Real-World Evidence on Disease Burden and Economic Impact of Sickle Cell Disease in Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
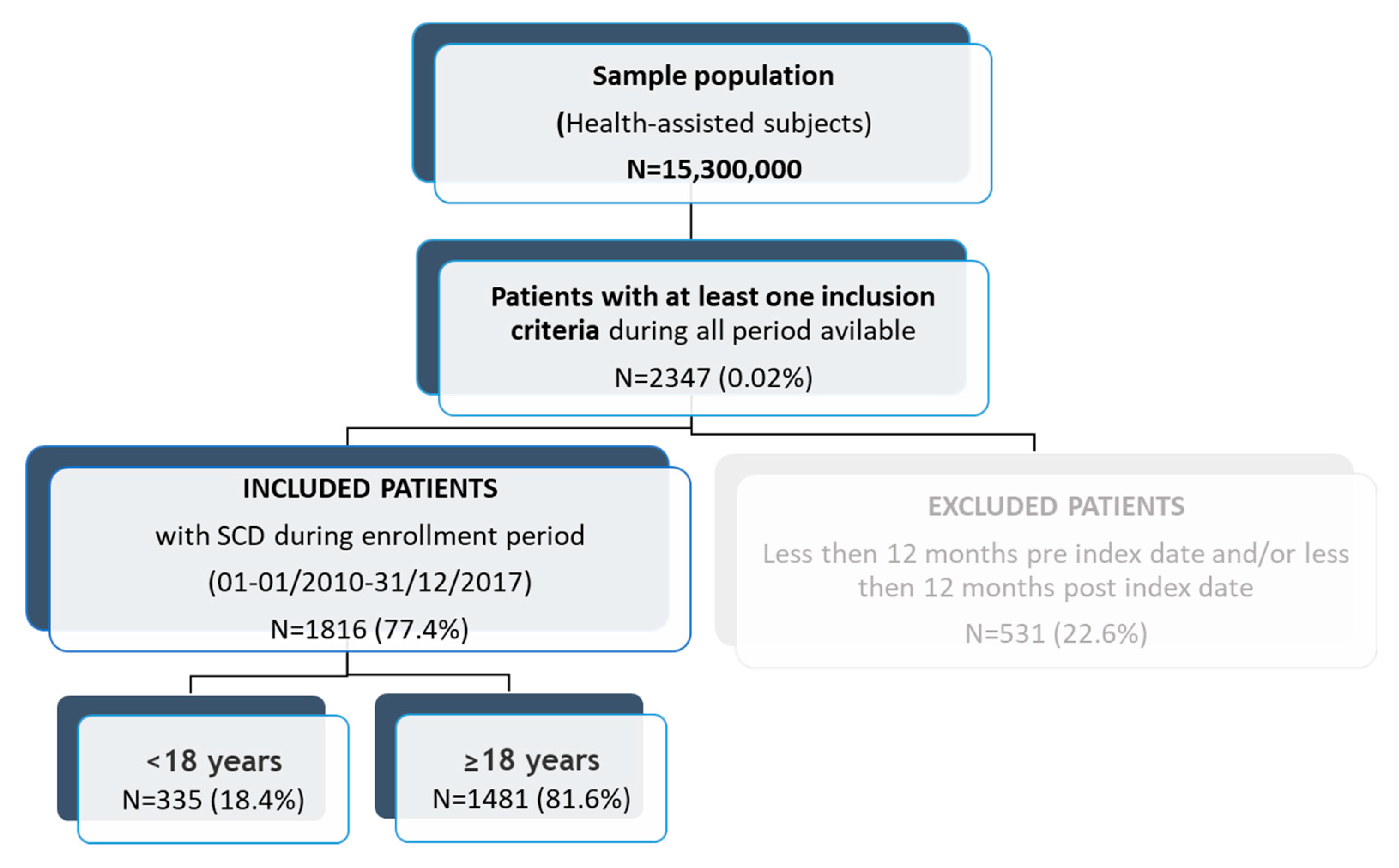
2.2. Study Design
2.3. Study Variables
2.4. Statistical Analysis
3. Results
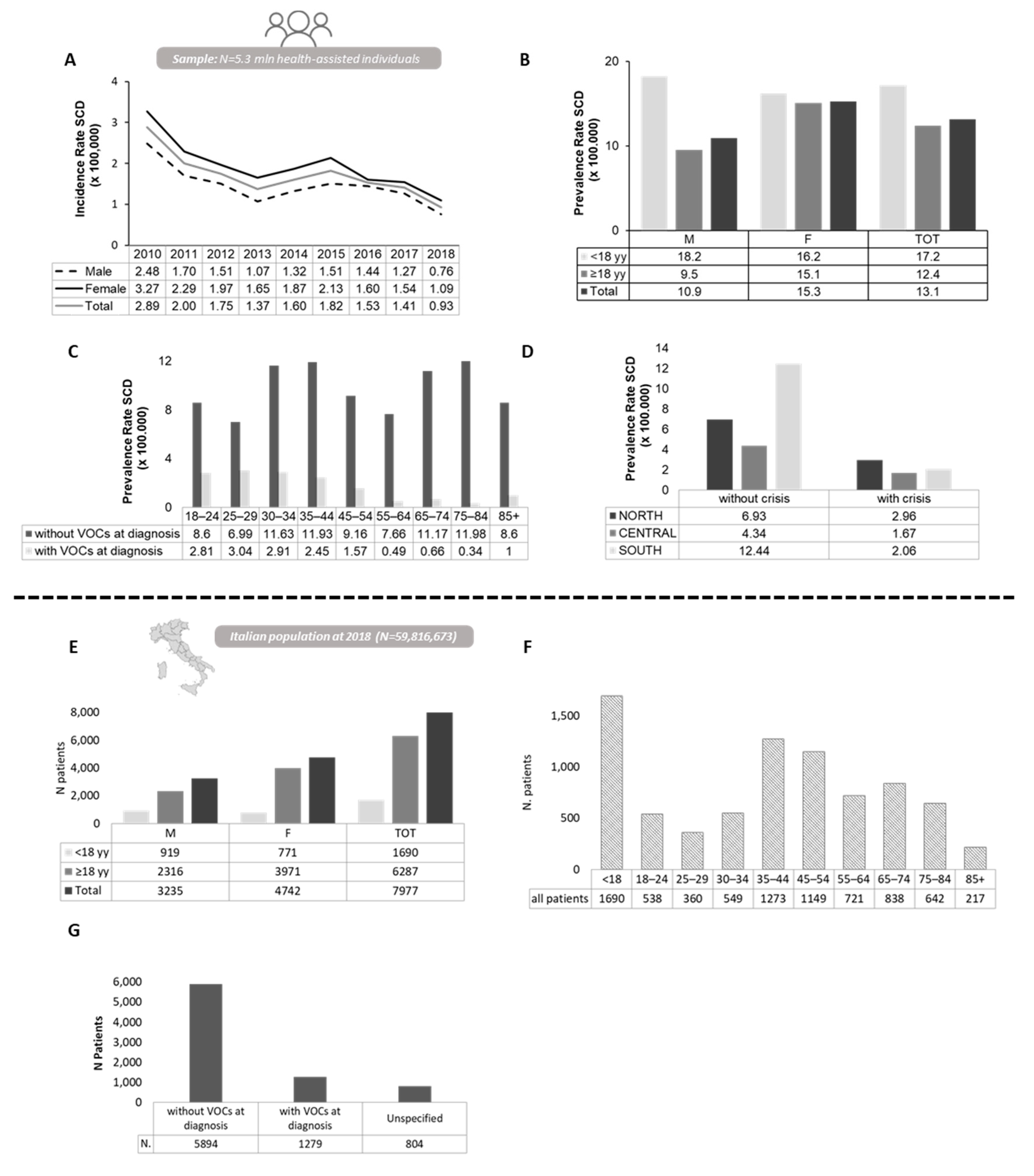
3.1. Epidemiology Estimates a High Prevalence of SCD in Italy
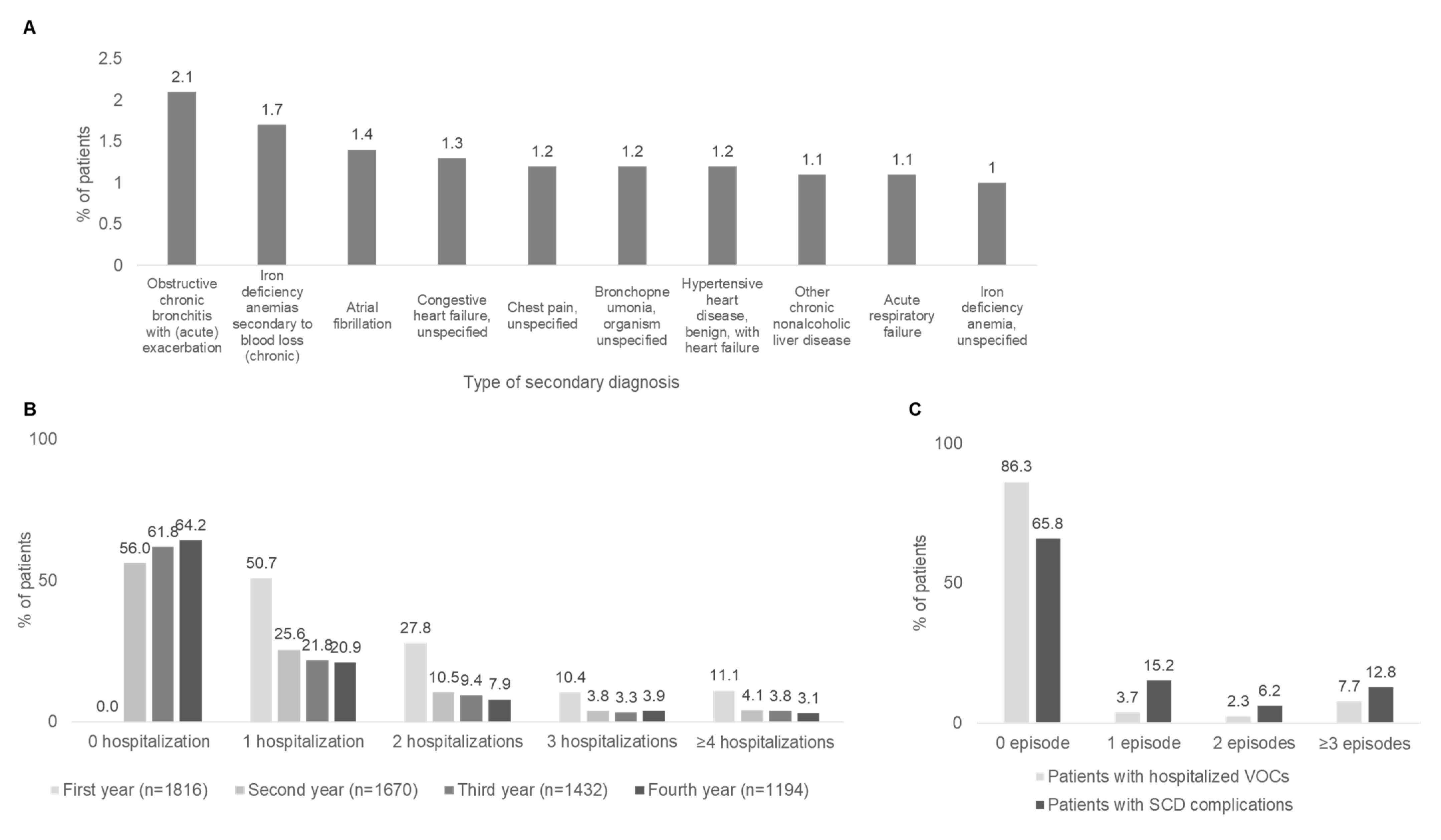
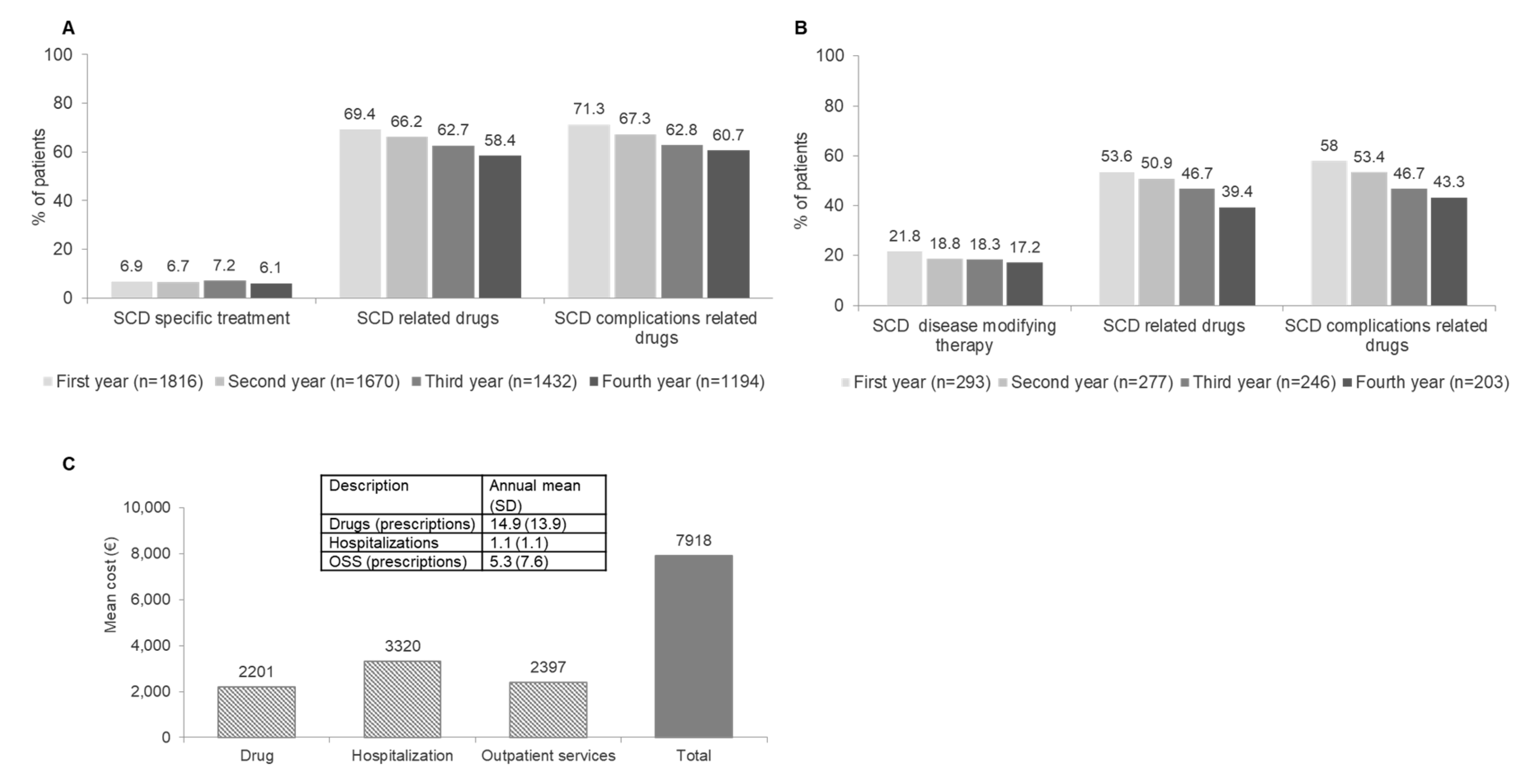
3.2. High Healthcare Utilization and Significant Direct Medical Costs Contribute to High Burden of Sickle Cell Disease in Italian Adult Population
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cordovil, K. Sickle Cell Disease: A Genetic Disorder of Beta-Globin. In Thalassemia and Other Hemolytic Anemias; AL-Zwaini, I., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-366-7. [Google Scholar]
- Brugnara, C.; Armsby, C.C.; De Franceschi, L.; Crest, M.; Martin Euclaire, M.-F.; Alper, S.L. Ca2+-Activated K+ Channels of Human and Rabbit Erythrocytes Display Distinctive Patterns of Inhibition by Venom Peptide Toxins. J. Membarin Biol. 1995, 147, 71–82. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, L.; Brugnara, C.; Rouyer-Fessard, P.; Jouault, H.; Beuzard, Y. Formation of Dense Erythrocytes in SAD Mice Exposed to Chronic Hypoxia: Evaluation of Different Therapeutic Regimens and of a Combination of Oral Clotrimazole and Magnesium Therapies. Blood 1999, 94, 4307–4313. [Google Scholar] [CrossRef] [PubMed]
- McNaughton-Smith, G.A.; Burns, J.F.; Stocker, J.W.; Rigdon, G.C.; Creech, C.; Arrington, S.; Shelton, T.; de Franceschi, L. Novel Inhibitors of the Gardos Channel for the Treatment of Sickle Cell Disease. J. Med. Chem. 2008, 51, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Kalish, B.T.; Matte, A.; Andolfo, I.; Iolascon, A.; Weinberg, O.; Ghigo, A.; Cimino, J.; Siciliano, A.; Hirsch, E.; Federti, E.; et al. Dietary ω-3 Fatty Acids Protect against Vasculopathy in a Transgenic Mouse Model of Sickle Cell Disease. Haematologica 2015, 100, 870–880. [Google Scholar] [CrossRef]
- De Franceschi, L.; Bertoldi, M.; Matte, A.; Santos Franco, S.; Pantaleo, A.; Ferru, E.; Turrini, F. Oxidative Stress and 𝛽-Thalassemic Erythroid Cells behind the Molecular Defect. Oxidative Med. Cell. Longev. 2013, 2013, e985210. [Google Scholar] [CrossRef]
- Shah, N.; Bhor, M.; Xie, L.; Paulose, J.; Yuce, H. Sickle Cell Disease Complications: Prevalence and Resource Utilization. PLoS ONE 2019, 14, e0214355. [Google Scholar] [CrossRef]
- Telen, M.J.; Malik, P.; Vercellotti, G.M. Therapeutic Strategies for Sickle Cell Disease: Towards a Multi-Agent Approach. Nat. Rev. Drug. Discov. 2019, 18, 139–158. [Google Scholar] [CrossRef]
- Russo, G.; De Franceschi, L.; Colombatti, R.; Rigano, P.; Perrotta, S.; Voi, V.; Palazzi, G.; Fidone, C.; Quota, A.; Graziadei, G.; et al. Current Challenges in the Management of Patients with Sickle Cell Disease–A Report of the Italian Experience. Orphanet J. Rare Dis. 2019, 14, 120. [Google Scholar] [CrossRef]
- Matte, A.; Cappellini, M.D.; Iolascon, A.; Enrica, F.; De Franceschi, L. Emerging Drugs in Randomized Controlled Trials for Sickle Cell Disease: Are We on the Brink of a New Era in Research and Treatment? Expert. Opin. Investig. Drugs 2020, 29, 23–31. [Google Scholar] [CrossRef]
- Matte, A.; Zorzi, F.; Mazzi, F.; Federti, E.; Olivieri, O.; De Franceschi, L. New Therapeutic Options for the Treatment of Sickle Cell Disease. Mediterr. J. Hematol. Infect Dis. 2019, 11, e2019002. [Google Scholar] [CrossRef]
- Peterson, E.E.; Salemi, J.L.; Dongarwar, D.; Salihu, H.M. Acute Care Utilization in Pediatric Sickle Cell Disease and Sickle Cell Trait in the USA: Prevalence, Temporal Trends, and Cost. Eur. J. Pediatr. 2020, 179, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.; Meier, E.R.; Hankins, J.S.; Paulukonis, S.T.; Snyder, A.B. Improving Outcomes for Patients With Sickle Cell Disease in the United States: Making the Case for More Resources, Surveillance, and Longitudinal Data. JAMA Health Forum. 2021, 2, e213467. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Bhor, M.; Xie, L.; Halloway, R.; Arcona, S.; Paulose, J.; Yuce, H. Treatment Patterns and Economic Burden of Sickle-Cell Disease Patients Prescribed Hydroxyurea: A Retrospective Claims-Based Study. Health Qual. Life Outcomes 2019, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, L.; Lux, C.; Piel, F.B.; Gianesin, B.; Bonetti, F.; Casale, M.; Graziadei, G.; Lisi, R.; Pinto, V.; Putti, M.C.; et al. Access to Emergency Departments for Acute Events and Identification of Sickle Cell Disease in Refugees. Blood 2019, 133, 2100–2103. [Google Scholar] [CrossRef] [PubMed]
- Colombatti, R.; Sainati, L. Management of Children With Sickle Cell Disease in Europe: Current Situation and Future Perspectives. Available online: https://emj.emg-health.com/hematology/article/management-of-children-with-sickle-cell-disease-in-europe-current-situation-and-future-perspectives/ (accessed on 23 November 2021).
- Italian Republic. Official Journal of the Italian Republic. Rome-Friday, 12 May 2017 (Gazzetta Ufficiale Della Repubblica Italiana Roma-Venerdì, 12 Maggio 2017). Available online: https://www.gazzettaufficiale.it/eli/gu/2017/05/12/109/sg/pdf (accessed on 24 April 2022).
- Reeves, S.; Garcia, E.; Kleyn, M.; Housey, M.; Stottlemyer, R.; Lyon-Callo, S.; Dombkowski, K.J. Identifying Sickle Cell Disease Cases Using Administrative Claims. Acad. Pediatr. 2014, 14, S61–S67. [Google Scholar] [CrossRef]
- Michalik, D.E.; Taylor, B.W.; Panepinto, J.A. Identification and Validation of a Sickle Cell Disease Cohort Within Electronic Health Records. Acad. Pediatr. 2017, 17, 283–287. [Google Scholar] [CrossRef]
- Singh, A.; Mora, J.; Panepinto, J.A. Identification of Patients with Hemoglobin SS/Sβ0 Thalassemia Disease and Pain Crises within Electronic Health Records. Blood. Adv. 2018, 2, 1172–1179. [Google Scholar] [CrossRef]
- Perrone, V.; Sangiorgi, D.; Andretta, M.; Ducci, G.; Forti, B.; Francesa Morel, P.; Gambera, M.; Maina, G.; Mencacci, C.; Mennini, F.; et al. Assessment of Patients Affected by Treatment-Resistant Depression: Findings from a Real-World Study in Italy. J. Psychiatry Psychiatr. Disord. 2020, 4, 104–117. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Perrone, V.; Sangiorgi, D.; Sinigaglia, L. Assessment of Patients Affected by Rheumatoid Arthritis Eligible for Biotechnological Agents and Evaluation of Their Healthcare Resource Utilization and Related Costs. Reumatismo 2021, 73, 5–14. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic. Dis 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Lanzkron, S.; Carroll, C.P.; Haywood, C. The Burden of Emergency Department Use for Sickle-Cell Disease: An Analysis of the National Emergency Department Sample Database. Am. J. Hematol. 2010, 85, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Leleu, H.; Arlet, J.B.; Habibi, A.; Etienne-Julan, M.; Khellaf, M.; Adjibi, Y.; Pirenne, F.; Pitel, M.; Granghaud, A.; Sinniah, C.; et al. Epidemiology and Disease Burden of Sickle Cell Disease in France: A Descriptive Study Based on a French Nationwide Claim Database. PLoS ONE 2021, 16, e0253986. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.L.; Glenthøj, A.; Möller, S.; Biemond, B.J.; Andersen, K.; Gaist, D.; Petersen, J.; Frederiksen, H. Prevalence of Congenital Hemolytic Disorders in Denmark, 2000-2016. Clin. Epidemiol. 2020, 12, 485–495. [Google Scholar] [CrossRef]
- Lodi, M.; Bigi, E.; Palazzi, G.; Vecchi, L.; Morandi, R.; Setti, M.; Borsari, S.; Bergonzini, G.; Iughetti, L.; Venturelli, D. Universal Screening Program in Pregnant Women and Newborns At-Risk for Sickle Cell Disease: First Report from Northern Italy. Hemoglobin 2017, 41, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Martella, M.; Viola, G.; Azzena, S.; Schiavon, S.; Biondi, A.; Basso, G.; Corti, P.; Colombatti, R.; Masera, N.; Sainati, L. Evaluation of Technical Issues in a Pilot Multicenter Newborn Screening Program for Sickle Cell Disease. Int. J. Neonatal. Screen. 2018, 5, 2. [Google Scholar] [CrossRef]
- Colombatti, R.; Martella, M.; Cattaneo, L.; Viola, G.; Cappellari, A.; Bergamo, C.; Azzena, S.; Schiavon, S.; Baraldi, E.; Barba, B.D.; et al. Results of a Multicenter Universal Newborn Screening Program for Sickle Cell Disease in Italy: A Call to Action. Pediatr. Blood Cancer 2019, 66, e27657. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C. Updated Worldwide Epidemiology of Inherited Erythrocyte Disorders. Acta. Haematol. 2020, 143, 196–203. [Google Scholar] [CrossRef]
- Rigano, P.; De Franceschi, L.; Sainati, L.; Piga, A.; Piel, F.B.; Cappellini, M.D.; Fidone, C.; Masera, N.; Palazzi, G.; Gianesin, B.; et al. Real-Life Experience with Hydroxyurea in Sickle Cell Disease: A Multicenter Study in a Cohort of Patients with Heterogeneous Descent. Blood Cells Mol. Dis. 2018, 69, 82–89. [Google Scholar] [CrossRef]
- Osunkwo, I.; Andemariam, B.; Minniti, C.P.; Inusa, B.P.D.; El Rassi, F.; Francis-Gibson, B.; Nero, A.; Trimnell, C.; Abboud, M.R.; Arlet, J.-B.; et al. Impact of Sickle Cell Disease on Patients’ Daily Lives, Symptoms Reported, and Disease Management Strategies: Results from the International Sickle Cell World Assessment Survey (SWAY). Am. J. Hematol. 2021, 96, 404–417. [Google Scholar] [CrossRef]
- Brousseau, D.C.; Owens, P.L.; Mosso, A.L.; Panepinto, J.A.; Steiner, C.A. Acute Care Utilization and Rehospitalizations for Sickle Cell Disease. JAMA 2010, 303, 1288–1294. [Google Scholar] [CrossRef]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle Cell Disease. Nat. Rev. Dis. Prim. 2018, 4, 18010. [Google Scholar] [CrossRef] [PubMed]
- Cannas, G. Sickle cell disease and infections in high- and low-income countries. Mediterr. J. Hematol. Infect Dis. 2019, 11, 02019042. [Google Scholar] [CrossRef] [PubMed]
- Claudio, A.M.; Foltanski, L.; Delay, T.; Britell, A.; Duckett, A.; Weeda, E.R.; Bohm, N. Antibiotic Use and Respiratory Pathogens in Adults With Sickle Cell Disease and Acute Chest Syndrome. Ann. Pharm. 2019, 53, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Vichinsky, E.P.; Neumayr, L.D.; Earles, A.N.; Williams, R.; Lennette, E.T.; Dean, D.; Nickerson, B.; Orringer, E.; McKie, V.; Bellevue, R.; et al. Causes and Outcomes of the Acute Chest Syndrome in Sickle Cell Disease. New. Engl. J. Med. 2000, 342, 1855–1865. [Google Scholar] [CrossRef]
- William, B.M.; Thawani, N.; Sae-Tia, S.; Corazza, G.R. Hyposplenism: A Comprehensive Review. Part II: Clinical Manifestations, Diagnosis, and Management. Hematology 2007, 12, 89–98. [Google Scholar] [CrossRef]
- Ferrari, R.; Duse, G.; Capraro, M.; Visentin, M. Risk Assessment of Opioid Misuse in Italian Patients with Chronic Noncancer Pain. Pain Res. Treat. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- De Franceschi, L.; Finco, G.; Vassanelli, A.; Zaia, B.; Ischia, S.; Corrocher, R. A Pilot Study on the Efficacy of Ketorolac plus Tramadol Infusion Combined with Erythrocytapheresis in the Management of Acute Severe Vaso-Occlusive Crises and Sickle Cell Pain. Haematologica 2004, 89, 1389–1391. [Google Scholar]
- De Franceschi, L.; Mura, P.; Schweiger, V.; Vencato, E.; Quaglia, F.M.; Delmonte, L.; Evangelista, M.; Polati, E.; Olivieri, O.; Finco, G. Fentanyl Buccal Tablet: A New Breakthrough Pain Medication in Early Management of Severe Vaso-Occlusive Crisis in Sickle Cell Disease. Pain. Pr. 2016, 16, 680–687. [Google Scholar] [CrossRef]
- Forni, G.L.; Finco, G.; Graziadei, G.; Balocco, M.; Rigano, P.; Perrotta, S.; Olivieri, O.; Cappellini, M.D.; De Franceschi, L. Development of Interactive Algorithm for Clinical Management of Acute Events Related to Sickle Cell Disease in Emergency Department. Orphanet. J. Rare Dis. 2014, 9, 91. [Google Scholar] [CrossRef]
- Honsel, V.; Khimoud, D.; Ranque, B.; Offredo, L.; Joseph, L.; Pouchot, J.; Arlet, J.-B. Comparison between Adult Patients with Sickle Cell Disease of Sub-Saharan African Origin Born in Metropolitan France and in Sub-Saharan Africa. J. Clin. Med. 2019, 8, 2173. [Google Scholar] [CrossRef]
- Lesage, N.; Deneux Tharaux, C.; Saucedo, M.; Habibi, A.; Galacteros, F.; Girot, R.; Bouvier Colle, M.H.; Kayem, G. Maternal Mortality among Women with Sickle-Cell Disease in France, 1996-2009. Eur. J. Obs. Gynecol. Reprod. Biol. 2015, 194, 183–188. [Google Scholar] [CrossRef]
- Graziadei, G.; De Franceschi, L.; Sainati, L.; Venturelli, D.; Masera, N.; Bonomo, P.; Vassanelli, A.; Casale, M.; Lodi, G.; Voi, V.; et al. Transfusional Approach in Multi-Ethnic Sickle Cell Patients: Real-World Practice Data From a Multicenter Survey in Italy. Front. Med. 2022, 9, 832154. [Google Scholar] [CrossRef]
- Snyder, A.B.; Lane, P.A.; Zhou, M.; Paulukonis, S.T.; Hulihan, M.M. The Accuracy of Hospital ICD-9-CM Codes for Determining Sickle Cell Disease Genotype. J. Rare Dis. Res. Treat. 2017, 2, 39–45. [Google Scholar] [CrossRef]
- Angelucci, E.; Antmen, A.; Losi, S.; Burrows, N.; Bartiromo, C.; Hu, X.H. Direct Medical Care Costs Associated with β-Thalassemia Care in Italy. Blood 2017, 130, 3368. [Google Scholar] [CrossRef]
- Colombo, C.; Daccò, V.; Alicandro, G.; Loi, S.; Mazzi, S.; Lucioni, C.; Ravasio, R. Cost of Cystic Fibrosis: Analysis of Treatment Costs in a Specialized Center in Northern Italy. Adv. Ther. 2013, 30, 165–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piel, F.B.; Steinberg, M.H.; Rees, D.C. Sickle Cell Disease. New Engl. J. Med. 2017, 376, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
| <18 years (N = 335) | ≥18 years (N = 1481) | Total (N = 1816) | |
|---|---|---|---|
| Age at index date, mean ± SD | 6.9 ± 5.2 | 52.1 ± 19.9 | 43.8 ± 25.2 |
| Female, n (%) | 158 (47.2) | 902 (60.9) | 1060 (58.4) |
| Charlson comorbidity index, mean ± SD | 0.4 ± 0.5 | 1.4 ± 1.5 | 1.2 ± 1.4 |
| Diagnosis with crisis, n (%) | 88 (26.3) | 205 (13.8) | 293 (16.1) |
| Diagnosis without crisis, n (%) | 181 (54.0) | 1168 (78.9) | 1349 (74.3) |
| Diagnosis with unspecified crisis, n (%) | 66 (19.7) | 108 (7.3) | 174 (9.6) |
| MDC | First Year Follow-Up (n = 1816) | Second Year Follow-Up (n = 1670) | Third Year Follow-Up (n = 1432) | Fourth Year Follow-Up (n = 1194) |
|---|---|---|---|---|
| Blood and blood-forming organs and immunological disorders, n (%) | 290 (16.0) | 81 (4.9) | 71 (5.0) | 45 (3.8) |
| Average length (in days), mean ± SD | 6.9 ± 6.4 | 5.3 ± 4.3 | 5.9 ± 5.4 | 11.6 ± 45.3 |
| Circulatory system, n (%) | 218 (12.0) | 71 (4.3) | 52 (3.6) | 31 (2.6) |
| Average length (in days), mean ± SD | 7.9 ± 8.5 | 8.1 ± 7.2 | 8.8 ± 6.6 | 10.4 ± 8.0 |
| Respiratory system, n (%) | 197 (10.8) | 46 (2.8) | 35 (2.4) | 41 (3.4) |
| Average length (in days), mean ± SD | 10.2 ± 7.3 | 10.6 ± 7.4 | 9.7 ± 5.3 | 11.1 ± 7.5 |
| Digestive system, n (%) | 165 (9.1) | 32 (1.9) | 27 (1.9) | 19 (1.6) |
| Average length (in days), mean ± SD | 7.0 ± 8.8 | 13.7 ± 19.3 | 8.0 ± 7.6 | 6.5 ± 5.2 |
| Hepatobiliary system and pancreas, n (%) | 154 (8.5) | 36 (2.2) | 25 (1.7) | 23 (1.9) |
| Average length (in days), mean ± SD | 8.5 ± 7.3 | 8.3 ± 5.9 | 8.2 ± 4.6 | 9.6 ± 6.0 |
| Musculoskeletal system and connective tissue, n (%) | 117 (6.4) | 42 (2.5) | 28 (2.0) | 23 (1.9) |
| Average length (in days), mean ± SD | 12.7 ± 13.3 | 9.1 ± 8.0 | 8.8 ± 8.8 | 20.1 ± 56.7 |
| Nervous system, n (%) | 91 (5.0) | 31 (1.9) | 22 (1.5 | 11 (0.9) |
| Average length (in days), mean ± SD | 13.0 ± 22.9 | 10.5 ± 11.0 | 17.6 ± 9.1 | 13.8 ± 10.8 |
| Kidney and urinary tract *, n (%) | 76 (4.2) | 25 (1.5) | - | 10 (0.8) |
| Average length (in days), mean ± SD | 8.3 ± 6.8 | 8.9 ± 10.2 | - | 14.5 ± 11.5 |
| Pregnancy, childbirth, and puerperium **, n (%) | 60 (3.3) | - | - | 9 (0.8) |
| Average length (in days), mean ± SD | 6.4 ± 8.2 | - | - | 4.9 ± 3.3 |
| Infectious and parasitic DDs, n (%) | 56 (3.1) | 20 (1.2) | 15 (1.0) | 9 (0.8) |
| Average length (in days), mean ± SD | 8.6 ± 12.1 | 14.3 ± 14.5 | 9.8 ± 7.5 | 5.0 ± 3.1 |
| 1st Year Follow-Up (n = 1816) | 2nd Year Follow-Up (n = 1670) | 3rd year Follow-Up (n = 1432) | 4th Year Follow-Up (n = 1194) | |||||
|---|---|---|---|---|---|---|---|---|
| Description | n | % | n | % | n | % | n | % |
| Antibacterials for systemic use | 1140 | 62.8 | 995 | 59.6 | 805 | 56.2 | 630 | 52.8 |
| Drugs for acid related disorders | 868 | 47.8 | 694 | 41.6 | 547 | 38.2 | 414 | 34.7 |
| Antithrombotic agents | 610 | 33.6 | 479 | 28.7 | 384 | 26.8 | 292 | 24.5 |
| Anti-inflammatory and antirheumatic products | 525 | 28.9 | 465 | 27.8 | 393 | 27.4 | 281 | 23.5 |
| Antianemic preparations | 442 | 24.3 | 353 | 21.1 | 285 | 19.9 | 223 | 18.7 |
| Drugs for obstructive airway diseases | 440 | 24.2 | 390 | 23.4 | 288 | 20.1 | 242 | 20.3 |
| Agents acting on RAAS | 436 | 24.0 | 383 | 22.9 | 306 | 21.4 | 261 | 21.9 |
| Corticosteroids for systemic use | 419 | 23.1 | 331 | 19.8 | 292 | 20.4 | 215 | 18.0 |
| Diuretics | 330 | 18.2 | 266 | 15.9 | 204 | 14.2 | 153 | 12.8 |
| Beta-blocking agents * | 281 | 15.5 | 255 | 15.3 | - | - | - | - |
| Vitamins ** | - | - | - | - | 241 | 16.8 | 200 | 16.8 |
| Analgesics | 209 | 11.5 | 188 | 11.3 | 134 | 9.4 | 104 | 8.7 |
| Opioids | 191 | 10.5 | 176 | 10.0 | 121 | 8.4 | 95 | 8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Franceschi, L.; Castiglioni, C.; Condorelli, C.; Valsecchi, D.; Premoli, E.; Fiocchi, C.; Perrone, V.; Esposti, L.D.; Forni, G.L.; on behalf of the GREATalyS Study Group. Real-World Evidence on Disease Burden and Economic Impact of Sickle Cell Disease in Italy. J. Clin. Med. 2023, 12, 117. https://doi.org/10.3390/jcm12010117
De Franceschi L, Castiglioni C, Condorelli C, Valsecchi D, Premoli E, Fiocchi C, Perrone V, Esposti LD, Forni GL, on behalf of the GREATalyS Study Group. Real-World Evidence on Disease Burden and Economic Impact of Sickle Cell Disease in Italy. Journal of Clinical Medicine. 2023; 12(1):117. https://doi.org/10.3390/jcm12010117
Chicago/Turabian StyleDe Franceschi, Lucia, Chiara Castiglioni, Claudia Condorelli, Diletta Valsecchi, Eleonora Premoli, Carina Fiocchi, Valentina Perrone, Luca Degli Esposti, Gian Luca Forni, and on behalf of the GREATalyS Study Group. 2023. "Real-World Evidence on Disease Burden and Economic Impact of Sickle Cell Disease in Italy" Journal of Clinical Medicine 12, no. 1: 117. https://doi.org/10.3390/jcm12010117
APA StyleDe Franceschi, L., Castiglioni, C., Condorelli, C., Valsecchi, D., Premoli, E., Fiocchi, C., Perrone, V., Esposti, L. D., Forni, G. L., & on behalf of the GREATalyS Study Group. (2023). Real-World Evidence on Disease Burden and Economic Impact of Sickle Cell Disease in Italy. Journal of Clinical Medicine, 12(1), 117. https://doi.org/10.3390/jcm12010117





