colley, H.E.; Lawson, M.A.; Rawlings, A.E. Targeted magnetic nanoparticle hyperthermia for the treatment of oral cancer. J. Oral Pathol. Med. 2019, 48, 803–809. [Google Scholar] [CrossRef]
- Tsai, M.-T.; Sun, Y.-S.; Keerthi, M.; Panda, A.K.; Dhawan, U.; Chang, Y.-H.; Lai, C.-F.; Hsiao, M.; Wang, H.-Y.; Chung, R.-J. Oral Cancer Theranostic Application of FeAu Bimetallic Nanoparticles Conjugated with MMP-1 Antibody. Nanomaterials 2021, 12, 61. [Google Scholar] [CrossRef]
- Keshri, S.; Kumar, V.; Wiśniewski, P.; Kamzin, A.S. Synthesis and characterization of LSMO manganite-based biocomposite. Phase Transitions 2013, 87, 468–476. [Google Scholar] [CrossRef]
- Kolovskaya, O.S.; Zamay, T.N.; Zamay, G.S.; Babkin, V.A.; Medvedeva, E.N.; Neverova, N.A.; Kirichenko, A.K.; Zamay, S.S.; Lapin, I.N.; Morozov, E.V.; et al. Aptamer-Conjugated Superparamagnetic Ferroarabinogalactan Nanoparticles for Targeted Magnetodynamic Therapy of Cancer. Cancers 2020, 12, 216. [Google Scholar] [CrossRef]
- Gu, T.; Wang, Y.; Lu, Y.; Cheng, L.; Feng, L.; Zhang, H.; Li, X.; Han, G.; Liu, Z. Platinum Nanoparticles to Enable Electrodynamic Therapy for Effective Cancer Treatment. Adv. Mater. 2019, 31, e1806803. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, J.; Fang, C.; Zhou, Y.; Li, X.; Han, G. Porous Pt Nanospheres Incorporated with GOx to Enable Synergistic Oxygen-Inductive Starvation/Electrodynamic Tumor Therapy. Adv. Sci. 2020, 7, 2001223. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, Q.; Feng, Z.; Xu, Q.; Zhang, X.; Yang, Y.; Zhang, Y.; Liang, X.-J.; Yu, Z.; Yu, M. Glutamine Antagonist Synergizes with Electrodynamic Therapy to Induce Tumor Regression and Systemic Antitumor Immunity. ACS Nano 2022, 16, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chu, Q.; Li, M.; Han, G.; Li, X. Fe3O4@Pt nanoparticles to enable combinational electrodynamic/chemodynamic therapy. J. Nanobiotechnol. 2021, 19, 206. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Yang, X.; Ainiwaer, M.; Chen, F.; Liu, J. Recent Clinical and Preclinical Advances in External Stimuli-Responsive Therapies for Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2023, 12, 173. https://doi.org/10.3390/jcm12010173
Jiang Z, Yang X, Ainiwaer M, Chen F, Liu J. Recent Clinical and Preclinical Advances in External Stimuli-Responsive Therapies for Head and Neck Squamous Cell Carcinoma. Journal of Clinical Medicine. 2023; 12(1):173. https://doi.org/10.3390/jcm12010173
Chicago/Turabian StyleJiang, Zheng, Xin Yang, Mailudan Ainiwaer, Fei Chen, and Jun Liu. 2023. "Recent Clinical and Preclinical Advances in External Stimuli-Responsive Therapies for Head and Neck Squamous Cell Carcinoma" Journal of Clinical Medicine 12, no. 1: 173. https://doi.org/10.3390/jcm12010173
APA StyleJiang, Z., Yang, X., Ainiwaer, M., Chen, F., & Liu, J. (2023). Recent Clinical and Preclinical Advances in External Stimuli-Responsive Therapies for Head and Neck Squamous Cell Carcinoma. Journal of Clinical Medicine, 12(1), 173. https://doi.org/10.3390/jcm12010173






