Clinicopathological Characteristics of Primary Pulmonary Hodgkin Lymphoma (PPHL): Two Institutional Experiences with Comprehensive Literature Review of 115 PPHL Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection and Clinicopathological Data Collection
2.2. Immunohistochemical Staining
2.3. EBER-ISH
2.4. Comprehensive Literature Review
2.5. Statistical Analysis
3. Results
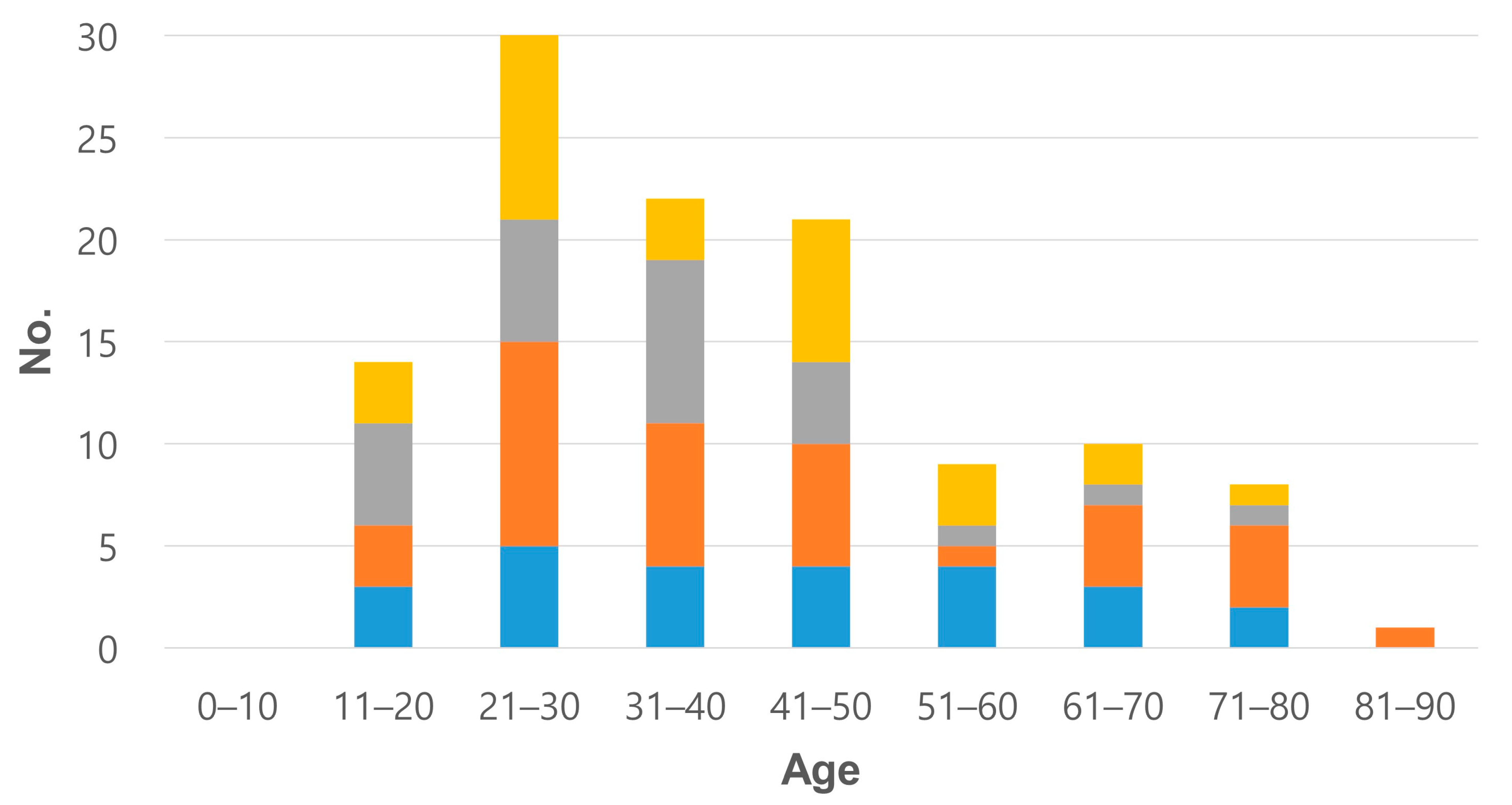
3.1. Clinical Features
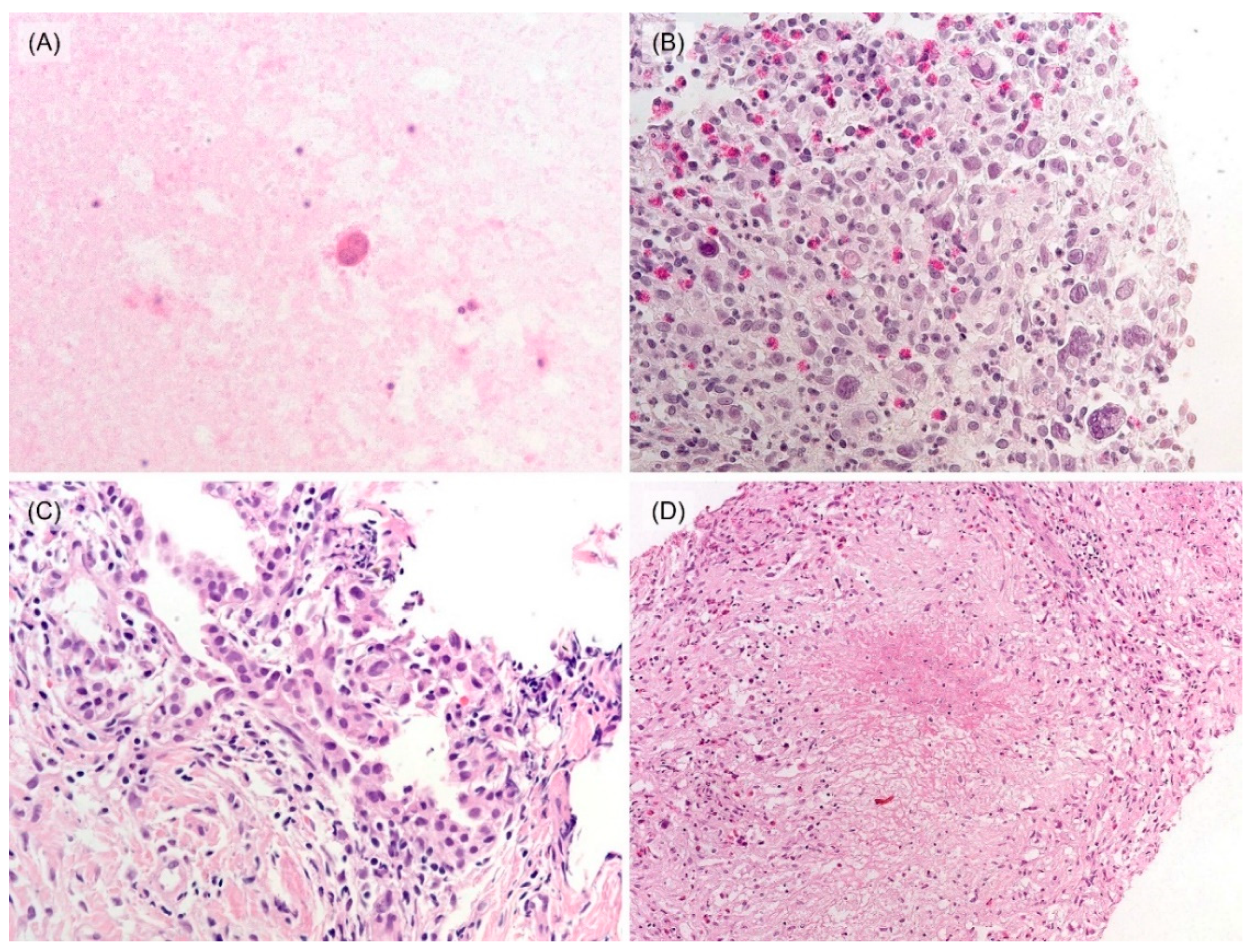
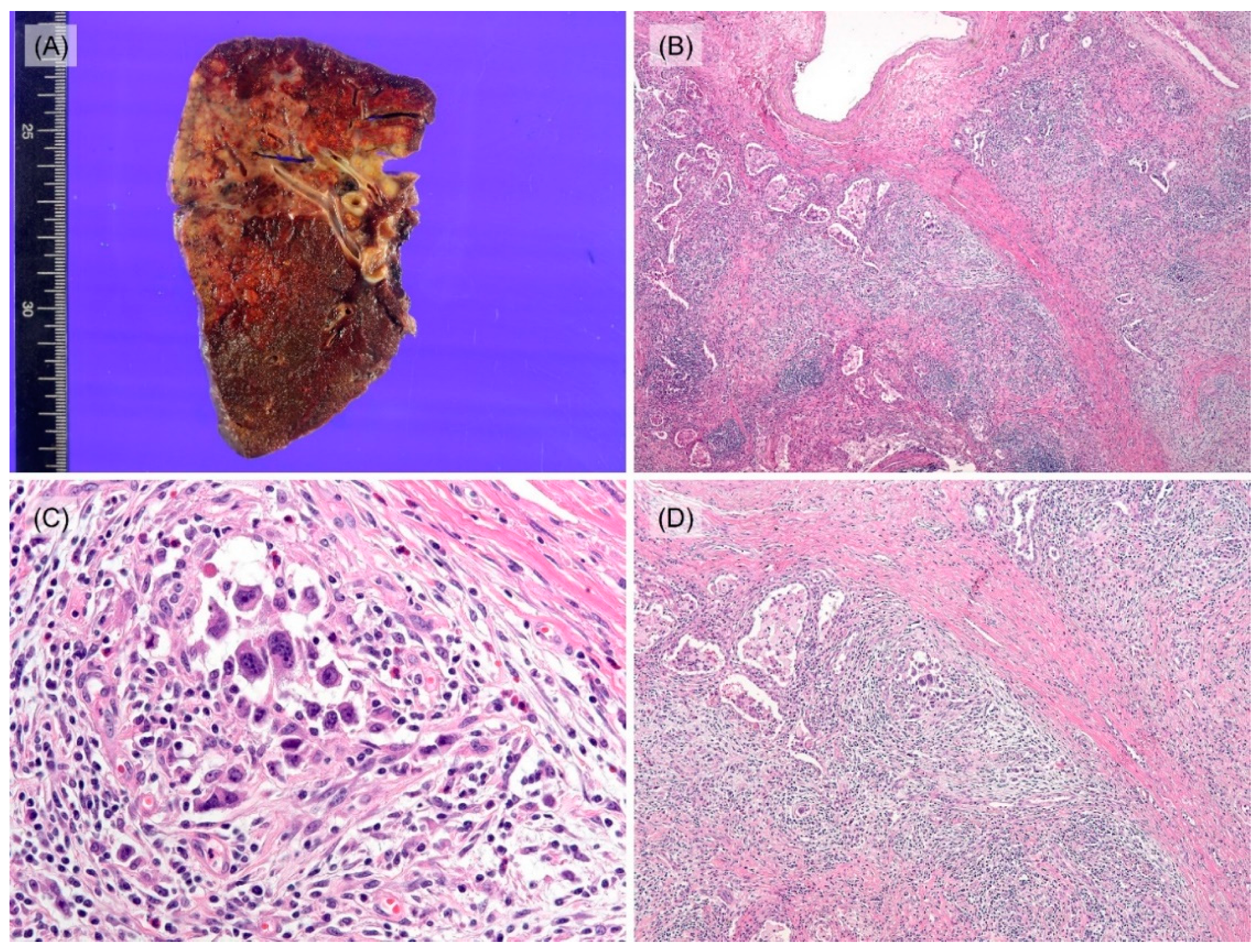
3.2. Histological and Immunohistochemical Features
3.3. Follow-Up and Survival Analysis
3.4. Literature Review: Clinicopathological Features of 115 Cases
3.5. Differences in Clinicopathological Characteristics between Our 10 and Previously Published 105 Cases of PPHL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hodgkin, D. Absorbent glands and spleen: On some morbid appearances of the absorbent glands and spleen. Boston Med. Surg. J. 1832, 6, 101–104. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; IARC: Lyon, France, 2008. [Google Scholar]
- Goldblum, J.R.; Lamps, L.W.; McKenney, J.K.; Myers, J.L. Rosai and Ackerman’s Surgical Pathology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Van der Schee, A.C.; Dinkla, B.A.; van Knapen, A. Primary pulmonary manifestation of Hodgkin’s disease. Respiration 1990, 57, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Radin, A.I. Primary pulmonary Hodgkin’s disease. Cancer 1990, 65, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Lluch-Garcia, R.; Briones-Gomez, A.; Castellano, E.M.; Sanchez-Toril, F.; Lopez, A.; Brotons, B. Primary pulmonary Hodgkin’s lymphoma. Can. Respir. J. 2010, 17, e106–e108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sidhu, H.; Mistry, R.; Shet, T. Primary pulmonary Hodgkin’s lymphoma: A rare pitfall in transthoracic fine needle aspiration cytology. Diagn. Cytopathol. 2008, 36, 666–669. [Google Scholar] [CrossRef]
- McElnay, P.J.; Pawade, J.; Chandratreya, L.; West, D. Giant thoracic mass: An unusual presentation of primary pulmonary Hodgkin’s lymphoma. BMJ Case Rep. 2013, 2013, bcr2013200909. [Google Scholar] [CrossRef]
- Chetty, R.; Slavin, J.L.; O’Leary, J.J.; Ansari, N.A.; Gatter, K.C. Primary Hodgkin’s disease of the lung. Pathology 1995, 27, 111–114. [Google Scholar] [CrossRef]
- Boshnakova, T.; Michailova, V.; Koss, M.; Georgiev, C.; Todorov, T.; Sarbinova, M. Primary pulmonary Hodgkin’s disease: Report of two cases. Respir. Med. 2000, 94, 830–831. [Google Scholar] [CrossRef]
- Saad, R.S.; Leon, M.E.; Olson, P.R. Pathologic Quiz Case: A localized pulmonary consolidation in a young woman. Arch. Pathol. Lab. Med. 2003, 127, e49–e50. [Google Scholar] [CrossRef]
- Codrich, D.; Monai, M.; Pelizzo, G.; Bussani, R.; Rabusin, M.; Guastalla, P.; Barbi, E.; Schleef, J. Primary pulmonary Hodgkin’s disease and tuberculosis in an 11-year-old boy: Case report and review of the literature. Pediatr. Pulmonol. 2006, 41, 694–698. [Google Scholar] [CrossRef]
- Pai, R.R.; Raghuveer, C.V.; Philipose, R.T.; Shetty, A.B. Primary pulmonary Hodgkin’s disease: A distinct entity. Ind. J. Chest Dis. Allied Sci. 2006, 48, 139–141. [Google Scholar]
- Rodriguez, J.; Tirabosco, R.; Pizzolitto, S.; Rocco, M.; Falconieri, G. Hodgkin lymphoma presenting with exclusive or preponderant pulmonary involvement: A clinicopathologic study of 5 new cases. Ann. Diagn. Pathol. 2006, 10, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Bakan, N.D.; Camsari, G.; Gur, A.; Ozkan, G.; Bayram, M.; Gorgulu, F.; Urer, N. A 21-year-old male with productive cough, hemoptysis, chest pain, and weight loss. Respiration 2007, 74, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Tillawi, I.S. Primary pulmonary Hodgkin’s lymphoma. A report of 2 cases and review of the literature. Saudi Med. J. 2007, 28, 943–948. [Google Scholar] [PubMed]
- Malur, P.R.; Gaude, G.S.; Bannur, H.B.; Anurshetru, S.B.; Suranagi, V.V.; Kangle, R.P.; Dhumale, A.J.; Patil, P.H.; Davanagere, R. Primary endobronchial Hodgkin’s disease. Lung India 2009, 26, 136–138. [Google Scholar] [CrossRef]
- Homma, M.; Yamochi-Onizuka, T.; Shiozawa, E.; Takimoto, M.; Ariizumi, H.; Nakashima, H.; Matsuda, I.; Nakamaki, T.; Kunimura, T.; Kushima, M. Primary pulmonary classical Hodgkin lymphoma with two recurrences in the mediastinum: A case report. J. Clin. Exp. Hematop. 2010, 50, 151–157. [Google Scholar] [CrossRef]
- Oka, K.; Shinonaga, M.; Nagayama, R.; Kashimura, H.; Yonekawa, N.; Tatebe, S.; Kuraoka, S.; Yatabe, Y.; Mori, N. Coexistence of primary pulmonary Hodgkin lymphoma and gastric MALT lymphoma associated with Epstein-Barr virus infection: A case report. Pathol. Int. 2010, 60, 520–523. [Google Scholar] [CrossRef]
- Binesh, F.; Halvani, H.; Taghipour, S.; Navabii, H. Primary pulmonary classic Hodgkin’s lymphoma. BMJ Case Rep. 2011, 2011, bcr0320113955. [Google Scholar] [CrossRef]
- Ezzine-Baccari, S.; Bacha, D.; Bouzaidi, K.; Ghrairi, H.; Sassi, S. Hodgkin lymphoma presenting with exclusive pulmonary involvement. Tunis. Med. 2012, 90, 833–834. [Google Scholar]
- Simon, Z.; Jóna, Á.; Miltényi, Z.; Páyer, E.; Lieber, A.; Szilasi, M.; Illés, Á. Diagnostic difficulties caused by a pulmonary infiltrate. Orv. Hetil. 2012, 153, 1077–1081. [Google Scholar] [CrossRef]
- Valizadeh, N.; Gholamnejad, M. Pulmonary Hodgkin lymphoma misdiagnosed as tuberculosis. Int. J. Hematol. Oncol. Stem. Cell Res. 2012, 6, 39–41. [Google Scholar]
- Fratoni, S.; Abruzzese, E.; Niscola, P.; Trawinska, M.M.; Mercadante, E.; Casullo, A.; de Fabritiis, P.; Perrotti, A.; Santeusanio, G. Primary pulmonary Hodgkin lymphoma simulating a mediastinal tumour: An uncommon occurrence. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013013. [Google Scholar] [CrossRef] [PubMed]
- Cooksley, N.; Judge, D.J.; Brown, J. Primary pulmonary Hodgkin’s lymphoma and a review of the literature since 2006. BMJ Case Rep. 2014, 2014, bcr2014204020. [Google Scholar] [CrossRef] [PubMed]
- Schild, M.H.; Wong, W.W.; Valdez, R.; Leis, J.F. Primary pulmonary classical Hodgkin lymphoma: A case report. J. Surg. Oncol. 2014, 110, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, S.; El Damati, A.; El Baz, A.; Alsayyah, A.; ElSharkawy, T.; Regal, M. Primary pulmonary Hodgkin lymphoma. Rare Tumors 2015, 7, 145–147. [Google Scholar] [CrossRef] [PubMed]
- El Hage, H.; Hossri, S.; Samra, B.; El-Sayegh, D. Primary pulmonary Hodgkin’s lymphoma: A rare etiology of a cavitary lung mass. Cureus 2017, 9, e1620. [Google Scholar] [CrossRef] [PubMed]
- Lowenthal, B.M.; Xu, X.; Subash, M.; Jih, L.J. Hodgkin’s lymphoma with unusual pulmonary presentations: Reporting two cases. Ind. J. Pathol. Microbiol. 2017, 60, 272–274. [Google Scholar] [CrossRef]
- Abid, H.; Khan, J.; Lone, N. Hodgkin’s Lymphoma presenting as an obstructing endobronchial mass: A rare presentation. BMJ Case Rep. 2018, 2018, bcr2017223809. [Google Scholar] [CrossRef]
- Aljehani, Y.; Al-Saif, H.; Al-Osail, A.; Al-Osail, E. Multiloculated cavitary primary pulmonary hodgkin lymphoma: Case series. Case Rep. Oncol. 2018, 11, 90–97. [Google Scholar] [CrossRef]
- Conti, L.; Pisani, D.; Gatt, A.; Montefort, S. Unusual case of primary pulmonary Hodgkin’s lymphoma presenting with a continuous murmur. BMJ Case Rep. 2018, 2018, bcr2018225674. [Google Scholar] [CrossRef]
- Parente, P.; Zanelli, M.; Zizzo, M.; Carosi, I.; Di Candia, L.; Sperandeo, M.; Lacedonia, D.; Fesce, V.F.; Ascani, S.; Graziano, P. Primary pulmonary Hodgkin lymphoma presenting as multiple cystic lung lesions: Diagnostic usefulness of cell block. Cytopathology 2020, 31, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, G.S.; Mehta, R.; Tyagi, R. Primary pulmonary Hodgkin’s lymphoma with pulmonary histoplasmosis. Med. J. Armed Forces India 2020, 76, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, P.; Lomangino, I.; Querzoli, G.; Bonalumi, A.; Bogina, G.S.; Terzi, A.C. Primary Hodgkin lymphoma of the lung arising with hemoptysis and pulmonary consolidation: A case report. Monaldi Arch. Chest Dis. 2021, 91, 1781. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.C.; Chen, S.H.; Chen, B.J.; Huang, Y.L.; Miserc, J.S.; Wei, C.H.; Lin, W.C. Primary pulmonary Hodgkin’s lymphoma: A rare etiology mimicking pulmonary tuberculosis. Pediatr. Neonatol. 2021, 62, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Kanitra, J.J.; Thampy, C.A.; Cullen, M.L. A decade’s experience of pediatric lung abscess and empyema at a community hospital. Pediatr. Pulmonol. 2021, 56, 1245–1251. [Google Scholar] [CrossRef]
- Sun, K.; Yu, Q.; Zhou, J.; Zhang, H.; Gao, L.; Nong, L.; Wang, M.; Que, C. Primary pulmonary Hodgkin’s lymphoma mimicking rheumatoid arthritis-associated organizing pneumonia: A case report. Thorac. Cancer 2021, 12, 1620–1624. [Google Scholar] [CrossRef]
- Tzankov, A.; Bourgau, C.; Kaiser, A.; Zimpfer, A.; Maurer, R.; Pileri, S.A.; Went, P.; Dirnhofer, S. Rare expression of T-cell markers in classical Hodgkin’s lymphoma. Mod. Pathol. 2005, 18, 1542–1549. [Google Scholar] [CrossRef]
- Venkataraman, G.; Song, J.Y.; Tzankov, A.; Dirnhofer, S.; Heinze, G.; Kohl, M.; Traverse-Glehen, A.; Eberle, F.C.; Hanson, J.C.; Raffeld, M.A.; et al. Aberrant T-cell antigen expression in classical Hodgkin lymphoma is associated with decreased event-free survival and overall survival. Blood 2013, 121, 1795–1804. [Google Scholar] [CrossRef]
- William, J.; Variakojis, D.; Yeldandi, A.; Raparia, K. Lymphoproliferative neoplasms of the lung: A review. Arch. Pathol. Lab. Med. 2013, 137, 382–391. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, B.; Diep, M.L.; Nandurkar, D. IgG4 related lung disease. Can. Respir. J. 2016, 2016, 1409281. [Google Scholar] [CrossRef]
- Hirano, K.; Kawabe, T.; Komatsu, Y.; Matsubara, S.; Togawa, O.; Arizumi, T.; Yamamoto, N.; Nakai, Y.; Sasahira, N.; Tsujino, T.; et al. High-rate pulmonary involvement in autoimmune pancreatitis. Int. Med. J. 2006, 36, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Yousem, S.A.; Weiss, L.M.; Colby, T.V. Primary pulmonary Hodgkin’s disease. A clinicopathologic study of 15 cases. Cancer 1986, 57, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Nakachi, S.; Nagasaki, A.; Owan, I.; Uchihara, T.; Fujita, J.; Ohshima, K.; Miyagi, T.; Taira, T.; Taira, N.; Takasu, N. Primary pulmonary Hodgkin lymphoma: Two case reports and a review of the literature. Gan Kagaku Ryoho. 2007, 34, 2279–2282. [Google Scholar]


| Characteristic | Number of Cases | |
|---|---|---|
| Age (years; range) | 41 (27–72) | |
| Sex | Man | 4 |
| Woman | 6 | |
| Smoking history | Never-smoker | 7 |
| Ex-smoker | 1 | |
| Current smoker | 2 | |
| Symptom | None | 3 |
| Cough | 6 | |
| Chest pain | 3 | |
| Weight loss | 1 | |
| Sputum | 1 | |
| CT findings | Single mass | 8 |
| Consolidation | 2 | |
| Mediastinal lymphadenopathy | Present | 9 |
| Absent | 1 | |
| Tumor location | Left upper lobe | 5 |
| Left lower lobe | 1 | |
| Right upper lobe | 4 | |
| Tumor size (cm; range) | 5.7 (4.1–10.5) | |
| Radiological impression | Carcinoma | 7 |
| Sarcoma | 1 | |
| Mycobacterium | 1 | |
| Pneumonia | 1 | |
| Treatment | Surgery followed by chemotherapy | 4 |
| Chemotherapy | 5 | |
| Not performed | 1 | |
| Treatment response | Complete remission | 8 |
| Not applicable | 2 | |
| Survival status (mean follow-up period) | Alive | 9 (6.4 years) |
| Dead | 1 (1 month) | |
| Sputum cytology | Not performed | 8 |
| Negative | 2 | |
| Bronchial washing cytology | Not performed | 3 |
| Negative | 7 | |
| Lung aspiration cytology | Not performed | 6 |
| Negative | 2 | |
| Atypical cells | 1 | |
| Suspicious for Hodgkin lymphoma | 1 | |
| Needle biopsy | Not performed | 1 |
| Chronic granulomatous inflammation, suspicious for tuberculosis | 1 | |
| Atypical pneumocytes, suspicious for adenocarcinoma | 1 | |
| Hodgkin lymphoma | 7 | |
| Subtype | Nodular sclerosis classical Hodgkin lymphoma | 4 |
| Mixed cellularity classical Hodgkin lymphoma | 2 | |
| Lymphocyte-rich classical Hodgkin lymphoma | 1 | |
| Classic Hodgkin lymphoma unspecified | 2 | |
| Hodgkin lymphoma, not otherwise specified | 1 | |
| Subtype | Number of Cases |
|---|---|
| Nodular sclerosis classical Hodgkin lymphoma | 43 |
| Mixed cellularity classical Hodgkin lymphoma | 20 |
| Lymphocyte-rich classical Hodgkin lymphoma | 2 |
| Nodular lymphocyte predominant Hodgkin lymphoma | 1 |
| Not applicable | 49 |
| Total | 115 |
| Category | Disease | Histologic Characteristics | IHC |
|---|---|---|---|
| Epithelial malignancy | Adenocarcinoma | Non-small cell carcinoma with glandular differentiation and variable architectural patterns (lepidic, acinar, papillary, micropapillary, and solid) | TTF1+, CK+ |
| Histiocytic and dendritic cell neoplasm | Histiocytic sarcoma | Malignant proliferation of mature, large histiocytes showing abundant cytoplasm and often hemophagocytosis | CD68+, CD163+, lysozyme+ |
| Langerhans cell histiocytosis | Aggregates of Langerhans cells with convoluted and grooved nuclei in the background of eosinophils | S100+, CD1a+ | |
| B-cell neoplasm | T-cell/histiocyte-rich large B-cell lymphoma | Scattered large B-cells in the background of many T-cells and histiocytes forming diffuse or vaguely nodular patten | CD19+, CD20+, CD79a+, IgD–, BCL6– |
| Lymphomatoid granulomatosis | Polymorphous lymphoid infiltrates with angiocentric distribution and often central necrosis | EBV+, CD20+, CD15– | |
| T-cell neoplasm | PTCL | Diffuse infiltrates of medium to large neoplastic T-cells and inflammatory background | CD4±, CD8±, PAX5– |
| ALCL | Large hallmark cells with horseshoe- or kidney-shaped nuclei | CD30+, ALK± | |
| Mesenchymal tumor | IMT | Spindle shaped tumor cells with prominent inflammatory stroma | SMA+, ALK± |
| Solitary fibrous tumor | Fibroblastic tumor cells with patternless architecture and staghorn vessels | STAT6 (+) CD34 (+) | |
| Infection | Tuberculosis | Central necrosis surrounded by epithelioid histiocytes | |
| Inflammatory lesion | Hypersensitivity pneumonitis | Airway-centered inflammatory reaction in the clinical setting of exposure by causative agents | |
| Bronchocentric granulomatosis | Necrotizing granulomatous inflammation involving bronchi and bronchioles | ||
| Interstitial lymphocytic pneumonitis | Diffuse interstitial infiltrates of lymphoplasma cells with lymphoid follicles and histiocytes | CD4+, CD8+ | |
| Drug-associated lung disease | Variable degree of interstitial pneumonia by drug | ||
| IgG4-related disease | Lymphoplasmacytic infiltrates with storiform fibrosis, and obliterative phlebitis | CD138+, IgG4+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.; Kim, H.-S.; Han, J.; Ko, Y.H.; Choi, Y.-D.; Lee, T. Clinicopathological Characteristics of Primary Pulmonary Hodgkin Lymphoma (PPHL): Two Institutional Experiences with Comprehensive Literature Review of 115 PPHL Cases. J. Clin. Med. 2023, 12, 126. https://doi.org/10.3390/jcm12010126
Jung H, Kim H-S, Han J, Ko YH, Choi Y-D, Lee T. Clinicopathological Characteristics of Primary Pulmonary Hodgkin Lymphoma (PPHL): Two Institutional Experiences with Comprehensive Literature Review of 115 PPHL Cases. Journal of Clinical Medicine. 2023; 12(1):126. https://doi.org/10.3390/jcm12010126
Chicago/Turabian StyleJung, Hera, Hyun-Soo Kim, Joungho Han, Young Hyeh Ko, Yoo-Duk Choi, and Taebum Lee. 2023. "Clinicopathological Characteristics of Primary Pulmonary Hodgkin Lymphoma (PPHL): Two Institutional Experiences with Comprehensive Literature Review of 115 PPHL Cases" Journal of Clinical Medicine 12, no. 1: 126. https://doi.org/10.3390/jcm12010126
APA StyleJung, H., Kim, H.-S., Han, J., Ko, Y. H., Choi, Y.-D., & Lee, T. (2023). Clinicopathological Characteristics of Primary Pulmonary Hodgkin Lymphoma (PPHL): Two Institutional Experiences with Comprehensive Literature Review of 115 PPHL Cases. Journal of Clinical Medicine, 12(1), 126. https://doi.org/10.3390/jcm12010126






