A Randomised Controlled Trial Comparing Induction of Labour with the Propess Vaginal System to the Prostin Vaginal Tablet in Premature Rupture of Membranes at Term
Abstract
1. Introduction
2. Materials and Methods
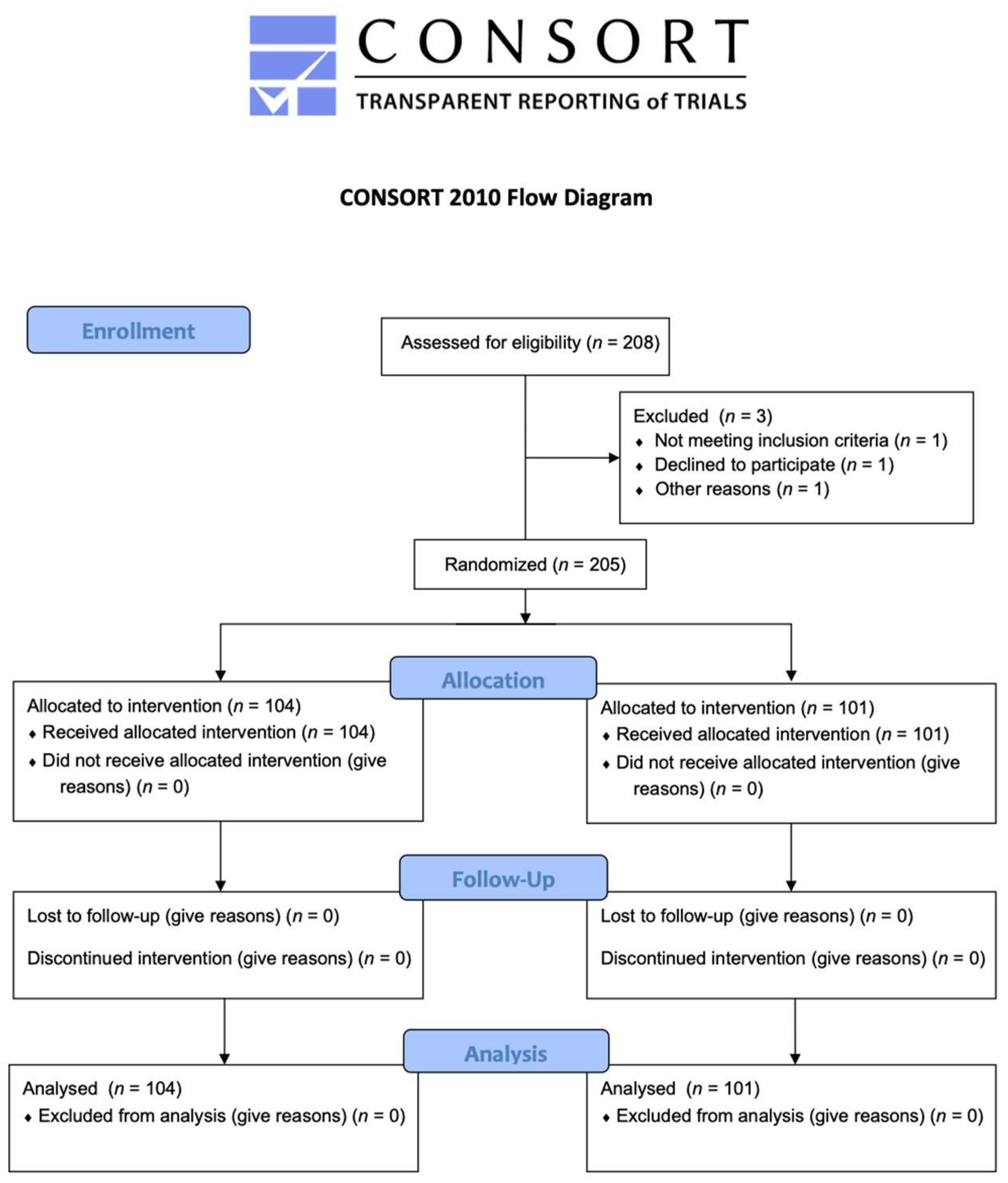
2.1. Enrollment of Patients
2.2. Random Allocation
2.3. Induction of Labour
2.4. Study Outcomes
2.5. Sample Size Estimation
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Principal Findings
4.2. Results in the Context of What Is Known
4.3. Clinical Implications
4.4. Research Implications
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pourali, L.; Saghafi, N.; Eslami Hasan Abadi, S.; Tara, F.; Vatanchi, A.M.; Motamedi, E. Induction of labour in term premature rupture of membranes; oxytocin versus sublingual misoprostol a randomised clinical trial. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2018, 38, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, L.; Kale, M.; Mannerfeldt, J.; Metcalfe, A. Oral Misoprostol for Induction of Labour in Term PROM: A Systematic Review. J. Obstet. Gynaecol. Can. 2020, 42, 1525–1531.e1. [Google Scholar] [CrossRef]
- Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 188: Prelabor Rupture of Membranes. Obstet. Gynecol. 2018, 131, e1–e14. [Google Scholar] [CrossRef]
- Levine, L.D. Cervical ripening: Why we do what we do. Semin. Perinatol. 2020, 44, 151216. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Dinoprostone Vaginal Insert: A Review in Cervical Ripening. Drugs 2018, 78, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Anzeljc, V.; Mujezinovic, F. Caesarean sections and outcomes of labor induction after the introduction of a new intravaginal device: Retrospective analysis. Clin. Exp. Obstet. Gynecol. 2021, 48, 615. [Google Scholar] [CrossRef]
- Propess 10 mg Vaginal Delivery System—Summary of Product Characteristics (SmPC)—(emc). Available online: https://www.medicines.org.uk/emc/medicine/16898/SPC/Propess+10mg+vaginal+delivery+system/#gref (accessed on 17 November 2022).
- Sealed Envelope|Power Calculator for Continuous Outcome Superiority Trial. Available online: https://www.sealedenvelope.com/power/continuous-superiority/ (accessed on 5 January 2021).
- Giorgi, F.M.; Ceraolo, C.; Mercatelli, D. The R Language: An Engine for Bioinformatics and Data Science. Life 2022, 12, 648. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, X.; Zhang, Y.; Guo, Y.; Yin, B.; Chen, D.; Chen, Y.; Yu, Y.; Zhu, B.; Qin, Y.; et al. Comparison of Dinoprostone and Oxytocin for the Induction of Labor in Late-Term Pregnancy and the Rate of Cesarean Section: A Retrospective Study in Ten Centers in South China. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 8554–8561. [Google Scholar] [CrossRef] [PubMed]
- López-Jiménez, N.; García-Sánchez, F.; Pailos, R.H.; Rodrigo-Álvaro, V.; Pascual-Pedreño, A.; Moreno-Cid, M.; Hernández-Martínez, A.; Molina-Alarcón, M. Use of Vaginal Dinoprostone (PGE2) in Patients with Premature Rupture of Membranes (PROM) Undergoing Induction of Labor: A Comparative Study. J. Clin. Med. 2022, 11, 2217. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yin, H.; Zhang, H.; Zhu, H.; Hu, R.; Gu, W. Dinoprostone pessary for labor induction in Chinese patients with premature rupture of membranes at term: A retrospective cohort. J. Obstet. Gynaecol. Res. 2021, 47, 2356–2362. [Google Scholar] [CrossRef]
- Rijal, H.; Manandhar, R.; Pradhan, N. A randomized study comparing intravaginal prostaglandin (PGE2) with oxytocin for induction of labour in premature rupture of membrane at term. Nepal Med. Coll. J. NMCJ 2012, 14, 199–203. [Google Scholar] [PubMed]
- Kunt, C.; Kanat-Pektas, M.; Gungor, A.N.C.; Kurt, R.K.; Ozat, M.; Gulerman, C.; Gungor, T.; Mollamahmutoglu, L. Randomized trial of vaginal prostaglandin E2 versus oxytocin for labor induction in term premature rupture of membranes. Taiwan. J. Obstet. Gynecol. 2010, 49, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Sobande, A.A.; Albar, H.M. Induced labour with prostaglandin E2 in different parity groups after premature rupture of membranes. East. Mediterr. Health J. 2003, 9, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Liu, Y.; Jiang, T.; Dai, Y.; Gong, Y.; Li, Q.; Wang, X. Effect of premature rupture of membranes on time to delivery and outcomes in full-term pregnancies with vaginal dinoprostone-induced labour. Arch. Gynecol. Obstet. 2020, 301, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Kehl, S.; Weiss, C.; Dammer, U.; Baier, F.; Faschingbauer, F.; Beckmann, M.W.; Sütterlin, M.; Pretscher, J. Effect of Premature Rupture of Membranes on Induction of Labor: A Historical Cohort Study. Geburtshilfe Frauenheilkd. 2017, 77, 1174–1181. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hannah, M.E.; Ohlsson, A.; Farine, D.; Hewson, S.A.; Hodnett, E.D.; Myhr, T.L.; Wang, E.E.; Weston, J.A.; Willan, A.R. Induction of labor compared with expectant management for prelabor rupture of the membranes at term. TERMPROM Study Group. N. Engl. J. Med. 1996, 334, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Melamed, N.; Berghella, V.; Ananth, C.V.; Lipworth, H.; Yoon, E.W.; Barrett, J. Optimal timing of labor induction after prelabor rupture of membranes at term: A secondary analysis of the TERMPROM study. Am. J. Obstet. Gynecol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, G.; Gyte, G.M.; Dahlen, H.G.; Thomson, G.; Singata-Madliki, M.; Clegg, A.; Downe, S. Routine vaginal examinations compared to other methods for assessing progress of labour to improve outcomes for women and babies at term. Cochrane Database Syst. Rev. 2022, 3, CD010088. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Prostin (n = 104) | Propess (n = 101) | Total (n = 205) | p Value | |||
|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | ||
| Age (years) | 30.4 | 4.3 | 31.1 | 5.2 | 30.7 | 4.7 | 0.36 |
| Height (cm) | 167.3 | 5.6 | 166.3 | 6.4 | 166.8 | 6.0 | 0.24 |
| Weight at conception (kg) | 68.4 | 14.4 | 66.1 | 15.0 | 67.3 | 14.7 | 0.25 |
| Weight at delivery (kg) | 83.3 | 14.2 | 80.3 | 16.2 | 81.8 | 15.2 | 0.17 |
| N | % | N | % | N | % | ||
| Number of pregnancies | |||||||
| One | 60 | 57.7 | 56 | 55.5 | 116 | 56.6 | 0.85 |
| Two and more | 44 | 42.3 | 45 | 44.5 | 89 | 43.1 | |
| Number of deliveries | |||||||
| One | 74 | 71.2 | 73 | 72.3 | 147 | 71.7 | 0.98 |
| Two or more | 30 | 28.9 | 28 | 27.7 | 58 | 28.3 | |
| Smoking (Yes) | 7 | 6.7 | 5 | 5.0 | 12 | 5.9 | 0.81 |
| Disease before pregnancies | |||||||
| Yes | 10 | 9.6 | 8 | 7.9 | 18 | 8.8 | 0.86 |
| No | 94 | 90.4 | 93 | 92.1 | 187 | 91.2 | |
| Diseases in pregnancies | |||||||
| Yes | 62 | 59.6 | 63 | 62.4 | 125 | 61.0 | 0.79 |
| No | 42 | 40.4 | 38 | 37.6 | 80 | 39.0 | |
| SGB positive | |||||||
| Yes | 5 | 4.8 | 10 | 9.9 | 15 | 7.3 | 0.26 |
| No | 99 | 95.2 | 91 | 90.1 | 190 | 92.7 | |
| Days in hospital | |||||||
| 4 or less | 74 | 71.2 | 71 | 70.3 | 145 | 70.7 | 1.0 |
| 5 or more | 30 | 28.8 | 30 | 29.7 | 60 | 29.3 | |
| Weeks of gestation | |||||||
| 37 | 13 | 12.5 | 10 | 9.9 | 23 | 11.2 | 0.36 |
| 38 | 18 | 17.3 | 26 | 25.7 | 44 | 21.5 | |
| 39 | 28 | 26.9 | 32 | 31.7 | 60 | 29.3 | |
| 40 | 37 | 35.6 | 29 | 28.7 | 66 | 32.2 | |
| 41 | 8 | 7.7 | 4 | 3.96 | 12 | 5.85 | |
| Bishop score at induction of labour | |||||||
| From 1 to 3 | 35 | 33.6 | 45 | 44.6 | 80 | 39.1 | 0.15 |
| From 4 to 6 | 69 | 66.4 | 56 | 55.4 | 125 | 60.9 | |
| Parameter | Prostin (n = 104) | Propess (n = 101) | Total (n = 205) | p Value | Effect Size (CI) | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Number of repetitions | ||||||||
| 1 | 69 | 66.3 | 101 | 100.0 | ||||
| 2 | 21 | 20.2 | 0 | 0.0 | ||||
| 3 | 14 | 13.5 | 0 | 0.0 | ||||
| Propess usage duration | ||||||||
| 12 h or less | 77 | 76.2 | ||||||
| 13 h and more | 24 | 23.8 | ||||||
| 24 h pause | ||||||||
| No | 104 | 100 | 101 | 100 | 205 | 100.0 | 1.00 | |
| Yes | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Failed induction | ||||||||
| No | 96 | 92.3 | 96 | 95.0 | 192 | 93.6 | 0.80 | 1.03 |
| Yes | 8 | 7.7 | 5 | 5.0 | 13 | 6.4 | (0.96–1.11) | |
| Cause of failed induction | ||||||||
| Unresponsive cervix | 3 | 37.5 | 0 | 0.0 | 3 | 23.1 | 0.38 | |
| Pathologic CTG | 5 | 62.5 | 5 | 100 | 10 | 76.9 | ||
| Total | 8 | 100.0 | 5 | 100.0 | 13 | 100.0 | ||
| Parameter | Prostin (n = 96) | Propess (n = 96) | Total (n = 192) | p | Effect Size (CI) | |||
|---|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | |||
| Oxytocin max. dosage | 9.1 | 5.0 | 9.2 | 5.3 | 9.4 | 4.9 | 0.47 | 0.02 (−0.26–0.30) |
| N | % | N | % | N | % | |||
| Oxytocin usage | ||||||||
| No | 37 | 38.5 | 35 | 36.5 | 72 | 37.5 | 0.96 | |
| First stage | 35 | 36.5 | 36 | 37.5 | 71 | 37.0 | 1.00 (0.69–1.45) | |
| Second stage | 24 | 25.0 | 25 | 26.0 | 49 | 25.5 | 1.04 (0.64–1.68) | |
| Fetal scalp sampling | ||||||||
| No | 83 | 86.5 | 83 | 87.5 | 167 | 87.0 | 1.0 | |
| Yes | 13 | 13.5 | 13 | 12.5 | 26 | 13.5 | 1.00 (0.89–1.12) | |
| pH level—fetal scalp blood sampling | ||||||||
| Equal and less then 7.24 | 9 | 69.2 | 7 | 58.3 | 16 | 61.5 | 0.67 | 0.78 (0.30–2.00) |
| 7.25 and more | 4 | 30.8 | 6 | 50.0 | 10 | 38.5 | ||
| Fetal leading part | ||||||||
| Occipit Anterior | 94 | 97.9 | 94 | 97.9 | 188 | 97.9 | 0.14 | |
| Occipit Posterior | 2 | 2.1 | 2 | 2.1 | 4 | 2.1 | 1.00 (0.14–6.96) | |
| Labor analgesia | ||||||||
| No | 7 | 7.3 | 7 | 7.3 | 14 | 7.3 | 0.26 | 1.00 (0.36–2.74) |
| Epidural | 42 | 43.8 | 53 | 55.2 | 95 | 49.5 | ||
| Intravenous | 47 | 49.0 | 36 | 37.5 | 83 | 43.2 |
| Parameter | Prostin (n = 96) | Propess (n = 96) | Total (n = 192) | p | Effect Size (CI) | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Delivery abnormalities | ||||||||
| No | 79 | 82.3 | 77 | 80.2 | 156 | 81.3 | 0.85 | |
| Yes | 17 | 17.7 | 19 | 19.8 | 36 | 18.8 | 1.1 (0.6–2.0) | |
| Operative delivery | ||||||||
| No | 82 | 85.4 | 79 | 82.3 | 161 | 83.9 | 0.78 | |
| Caesarean section | 4 | 4.2 | 6 | 6.3 | 10 | 5.2 | 1.5 (0.4–5.2) | |
| Vacuum extraction | 10 | 10.4 | 11 | 11.5 | 21 | 10.9 | 1.1 (0.5–2.5) | |
| Cervical dilatation in time of caesarean section (cm) | ||||||||
| 6 or less | 2 | 50.0 | 4 | 66.7 | 6 | 60.0 | 0.78 | |
| 7 or more | 2 | 50.0 | 2 | 18.2 | 4 | 40.0 | ||
| Episiotomy | ||||||||
| No | 61 | 63.5 | 56 | 58.3 | 117 | 60.9 | 0.55 | |
| Mediolateral | 35 | 36.5 | 40 | 41.7 | 75 | 39.1 | 1.1 (0.8-1.6) | |
| Rupture | ||||||||
| No | 63 | 65.6 | 58 | 60.4 | 121 | 63.0 | 0.75 | |
| Small vaginal rupture | 31 | 32.3 | 36 | 37.5 | 67 | 34.9 | 1.2 (0.8–1.7) | |
| III- and IV-degree perineal rupture | 2 | 2.1 | 2 | 2.1 | 4 | 2.1 | 1.0 (0.1–7.0) | |
| Parameter | Prostin (n = 104) | Propess (n = 101) | Total (n = 205) | p Value | Effect Size (CI) | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Apgar in 1. min | ||||||||
| 7 and lower | 9 | 8.7 | 9 | 8.9 | 18 | 8.8 | 1.0 | 1.0 (0.4–2.5) |
| 8 and higher | 95 | 91.3 | 92 | 91.1 | 187 | 91.2 | ||
| Apgar in 5. min | ||||||||
| 7 and lower | 3 | 2.9 | 3 | 2.97 | 6 | 2.93 | 1.0 | 1.0 (0.2–5.0) |
| 8 and higher | 101 | 97.1 | 98 | 97.03 | 199 | 97.07 | ||
| Apgar in 10. min | ||||||||
| 7 and lower | 0 | 0.0 | 2 | 1.98 | 2 | 0.98 | 0.46 | 5.1 (0.3–105.9) |
| 8 and higher | 104 | 100.0 | 99 | 98.02 | 203 | 98.02 | ||
| Admission to intensive care unit | ||||||||
| No | 104 | 100.0 | 100 | 99.01 | 204 | 99.5 | 0.89 | |
| Yes | 0 | 100.0 | 1 | 0.99 | 1 | 0.5 | 3.1 (0.1–74.9) | |
| (a) Mean values and standard deviations of the individual delivery interval lengths compared across research groups. | |||||||||
| Parameter | Prostin (n = 96) | Propess (n = 96) | Total (n = 192) | pValue | Effect Size (CI) | ||||
| Average | SD | Average | SD | Average | SD | ||||
| PROM—Induction (min) | 480 | 395 | 473 | 323 | 476 | 360 | 0.94 | −0.02 (−0.3–0.3) | |
| Prostin (n = 94) | Propess (n = 92) | Total (n = 186) | |||||||
| Average | SD | Average | SD | Average | SD | ||||
| Induction—Active phase (min) | 683 | 413 | 657 | 486 | 670 | 450 | 0.70 | −0.06 (−0.3–0.2) | |
| Prostin (n = 92) | Propess (n = 90) | Total (n = 182) | |||||||
| Average | SD | Average | SD | Average | SD | ||||
| Active phase—Delivery (min) | 128 | 89 | 138 | 79 | 133 | 84 | 0.43 | 0.12 (−0.17–0.41) | |
| Prostin (n = 92) | Propess (n = 90) | Total (n = 182) | |||||||
| Average | SD | Average | SD | Average | SD | ||||
| PROM—Delivery (min) | 1299 | 577 | 1252 | 607 | 1276 | 590 | 0.60 | −0.08 (−0.4–0.2) | |
| Prostin (n = 92) | Propess (n = 90) | Total (n = 182) | |||||||
| Average | SD | Average | SD | Average | SD | ||||
| Induction—Delivery (min) | 817 | 440 | 799 | 519 | 808 | 479 | 0.80 | −0.04 (−0.32–0.25) | |
| (b) Mean values and standard deviations of the individual delivery interval lengths compared across research groups by Bishop score. | |||||||||
| Parameter | Group | Bishop Three or Less | Bishop Four or More | Bishop (n = 192) | pValue | ||||
| Average | SD | Average | SD | Average | SD | ||||
| PROM—Induction (min) | All (n = 192) | 467 | 310 | 481 | 388 | 476 | 360 | 0.58 | |
| Prostin (n = 96) | 476 | 307 | 481 | 434 | 480 | 395 | 0.61 | ||
| Propess (n = 96) | 459 | 316 | 482 | 331 | 472 | 323 | 0.91 | ||
| Average | SD | Average | SD | Average | SD | ||||
| Induction—Active phase (min) | All (n = 186) | 853 | 511 | 563 | 372 | 670 | 450 | 0.0001 | |
| Prostin (n = 94) | 947 | 458 | 559 | 327 | 683 | 413 | 0.0001 | ||
| Propess (n = 92) | 780 | 422 | 566 | 422 | 657 | 487 | 0.04 | ||
| Average | SD | Average | SD | Average | SD | ||||
| Active phase—Delivery (min) | All (n = 182) | 142 | 91 | 127 | 80 | 133 | 85 | 0.35 | |
| Prostin (n = 92) | 148 | 111 | 119 | 76 | 128 | 89 | 0.42 | ||
| Propess (n = 90) | 138 | 75 | 138 | 84 | 138 | 80 | 0.81 | ||
| Average | SD | Average | SD | Average | SD | ||||
| PROM—Delivery (min) | All (n = 182) | 1495 | 664 | 1152 | 505 | 1276 | 590 | 0.0001 | |
| Prostin (n = 92) | 1612 | 597 | 1155 | 508 | 1299 | 575 | 0.0001 | ||
| Propess (n = 90) | 1402 | 707 | 1148 | 508 | 1253 | 607 | 0.07 | ||
| Average | SD | Average | SD | Average | SD | ||||
| Induction—Delivery (min) | All (n = 182) | 1013 | 550 | 691 | 391 | 808 | 479 | <0.0001 | |
| Prostin (n = 92) | 1114 | 478 | 680 | 348 | 817 | 440 | <0.0002 | ||
| Propess (n = 90) | 934 | 595 | 705 | 439 | 799 | 519 | 0.051 | ||
| Parameter | Values |
|---|---|
| PROM Induction vs. Induction Active phase interval | |
| Spearman (rho) | 0.050 |
| p | 0.490 |
| PROM Induction vs. Active phase Delivery interval | |
| Spearman | 0.076 |
| p | 0.306 |
| PROM Induction vs. PROM Delivery interval | |
| Spearman | 0.076 |
| p | 0.31 |
| PROM Induction vs. Induction delivery interval | |
| Spearman | 0.07 |
| p | 0.37 |
| Induction Active phase vs. PROM Delivery interval | |
| Spearman | 0.83 |
| p | <0.0001 |
| Induction Active phase vs. Induction Delivery interval | |
| Spearman | 0.98 |
| p | <0.0001 |
| (a) Logistic regressions analysis for risk of operative delivery. | ||||
| Predictor Factor | Regression Coefficient (Beta) | Significance Level (p) | aOR = Exp (Beta) | |
| Constant | −3.12 | <0.001 | 0.04 | |
| Bishop Score | −0.42 | 0.256 | 0.66 | |
| Primiparous women | 1.76 | 0.005 | 5.81 | |
| Induction Delivery Interval (ID interval) | 0.0002 | 0.737 | 1.00 | |
| PROM—Delivery Interval (PD interval) | 0.0003 | 0.0552 | 1.00 | |
| (b) Linear regression analysis for induction to delivery interval | ||||
| Adjusted R-Squared: 0.976, p < 0.0001 | ||||
| Predictor Factor | Coefficient | SE | tValue | pValue |
| Intercept | 57.82 | 17.38 | 3.33 | 0.001 |
| Induction—Active phase interval | 1.02 | 0.01 | 77.6 | <0.001 |
| Propess | 9.38 | 11.11 | 0.84 | 0.399 |
| Bishop score | 0.6 | 12.22 | 0.05 | 0.956 |
| Primiparous women | 81.61 | 12.14 | 6.72 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anzeljc, V.; Mujezinović, F. A Randomised Controlled Trial Comparing Induction of Labour with the Propess Vaginal System to the Prostin Vaginal Tablet in Premature Rupture of Membranes at Term. J. Clin. Med. 2023, 12, 174. https://doi.org/10.3390/jcm12010174
Anzeljc V, Mujezinović F. A Randomised Controlled Trial Comparing Induction of Labour with the Propess Vaginal System to the Prostin Vaginal Tablet in Premature Rupture of Membranes at Term. Journal of Clinical Medicine. 2023; 12(1):174. https://doi.org/10.3390/jcm12010174
Chicago/Turabian StyleAnzeljc, Veronika, and Faris Mujezinović. 2023. "A Randomised Controlled Trial Comparing Induction of Labour with the Propess Vaginal System to the Prostin Vaginal Tablet in Premature Rupture of Membranes at Term" Journal of Clinical Medicine 12, no. 1: 174. https://doi.org/10.3390/jcm12010174
APA StyleAnzeljc, V., & Mujezinović, F. (2023). A Randomised Controlled Trial Comparing Induction of Labour with the Propess Vaginal System to the Prostin Vaginal Tablet in Premature Rupture of Membranes at Term. Journal of Clinical Medicine, 12(1), 174. https://doi.org/10.3390/jcm12010174





