Feasibility of Combining Disease-Specific and Balance-Related Measures as Risk Predictors of Future Falls in Patients with Parkinson’s Disease
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Patient Selection
2.2. Clinical Assessment of PD
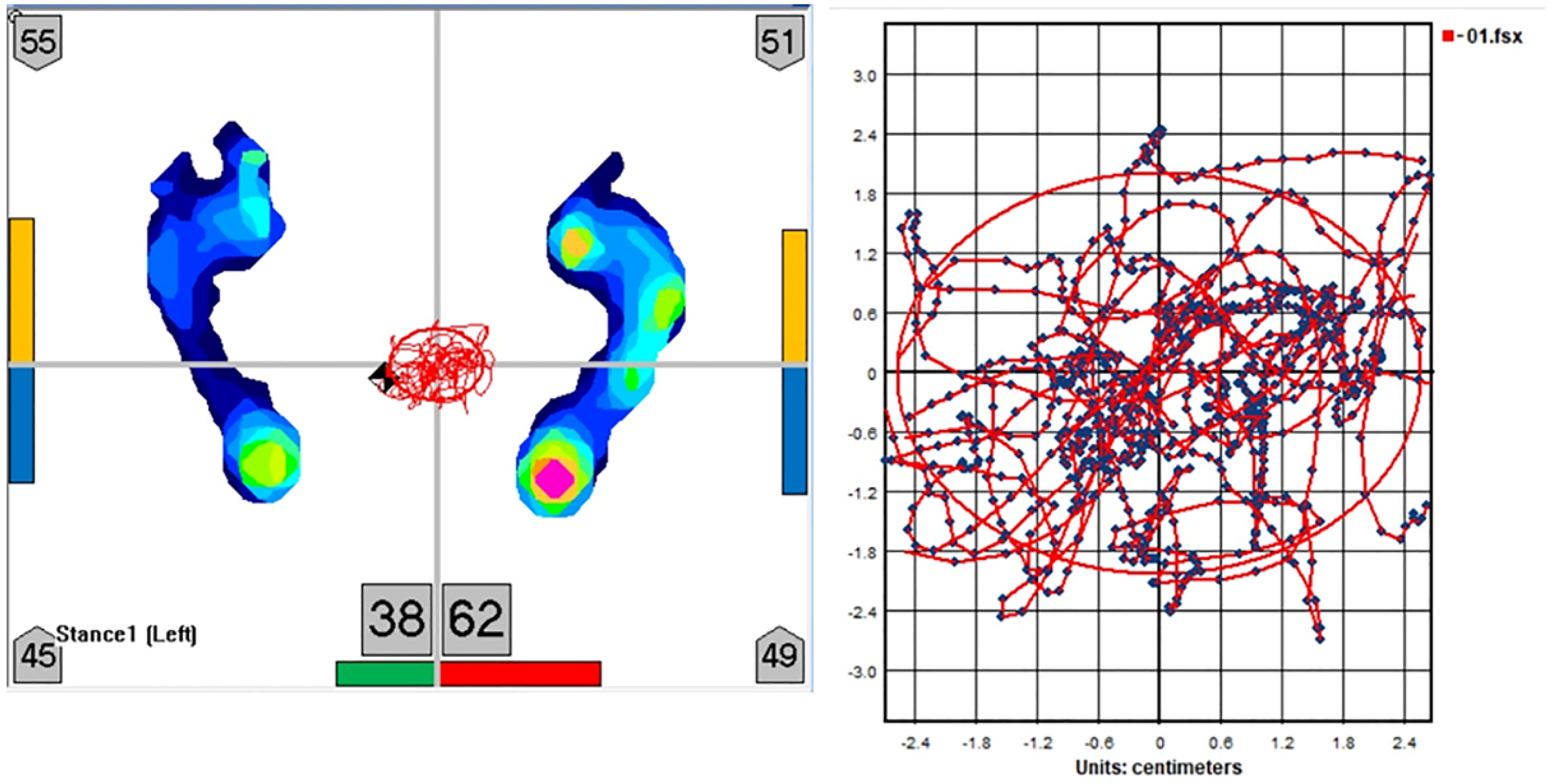
2.3. Measurement of Postural Sway
2.4. Assessment of the Moment of Inertia
2.5. Falls Assessments
2.6. Statistical Analysis
3. Results
3.1. General Characteristics of Patients with PD
3.2. L-Dopa-Induced Dyskinesia Increases Postural Sway
3.3. Dopaminergic Therapy Improved Functional and Fall Risk Scores
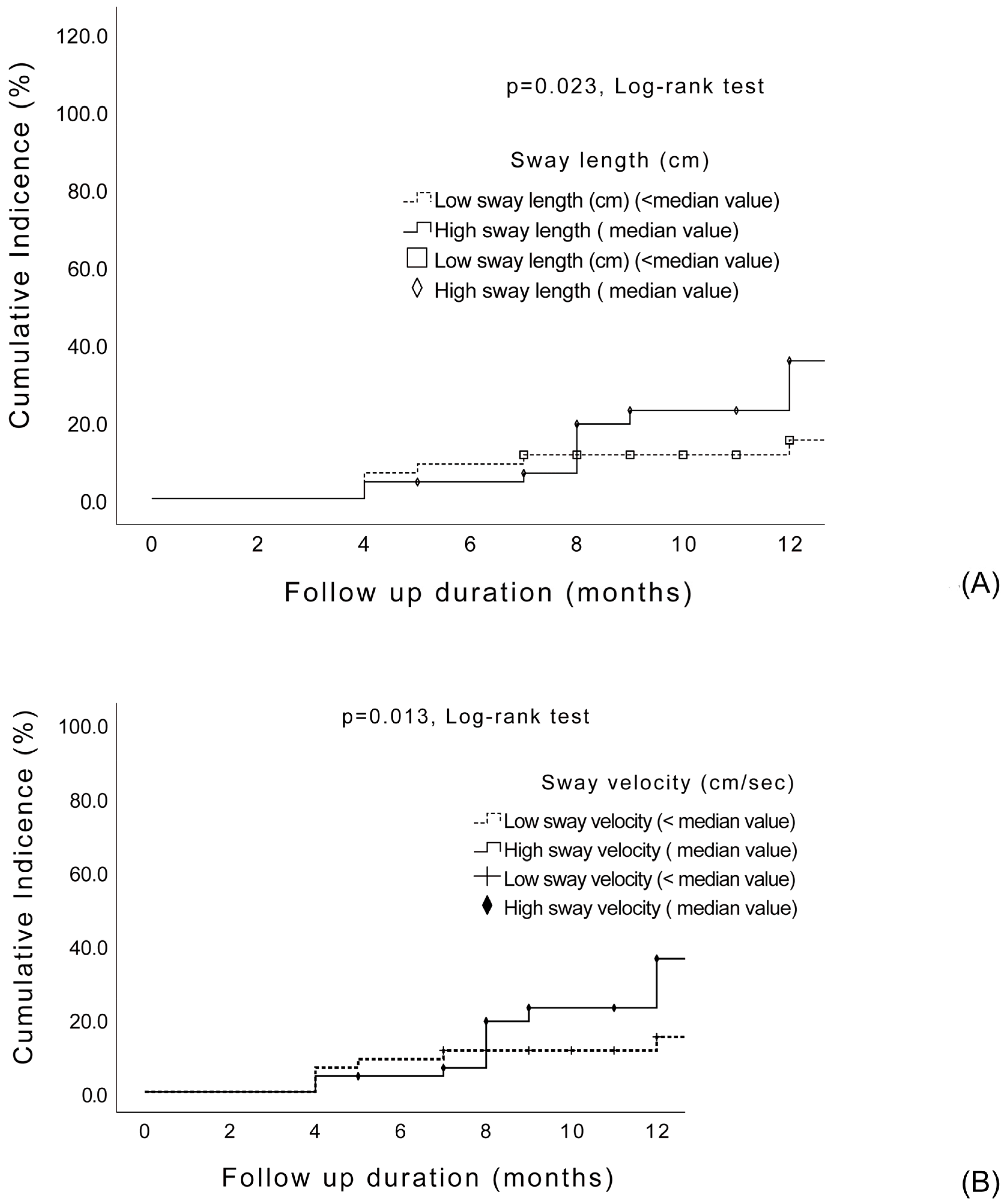
3.4. Risk Factors Associated with Subsequent Falls in the Cox Proportional Hazards Model
3.5. Partial Correlation Analyses of Standing Postural Sway, LEDD, and UPDRS Total Score after Controlling with Moment of Inertia
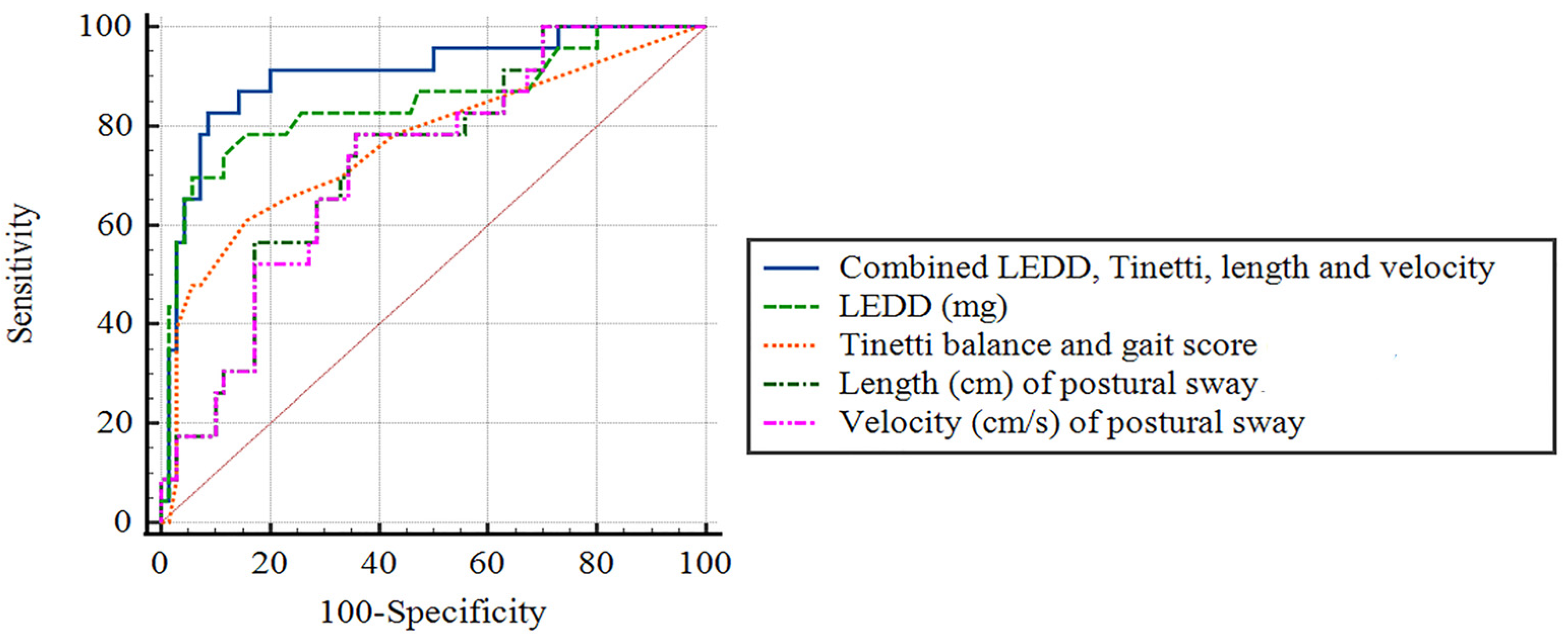
3.6. Diagnostic Accuracy for Predicting the Presence of Prospective Falls, Using Receiver-Operating Characteristic Curve Analysis
4. Discussion
4.1. Major Findings
4.2. The Effects of Clinical Disease Severity on Standing Postural Sway
4.3. The Effects of Dopaminergic Therapy on Standing Postural Sway
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Brown, L.A.; Cooper, S.A.; Doan, J.B.; Dickin, D.C.; Whishaw, I.Q.; Pellis, S.M.; Suchowersky, O. Parkinsonian deficits in sensory integration for postural control: Temporal response to changes in visual input. Park. Relat. Disord. 2006, 12, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Frenklach, A.; Louie, S.; Koop, M.M.; Bronte-Stewart, H. Excessive postural sway and the risk of falls at different stages of Parkinson’s disease. Mov. Disord. 2009, 24, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Tigrini, A.; Verdini, F.; Fioretti, S.; Mengarelli, A. Center of pressure plausibility for the double-link human stance model under the intermittent control paradigm. J. Biomech. 2021, 128, 110725. [Google Scholar] [CrossRef] [PubMed]
- Schieppati, M.; Nardone, A. Free and supported stance in Parkinson’s disease. The effect of posture and ‘postural set’ on leg muscle responses to perturbation, and its relation to the severity of the disease. Brain 1991, 114 Pt 3, 1227–1244. [Google Scholar] [CrossRef]
- Burleigh, A.; Horak, F.; Nutt, J.; Frank, J. Levodopa reduces muscle tone and lower extremity tremor in Parkinson’s disease. Can. J. Neurol. Sci. 1995, 22, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; Collins, J.J.; De Luca, C.J.; Burrows, A.; Lipsitz, L.A. Open-loop and closed-loop postural control mechanisms in Parkinson’s disease: Increased mediolateral activity during quiet standing. Neurosci. Lett. 1995, 197, 133–136. [Google Scholar] [CrossRef]
- Viitasalo, M.K.; Kampman, V.; Sotaniemi, K.A.; Leppavuori, S.; Myllyla, V.V.; Korpelainen, J.T. Analysis of sway in Parkinson’s disease using a new inclinometry-based method. Mov. Disord. 2002, 17, 663–669. [Google Scholar] [CrossRef]
- Contin, M.; Riva, R.; Baruzzi, A.; Albani, F.; Macri, S.; Martinelli, P. Postural stability in Parkinson’s disease: The effects of disease severity and acute levodopa dosing. Park. Relat. Disord. 1996, 2, 29–33. [Google Scholar] [CrossRef]
- Matinolli, M.; Korpelainen, J.T.; Korpelainen, R.; Sotaniemi, K.A.; Virranniemi, M.; Myllyla, V.V. Postural sway and falls in Parkinson’s disease: A regression approach. Mov. Disord. 2007, 22, 1927–1935. [Google Scholar] [CrossRef]
- Mancini, M.; Carlson-Kuhta, P.; Zampieri, C.; Nutt, J.G.; Chiari, L.; Horak, F.B. Postural sway as a marker of progression in Parkinson’s disease: A pilot longitudinal study. Gait. Posture 2012, 36, 471–476. [Google Scholar] [CrossRef]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease. Mov. Disord. 2015, 30, 1361–1370. [Google Scholar] [CrossRef]
- Rochester, L.; Baker, K.; Nieuwboer, A.; Burn, D. Targeting dopa-sensitive and dopa-resistant gait dysfunction in Parkinson’s disease: Selective responses to internal and external cues. Mov. Disord. 2011, 26, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Bekkers, E.M.J.; Dijkstra, B.W.; Heremans, E.; Verschueren, S.M.P.; Bloem, B.R.; Nieuwboer, A. Balancing between the two: Are freezing of gait and postural instability in Parkinson’s disease connected? Neurosci. Biobehav. Rev. 2018, 94, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Shivitz, N.; Koop, M.M.; Fahimi, J.; Heit, G.; Bronte-Stewart, H.M. Bilateral subthalamic nucleus deep brain stimulation improves certain aspects of postural control in Parkinson’s disease, whereas medication does not. Mov. Disord. 2006, 21, 1088–1097. [Google Scholar] [CrossRef]
- Jahn, K.; Deutschlander, A.; Stephan, T.; Kalla, R.; Wiesmann, M.; Strupp, M.; Brandt, T. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage 2008, 39, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.K.; Worringham, C.J.; Cole, M.H.; Lacherez, P.F.; Wood, J.M.; Silburn, P.A. Predictors of future falls in Parkinson disease. Neurology 2010, 75, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Heim, B.; Krismer, F.; De Marzi, R.; Seppi, K. Magnetic resonance imaging for the diagnosis of Parkinson’s disease. J. Neural. Transm. 2017, 124, 915–964. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Gil-Nagel, A.; Gracia, L.M.; Gomez, J.B.; Martinez-Sarries, J.; Bermejo, F. Unified Parkinson’s Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov. Disord. 1994, 9, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; McDermott, M.; Carter, J.; Gauthier, S.; Goetz, C.; Golbe, L.; Huber, S.; Koller, W.; Olanow, C.; Shoulson, I.; et al. Variable expression of Parkinson’s disease: A base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990, 40, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Chang, C.C.; Chang, W.N.; Tsai, N.W.; Huang, C.C.; Chang, Y.T.; Wang, H.C.; Kung, C.T.; Su, Y.J.; Lin, W.C.; et al. Neuropsychiatric symptoms in Alzheimer’s disease: Associations with caregiver burden and treatment outcomes. QJM 2017, 110, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Smith, C.E.; Suzuki, Y.; Kiyono, K.; Tanahashi, T.; Sakoda, S.; Morasso, P.; Nomura, T. Universal and individual characteristics of postural sway during quiet standing in healthy young adults. Physiol. Rep. 2015, 3, e12329. [Google Scholar] [CrossRef]
- Paul, S.S.; Canning, C.G.; Sherrington, C.; Lord, S.R.; Close, J.C.; Fung, V.S. Three simple clinical tests to accurately predict falls in people with Parkinson’s disease. Mov. Disord. 2013, 28, 655–662. [Google Scholar] [CrossRef]
- Youn, J.; Okuma, Y.; Hwang, M.; Kim, D.; Cho, J.W. Falling Direction can Predict the Mechanism of Recurrent Falls in Advanced Parkinson’s Disease. Sci. Rep. 2017, 7, 3921. [Google Scholar] [CrossRef]
- Revilla, F.J.; Larsh, T.R.; Mani, A.; Duker, A.P.; Cox, C.; Succop, P.; Gartner, M.; Jarrin Tejada, C.; Bhattacharya, A. Effect of dopaminergic medication on postural sway in advanced Parkinson’s disease. Front. Neurol. 2013, 4, 202. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Simundic, A.M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. [Google Scholar]
- Johnson, L.; James, I.; Rodrigues, J.; Stell, R.; Thickbroom, G.; Mastaglia, F. Clinical and posturographic correlates of falling in Parkinson’s disease. Mov. Disord. 2013, 28, 1250–1256. [Google Scholar] [CrossRef]
- Lindholm, B.; Nilsson, M.H.; Hansson, O.; Hagell, P. External validation of a 3-step falls prediction model in mild Parkinson’s disease. J. Neurol. 2016, 263, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, B.; Hagell, P.; Hansson, O.; Nilsson, M.H. Prediction of falls and/or near falls in people with mild Parkinson’s disease. PLoS ONE 2015, 10, e0117018. [Google Scholar] [CrossRef] [PubMed]
- Nantel, J.; McDonald, J.C.; Bronte-Stewart, H. Effect of medication and STN-DBS on postural control in subjects with Parkinson’s disease. Park. Relat. Disord. 2012, 18, 285–289. [Google Scholar] [CrossRef]
- Rocchi, L.; Chiari, L.; Horak, F.B. Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Merello, M.; Balej, J.; Delfino, M.; Cammarota, A.; Betti, O.; Leiguarda, R. Apomorphine induces changes in GPi spontaneous outflow in patients with Parkinson’s disease. Mov. Disord. 1999, 14, 45–49. [Google Scholar] [CrossRef]
- Filion, M.; Tremblay, L.; Bedard, P.J. Effects of dopamine agonists on the spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991, 547, 152–161. [Google Scholar] [CrossRef]
- Bronte-Stewart, H.M.; Minn, A.Y.; Rodrigues, K.; Buckley, E.L.; Nashner, L.M. Postural instability in idiopathic Parkinson’s disease: The role of medication and unilateral pallidotomy. Brain 2002, 125, 2100–2114. [Google Scholar] [CrossRef]
- Vitek, J.L.; Giroux, M. Physiology of hypokinetic and hyperkinetic movement disorders: Model for dyskinesia. Ann. Neurol. 2000, 47, S131–S140. [Google Scholar]
- St George, R.J.; Nutt, J.G.; Burchiel, K.J.; Horak, F.B. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology 2010, 75, 1292–1299. [Google Scholar] [CrossRef]
- Buhmann, C.; Huckhagel, T.; Engel, K.; Gulberti, A.; Hidding, U.; Poetter-Nerger, M.; Goerendt, I.; Ludewig, P.; Braass, H.; Choe, C.U.; et al. Adverse events in deep brain stimulation: A retrospective long-term analysis of neurological, psychiatric and other occurrences. PLoS ONE 2017, 12, e0178984. [Google Scholar] [CrossRef]
- Johansson, J.; Nordstrom, A.; Gustafson, Y.; Westling, G.; Nordstrom, P. Increased postural sway during quiet stance as a risk factor for prospective falls in community-dwelling elderly individuals. Age Ageing 2017, 46, 964–970. [Google Scholar] [CrossRef] [PubMed]

| Previous Falls | p-Value | ||
|---|---|---|---|
| Non-Fallers (n = 57) (n, %) | Fallers (n = 38) (n, %) | ||
| Age, years | 67.6 ± 10.1 | 69.0 ± 9.5 | 0.48 |
| Sex (men/women) | 32/27 | 15/21 | 0.16 |
| Body mass index (kg/m2) | 25.4 ± 4.2 | 24.9 ± 4.1 | 0.62 |
| Disease duration, years | 4.0 ± 3.5 | 7.7 ± 5.0 | <0.0001 |
| Presence of dyskinesia | 8 | 12 | 0.03 |
| Freezing of gait | 14 | 22 | 0.001 |
| Orthostatic hypotension | 5 | 1 | 0.4 |
| Fear of fall | 14 | 25 | <0.0001 |
| UPDRS total score (off-state) | 34.2 ± 18.8 | 46.4 ± 16.9 | 0.002 |
| UPDRS I (Mentation, behavior, and mood) | 1.4 ± 1.0 | 2.2 ± 1.7 | 0.01 |
| UPDRS II (ADL score) | 8.0 ± 4.5 | 13.0 ± 5.1 | <0.0001 |
| UPDRS III (motor score) (off-state) | 16.7 ± 10.3 | 19.9 ± 8.0 | 0.11 |
| UPDRS IV (motor complications) | 8.1 ± 5.3 | 11.3 ± 5.1 | 0.004 |
| UPDRS-derived PIGD score | 3.4 ± 2.6 | 7.0 ± 3.6 | <0.0001 |
| Hoehn and Yahr Staging | 1.9 ± 0.8 | 2.4 ± 1.0 | 0.006 |
| Cognitive Abilities Screening Instrument | 83.5 ± 14.3 | 81.7 ± 12.4 | 0.59 |
| Tinetti balance score | 9.5 ± 2.8 | 6.9 ± 2.9 | <0.0001 |
| Tinetti gait score | 14.4 ± 2.6 | 12.2 ± 3.4 | 0.001 |
| Tinetti balance and gait score | 24.0 ± 5.0 | 19.1 ± 2.6 | <0.0001 |
| Anti-Parkinsonian medications Φ | |||
| LEDD (mg/day) | 604.3 ± 365.4 | 1146.9 ± 684.2 | <0.0001 |
| Levodopa | 50 (87.7%) | 24 (63.2%) | |
| Dopamine agonist (Pramipexole/Ropinirole) | 35 (61.4%) | 28 (73.7%) | |
| MAO-B inhibitors (Selegiline/Rasagiline) | 24 (42.1%) | 13 (34.2%) | |
| COMT inhibitors (Entacapone) | 3 (5.3%) | 9 (23.7%) | |
| Amantadine | 2 (3.5%) | 7 (18.4%) | |
| Falling frequency | |||
| Once/per year | -- | 23 (60.5%) | |
| Once/per month | -- | 11 (28.9%) | |
| Once/per week | -- | 1 (2.6%) | |
| ≥Once/per day | -- | 3 (7.9%) | |
| Falling severity | |||
| Mild | -- | 24 (63.2%) | |
| Moderate | -- | 13 (34.2%) | |
| Severe | -- | 1 (2.6%) | |
| Falling situations | |||
| Sitting/standing | -- | 11 (26.3%) | |
| Walking | -- | 25 (65.8%) | |
| Turning | -- | 3 (7.9%) | |
| PD | Healthy Control (n = 23) | p-Value Ω | |||
|---|---|---|---|---|---|
| With Dyskinesia α (n = 20) | Without Dyskinesia α (n = 75) | p-Value β,κ | |||
| Age, years | 65.4 ± 7.8 | 68.9 ± 10.4 | 0.16 | 67.2 ± 9.7 | 0.11 |
| Disease duration, years | 9.7 ± 4.7 | 4.3 ± 3.7 | <0.0001 | - | |
| LEDD (mg) | 1477.1 ± 505.87 | 632.4 ± 443.2 | <0.0001 | - | |
| Parameters of postural sway | |||||
| Area (cm2) | |||||
| Off-state | 2.4 ± 1.5 * | 2.0 ± 1.2 * | 0.33 ‖ | 1.2 ± 0.8 * | 0.007 † |
| On-state | 5.0 ± 3.3 | 3.0 ± 2.0 | 0.001 | ||
| Velocity (cm/s) | |||||
| Off-state | 1.2 ± 0.4 * | 1.2 ± 0.9 | 0.9 | 0.8 ± 0.2 | 0.049 † |
| On-state | 1.7 ± 0.7 | 1.2 ± 0.6 | 0.004 | ||
| Length (cm) | |||||
| Off-state | 36.7 ± 27.4 * | 36.2 ± 11.8 | 0.94 | 23.5 ± 5.0 | 0.048 † |
| On-state | 50.3 ± 20.6 | 35.9 ± 17.6 | 0.002 | ||
| Length y (cm) | |||||
| Off-state | 2.5 ± 0.8 * | 2.4 ± 0.6 * | 0.44 ‖ | 2.1 ± 0.7 | 0.44 |
| On-state | 3.4 ± 1.0 | 2.7 ± 0.9 | 0.01 | ||
| Length x (cm) | |||||
| Off-state | 1.8 ± 0.7 * | 1.7 ± 0.7 * | 0.49 ‖ | 1.2 ± 0.3 | 0.001 † |
| On-state | 2.9 ± 1.3 | 2.0 ± 0.9 | 0.01 | ||
| Clinical scores | |||||
| PIGD score | |||||
| Off-state | 7.5 ± 2.4 * | 4.1 ± 3.9 * | <0.0001 ‖ | ||
| On-state | 4.1 ± 2.9 | 2.6 ± 2.5 | 0.03 | ||
| UPDRS III | |||||
| Off-state | 20.3 ± 8.1 * | 17.3 ± 10.0 * | 0.22 | ||
| On-state | 10.9 ± 6.8 | 11.5 ± 7.4 | 0.74 | ||
| UPDRS total score | |||||
| Off-state | 48.1 ± 12.3 * | 36.4 ± 19.9 * | 0.02 ‖ | ||
| On-state | 29.4 ± 11.0 | 21.2 ± 11.9 | 0.74 | ||
| Falls risk score | |||||
| Tinetti balance score | |||||
| Off-state | 7.7 ± 2.5 * | 8.7 ± 3.2 * | 0.20 | ||
| On-state | 11.1 ± 1.4 | 10.4 ± 2.0 | 0.15 | ||
| Tinetti gait score | |||||
| Off-state | 13.0 ± 2.5 * | 13.7 ± 3.3 * | 0.36 | ||
| On-state | 15.1 ± 1.6 | 15.2 ± 1.7 | 0.79 | ||
| Tinetti balance and gait score | |||||
| Off-state | 20.7 ± 4.0 * | 22.5 ± 6.1 * | 0.24 | ||
| On-state | 26.2 ± 2.6 | 25.6 ± 3.5 | 0.48 | ||
| Univariate | Prospective Falls (n = 24) | No Prospective Falls (n = 71) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |||
| Age, years | 68.8 ± 10.3 | 67.9 ± 9.7 | 1.01 (0.97–1.06) | 0.56 | ||
| Disease duration (years) | 8.4 ± 5.4 | 4.4 ± 3.6 | 1.13 (1.04–1.22) | 0.001 * | ||
| LEDD (mg/day) | 1377.1 ± 604.8 | 615.1 ± 410.8 | 1.0 (1.0–1.01) | <0.0001 * | 1.0 (1.0–1.01) | 0.008 * |
| Previous falls history | 24 | 14 | 130.0 (3.8–4443.4) | 0.007 * | 139.4 (3.6–5392.0) | <0.0001 * |
| Height (m) | 1.59 ± 0.08 | 1.59 ± 0.09 | 0.53 (0.003–101.15) | 0.82 | ||
| Body weight (kg) | 63.0 ± 11.9 | 63.8 ± 11.2 | 0.99 (0.96–1.02) | 0.52 | ||
| Body mass index | 24.9 ± 4.1 | 25.2 ± 4.2 | 0.97 (0.89–1.07) | 0.53 | ||
| Presence of dyskinesia | 11 (45.8%) | 9 (12.7%) | 2.87 (1.27–6.5) | 0.01 | ||
| Orthostatic hypotension | 5 | 1 | 1.18 (0.168.79) | 0.88 | ||
| Fear of fall | 20 | 19 | 10.7 (3.5931.9) | <0.0001 | ||
| Freezing of gait | 18 | 18 | 5.01 (2.0112.86) | 0.001 | ||
| Clinical scores | ||||||
| UPDRS total score | ||||||
| Off-state | 50.6 ± 16.1 | 34.6 ± 18.2 | 1.03 (1.02–1.05) | <0.0001 * | ||
| On-state | 31.4 ± 12.1 | 20.0 ± 10.5 | 1.07 (1.04–1.1) | <0.0001 * | ||
| Tinetti balance and gait score | ||||||
| Off-state | 17.7 ± 4.7 | 23.7 ± 5.3 | 0.83 (0.76–0.90) | <0.0001 * | ||
| On-state | 23.6 ± 3.5 | 26.4 ± 2.9 | 0.82 (0.75–0.89) | <0.0001 * | 0.82 (0.74–0.91) | 0.04 * |
| Cognitive Abilities Screening Instrument | 83.1 ± 11.1 | 82.6 ± 14.3 | 1.0 (0.97–1.04) | 0.81 | ||
| Standing postural sway, off-state | ||||||
| Area (cm2) | 2.3 ± 1.5 | 2.0 ± 1.2 | 1.23 (0.92–1.63) | 0.16 | ||
| Velocity (cm/s) | 1.4 ± 0.8 | 1.1 ± 0.8 | 1.43 (0.99–2.03) | 0.051 | ||
| Length (cm) | 41.7 ± 24.1 | 34.4 ± 24.8 | 1.01 (1.0–1.02) | 0.053 | ||
| Length y (cm) | 2.6 ± 0.8 | 2.4 ± 0.6 | 1.65 (0.91–3.01) | 0.1 | ||
| Length x (cm) | 1.9 ± 0.8 | 1.6 ± 0.6 | 1.62 (0–2.91) | 0.11 | ||
| Standing postural sway, on-state | ||||||
| Area (cm2) | 3.7 ± 2.5 | 3.4 ± 2.4 | 1.05 (0.91–1.21) | 0.54 | ||
| Velocity (cm/s) | 1.6 ± 0.8 | 1.2 ± 0.5 | 1.72 (1.1–2.68) | 0.016 * | ||
| Length (cm) | 47.8 ± 27.1 | 35.5 ± 15.7 | 1.02 (1.0–1.03) | 0.011 * | ||
| Length y (cm) | 3.0 ± 0.9 | 2.8 ± 1.0 | 1.1 (0.78–1.62) | 0.54 | ||
| Length x (cm) | 2.4 ± 1.0 | 2.1 ± 1.0 | 1.16 (0.82–1.64) | 0.4 | ||
| Spearman Correlation | LEDD | UPDRS Total Score, on Stage | Tinetti Balance and Gait Score | |||
|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | |
| Baseline characteristics | ||||||
| Disease duration (years) | 0.62 | <0.0001 | 0.33 | 0.001 | −0.09 | 0.42 |
| UPDRS total score, on-state | 0.48 | <0.0001 | -- | -- | −0.54 | <0.0001 |
| LEDD (mg/day) | -- | -- | 0.48 | <0.0001 | −0.25 | 0.02 |
| Standing postural sway, on-state | ||||||
| Length (cm) | 0.39 | <0.0001 | 0.42 | <0.0001 | −0.38 | <0.0001 |
| Velocity (cm/s) | 0.38 | <0.0001 | 0.41 | <0.0001 | −0.38 | <0.0001 |
| Significant Parameters † | Cut-Off Value | Sensitivity (%) | Specificity (%) | AUC (95% CI) | p-Value |
|---|---|---|---|---|---|
| Combined LEDD, Tinetti balance and gait score, and length and velocity of postural sway | -- | 91.3% | 80% | 0.90 (0.83–0.98) | <0.0001 |
| LEDD (mg) | 895 | 82.6% | 74.3% | 0.85 (0.74–0.96) | <0.0001 |
| Tinetti balance and gait score (on-state) | 24.5 | 84.3% | 60.0% | 0.76 (0.64–0.89) | <0.0001 |
| Length (cm) of postural sway, on-state | 35.7 | 78.3% | 62.9% | 0.73 (0.62–0.84) | 0.001 |
| Velocity (cm/s) of postural sway, on-state | 1.19 | 73.9% | 65.7% | 0.72 (0.61–0.84) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-L.; Lai, Y.-R.; Lien, C.-Y.; Huang, C.-C.; Chiu, W.-C.; Chen, Y.-S.; Yu, C.-C.; Cheng, B.-C.; Chiang, Y.-F.; Chang, H.-W.; et al. Feasibility of Combining Disease-Specific and Balance-Related Measures as Risk Predictors of Future Falls in Patients with Parkinson’s Disease. J. Clin. Med. 2023, 12, 127. https://doi.org/10.3390/jcm12010127
Tsai C-L, Lai Y-R, Lien C-Y, Huang C-C, Chiu W-C, Chen Y-S, Yu C-C, Cheng B-C, Chiang Y-F, Chang H-W, et al. Feasibility of Combining Disease-Specific and Balance-Related Measures as Risk Predictors of Future Falls in Patients with Parkinson’s Disease. Journal of Clinical Medicine. 2023; 12(1):127. https://doi.org/10.3390/jcm12010127
Chicago/Turabian StyleTsai, Chang-Lin, Yun-Ru Lai, Chia-Yi Lien, Chih-Cheng Huang, Wen-Chan Chiu, Yueh-Sheng Chen, Chiun-Chieh Yu, Ben-Chung Cheng, Yi-Fang Chiang, Hsueh-Wen Chang, and et al. 2023. "Feasibility of Combining Disease-Specific and Balance-Related Measures as Risk Predictors of Future Falls in Patients with Parkinson’s Disease" Journal of Clinical Medicine 12, no. 1: 127. https://doi.org/10.3390/jcm12010127
APA StyleTsai, C.-L., Lai, Y.-R., Lien, C.-Y., Huang, C.-C., Chiu, W.-C., Chen, Y.-S., Yu, C.-C., Cheng, B.-C., Chiang, Y.-F., Chang, H.-W., & Lu, C.-H. (2023). Feasibility of Combining Disease-Specific and Balance-Related Measures as Risk Predictors of Future Falls in Patients with Parkinson’s Disease. Journal of Clinical Medicine, 12(1), 127. https://doi.org/10.3390/jcm12010127







