Use of Hyperoncotic Human Albumin Solution in Severe Traumatic Brain Injury Revisited—A Narrative Review and Meta-Analysis
Abstract
:1. Introduction
Use of Human Albumin Solutions in Fluid Management of TBI
2. Methods
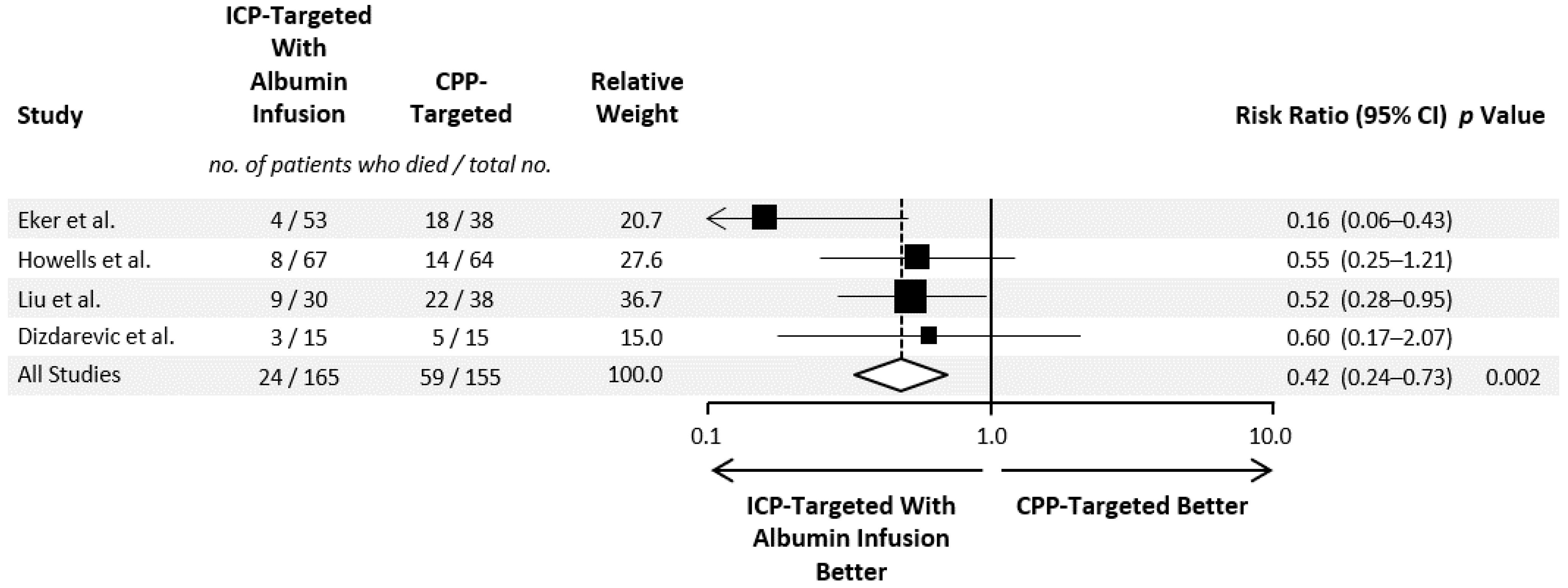
3. Results
3.1. Clinical Trials on Fluid Regimens Incorporating Hyperoncotic HAS Infusion in Severe TBI
3.2. Evidence Synthesis of Hyperoncotic HAS Administration in TBI
4. Mechanistic Considerations for the Use of Hyperoncotic HAS in TBI
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, H.C.; Bouamra, O.; Woodford, M.; King, A.T.; Yates, D.W.; Lecky, F.E. Trends in Head Injury Outcome from 1989 to 2003 and the Effect of Neurosurgical Care: An Observational Study. Lancet 2005, 366, 1538–1544. [Google Scholar] [CrossRef]
- Beck, B.; Gantner, D.; Cameron, P.A.; Braaf, S.; Saxena, M.; Cooper, D.J.; Gabbe, B.J. Temporal Trends in Functional Outcomes after Severe Traumatic Brain Injury: 2006–2015. J. Neurotrauma 2018, 35, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K. Traumatic Brain Injury: Pathophysiology for Neurocritical Care. J. Intensive Care 2016, 4, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juul, N.; Morris, G.F.; Marshall, S.B.; Marshall, L.F. Intracranial Hypertension and Cerebral Perfusion Pressure: Influence on Neurological Deterioration and Outcome in Severe Head Injury. The Executive Committee of the International Selfotel Trial. J. Neurosurg. 2000, 92, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stocchetti, N.; Carbonara, M.; Citerio, G.; Ercole, A.; Skrifvars, M.B.; Smielewski, P.; Zoerle, T.; Menon, D.K. Severe Traumatic Brain Injury: Targeted Management in the Intensive Care Unit. Lancet Neurol. 2017, 16, 452–464. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Picetti, E.; Zoerle, T.; Carbonara, M.; Zanier, E.R.; Stocchetti, N. Fluid Management in Acute Brain Injury. Curr. Neurol. Neurosci. Rep. 2018, 18, 74. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Phases of Fluid Management and the Roles of Human Albumin Solution in Perioperative and Critically Ill Patients. Curr. Med. Res. Opin. 2020, 36, 1961–1973. [Google Scholar] [CrossRef]
- Haynes, G. Growing Evidence for Hyperoncotic 20% Albumin Solution for Volume Resuscitation. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1702–1703. [Google Scholar] [CrossRef] [Green Version]
- Tomita, H.; Ito, U.; Tone, O.; Masaoka, H.; Tominaga, B. High Colloid Oncotic Therapy for Contusional Brain Edema. Acta Neurochirurgica Suppl. 1994, 60, 547–549. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Nakayama, K.; Matsumori, K.; Beppu, T.; Tanabe, T.; Kitahara, T.; Saito, T. Albumin therapy for patients with increased intracranial pressure: Oncotic therapy. No Shinkei Geka 1983, 11, 947–954. [Google Scholar]
- Grände, P.-O. The “Lund Concept” for the Treatment of Severe Head Trauma—Physiological Principles and Clinical Application. Intensive Care Med. 2006, 32, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Grände, P.-O.; Juul, N. Guidelines for Treatment of Patients with Severe Traumatic Brain Injury. In Management of Severe Traumatic Brain Injury; Springer: Berlin/Heidelberg, Germany, 2020; pp. 395–401. [Google Scholar]
- Ma, H.K.; Bebawy, J.F. Albumin Use in Brain-Injured and Neurosurgical Patients: Concepts, Indications, and Controversies. J. Neurosurg. Anesthesiol. 2021, 33, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Poole, D.; Helbok, R.; Meyfroidt, G.; Stocchetti, N.; Bouzat, P.; Cecconi, M.; Geeraerts, T.; Martin-Loeches, I.; Quintard, H.; et al. Fluid Therapy in Neurointensive Care Patients: ESICM Consensus and Clinical Practice Recommendations. Intensive Care Med. 2018, 44, 449–463. [Google Scholar] [CrossRef] [PubMed]
- SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health; Myburgh, J.; Cooper, D.J.; Finfer, S.; Bellomo, R.; Norton, R.; Bishop, N.; et al. Saline or Albumin for Fluid Resuscitation in Patients with Traumatic Brain Injury. N. Engl. J. Med. 2007, 357, 874–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gantner, D.; Moore, E.M.; Cooper, D.J. Intravenous Fluids in Traumatic Brain Injury: What’s the Solution? Curr. Opin. Crit. Care 2014, 20, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J.C.; Patel, P.M.; Lemkuil, B. Proscribing the Use of Albumin in the Head-Injured Patient Is Not Warranted. Anesth. Analg. 2011, 113, 426–427, author reply 427–428. [Google Scholar] [CrossRef]
- Cooper, D.J.; Myburgh, J.; Heritier, S.; Finfer, S.; Bellomo, R.; Billot, L.; Murray, L.; Vallance, S.; SAFE-TBI Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Albumin Resuscitation for Traumatic Brain Injury: Is Intracranial Hypertension the Cause of Increased Mortality? J. Neurotrauma 2013, 30, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Iguchi, N.; Kosaka, J.; Bertolini, J.; May, C.N.; Lankadeva, Y.R.; Bellomo, R. Differential Effects of Isotonic and Hypotonic 4% Albumin Solution on Intracranial Pressure and Renal Perfusion and Function. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2018, 20, 48–53. [Google Scholar]
- Briegel, J. Albumin in traumatic brain injury-osmolarity is what matters! Med. Klin. Intensivmed. und Notf. 2022, 117, 69–70. [Google Scholar] [CrossRef]
- Zampieri, F.G.; Machado, F.R.; Biondi, R.S.; Freitas, F.G.R.; Veiga, V.C.; Figueiredo, R.C.; Lovato, W.J.; Amêndola, C.P.; Serpa-Neto, A.; Paranhos, J.L.R.; et al. Effect of Intravenous Fluid Treatment With a Balanced Solution vs 0.9% Saline Solution on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA 2021, 326, 818–829. [Google Scholar] [CrossRef]
- Weinberg, L.; Collins, N.; Van Mourik, K.; Tan, C.; Bellomo, R. Plasma-Lyte 148: A Clinical Review. World J. Crit. Care Med. 2016, 5, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Asgeirsson, B.; Grände, P.O.; Nordström, C.H. A New Therapy of Post-Trauma Brain Oedema Based on Haemodynamic Principles for Brain Volume Regulation. Intensive Care Med. 1994, 20, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Eker, C.; Asgeirsson, B.; Grände, P.O.; Schalén, W.; Nordström, C.H. Improved Outcome after Severe Head Injury with a New Therapy Based on Principles for Brain Volume Regulation and Preserved Microcirculation. Crit. Care Med. 1998, 26, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Naredi, S.; Edén, E.; Zäll, S.; Stephensen, H.; Rydenhag, B. A Standardized Neurosurgical Neurointensive Therapy Directed toward Vasogenic Edema after Severe Traumatic Brain Injury: Clinical Results. Intensive Care Med. 1998, 24, 446–451. [Google Scholar] [CrossRef]
- Naredi, S.; Olivecrona, M.; Lindgren, C.; Ostlund, A.L.; Grände, P.O.; Koskinen, L.O. An Outcome Study of Severe Traumatic Head Injury Using the “Lund Therapy” with Low-Dose Prostacyclin. Acta Anaesthesiol. Scand. 2001, 45, 402–406. [Google Scholar] [CrossRef]
- Wahlström, M.R.; Olivecrona, M.; Koskinen, L.-O.D.; Rydenhag, B.; Naredi, S. Severe Traumatic Brain Injury in Pediatric Patients: Treatment and Outcome Using an Intracranial Pressure Targeted Therapy—The Lund Concept. Intensive Care Med. 2005, 31, 832–839. [Google Scholar] [CrossRef] [Green Version]
- Olivecrona, M.; Rodling-Wahlström, M.; Naredi, S.; Koskinen, L.-O.D. Effective ICP Reduction by Decompressive Craniectomy in Patients with Severe Traumatic Brain Injury Treated by an ICP-Targeted Therapy. J. Neurotrauma 2007, 24, 927–935. [Google Scholar] [CrossRef]
- Olivecrona, M.; Rodling-Wahlström, M.; Naredi, S.; Koskinen, L.-O.D. Prostacyclin Treatment in Severe Traumatic Brain Injury: A Microdialysis and Outcome Study. J. Neurotrauma 2009, 26, 1251–1262. [Google Scholar] [CrossRef] [Green Version]
- Olivecrona, M.; Rodling-Wahlström, M.; Naredi, S.; Koskinen, L.-O.D. Prostacyclin Treatment and Clinical Outcome in Severe Traumatic Brain Injury Patients Managed with an ICP-Targeted Therapy: A Prospective Study. Brain Inj. 2012, 26, 67–75. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Huser, M.C.; Smoll, N.R.; Maedler, S.; Bednarz, S.; von Hessling, A.; Lussmann, R.; Hildebrandt, G.; Seule, M.A. Long-Term Neurological and Neuropsychological Outcome in Patients with Severe Traumatic Brain Injury. Clin. Neurol. Neurosurg. 2013, 115, 2482–2488. [Google Scholar] [CrossRef]
- Stenberg, M.; Koskinen, L.-O.; Levi, R.; Stålnacke, B.-M. Severe Traumatic Brain Injuries in Northern Sweden: A Prospective 2-Year Study. J. Rehabil. Med. 2013, 45, 792–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koskinen, L.-O.D.; Olivecrona, M.; Grände, P.O. Severe Traumatic Brain Injury Management and Clinical Outcome Using the Lund Concept. Neuroscience 2014, 283, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic Brain Injury: Integrated Approaches to Improve Prevention, Clinical Care, and Research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [Green Version]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—a Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrier, I.; Boivin, J.-F.; Steele, R.J.; Platt, R.W.; Furlan, A.; Kakuma, R.; Brophy, J.; Rossignol, M. Should Meta-Analyses of Interventions Include Observational Studies in Addition to Randomized Controlled Trials? A Critical Examination of Underlying Principles. Am. J. Epidemiol. 2007, 166, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying Heterogeneity in a Meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Tone, O.; Ito, U.; Tomita, H.; Masaoka, H.; Tominaga, B. High Colloid Oncotic Therapy for Brain Edema with Cerebral Hemorrhage. Acta Neurochir. Suppl. 1994, 60, 568–570. [Google Scholar] [CrossRef]
- Gürkan, F.; Haspolat, K.; Yaramiş, A.; Ece, A. Beneficial Effect of Human Albumin on Neonatal Cerebral Edema. Am. J. Ther. 2001, 8, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Schalén, W.; Sonesson, B.; Messeter, K.; Nordström, G.; Nordström, C.H. Clinical Outcome and Cognitive Impairment in Patients with Severe Head Injuries Treated with Barbiturate Coma. Acta Neurochir. 1992, 117, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Schalén, W.; Messeter, K.; Nordström, C.H. Complications and Side Effects during Thiopentone Therapy in Patients with Severe Head Injuries. Acta Anaesthesiol. Scand. 1992, 36, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Eker, C.; Schalén, W.; Asgeirsson, B.; Grände, P.O.; Ranstam, J.; Nordström, C.H. Reduced Mortality after Severe Head Injury Will Increase the Demands for Rehabilitation Services. Brain Inj. 2000, 14, 605–619. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 30 June 2021).
- Liu, C.-W.; Zheng, Y.-K.; Lu, J.; Yu, W.-H.; Wang, B.; Hu, W.; Zhu, K.-Y.; Zhu, Y.; Hu, W.-H.; Wang, J.-R.; et al. Application of Lund concept in treating brain edema after severe head injury. Chin. Crit. Care Med. 2010, 22, 610–613. [Google Scholar]
- Dizdarevic, K.; Hamdan, A.; Omerhodzic, I.; Kominlija-Smajic, E. Modified Lund Concept versus Cerebral Perfusion Pressure-Targeted Therapy: A Randomised Controlled Study in Patients with Secondary Brain Ischaemia. Clin. Neurol. Neurosurg. 2012, 114, 142–148. [Google Scholar] [CrossRef]
- Howells, T.; Elf, K.; Jones, P.A.; Ronne-Engström, E.; Piper, I.; Nilsson, P.; Andrews, P.; Enblad, P. Pressure Reactivity as a Guide in the Treatment of Cerebral Perfusion Pressure in Patients with Brain Trauma. J. Neurosurg. 2005, 102, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Elf, K.; Nilsson, P.; Enblad, P. Outcome after Traumatic Brain Injury Improved by an Organized Secondary Insult Program and Standardized Neurointensive Care. Crit. Care Med. 2002, 30, 2129–2134. [Google Scholar] [CrossRef]
- Muzevic, D.; Splavski, B. The Lund Concept for Severe Traumatic Brain Injury. Cochrane Database Syst. Rev. 2013, 16, CD010193. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, R.; Mateu, X.; Maseda, E.; Yebenes, J.C.; Aldecoa, C.; De Haro, C.; Ruiz-Rodriguez, J.C.; Garnacho-Montero, J. Non-Oncotic Properties of Albumin. A Multidisciplinary Vision about the Implications for Critically Ill Patients. Expert Rev. Clin. Pharmacol. 2018, 11, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Hariri, G.; Joffre, J.; Deryckere, S.; Bige, N.; Dumas, G.; Baudel, J.L.; Maury, E.; Guidet, B.; Ait-Oufella, H. Albumin Infusion Improves Endothelial Function in Septic Shock Patients: A Pilot Study. Intensive Care Med. 2018, 44, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-J.; Fei, M.-M.; Ye, W.; Pan, A.-J.; Liu, B. Effect of albumin and hemoglobin level on prognosis of patients with uncomplicated severe traumatic brain injury: A retrospective cohort study. Chin. Crit. Care Med. 2013, 25, 301–305. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G.; et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. N. Engl. J. Med. 2014, 370, 1412–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.H.; Kim, W.J.; Kim, J.Y.; Chin, J.H.; Choi, D.K.; Sim, J.Y.; Choo, S.J.; Chung, C.H.; Lee, J.W.; Choi, I.C. Effect of Exogenous Albumin on the Incidence of Postoperative Acute Kidney Injury in Patients Undergoing Off-Pump Coronary Artery Bypass Surgery with a Preoperative Albumin Level of Less than 4.0 g/dL. Anesthesiology 2016, 124, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Marklund, N. Pharmacological Neuroprotection. In Management of Severe Traumatic Brain Injury: Evidence, Tricks, and Pitfalls; Sundstrøm, T., Grände, P.-O., Luoto, T., Rosenlund, C., Undén, J., Wester, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 409–419. ISBN 978-3-030-39383-0. [Google Scholar]
- Khatri, R.; Afzal, M.R.; Rodriguez, G.J.; Maud, A.; Miran, M.S.; Qureshi, M.A.; Cruz-Flores, S.; Qureshi, A.I. Albumin-Induced Neuroprotection in Focal Cerebral Ischemia in the ALIAS Trial: Does Severity, Mechanism, and Time of Infusion Matter? Neurocrit. Care 2018, 28, 60–64. [Google Scholar] [CrossRef]
- Rodling Wahlström, M.; Olivecrona, M.; Nyström, F.; Koskinen, L.-O.D.; Naredi, S. Fluid Therapy and the Use of Albumin in the Treatment of Severe Traumatic Brain Injury. Acta Anaesthesiol. Scand. 2009, 53, 18–25. [Google Scholar] [CrossRef] [Green Version]
| Set | Query |
|---|---|
| 1 | brain OR head OR cerebr* OR cranial OR intracranial |
| 2 | injur* OR trauma* OR contusion* OR concussion* OR damage OR herniat* |
| 3 | #1 AND #2 |
| 4 | Lund [tiab] OR “intracranial pressure-targeted” OR “ICP-targeted” |
| 5 | mortality OR surviv* OR death* OR died OR neurological outcome OR “Glasgow outcome scale” OR GOS |
| 6 | random* [tiab] OR “random allocation” [mh] OR “randomized controlled trial” [pt] |
| 7 | control* [tiab] OR “controlled clinical trial” [pt] |
| 8 | #6 OR #7 |
| 9 | #3 AND #4 AND #5 AND #8 |
| Study | Patients | Indication | Fluid Regimen |
|---|---|---|---|
| Eker et al. 1998 [23,24,43,44,45] | 91 | Head injury with GCS < 8 and ICP > 25 mm Hg | ICP-targeted therapy with albumin infusion to maintain serum albumin ≤ 40 g·L−1 vs. conventional treatment |
| Howells et al. 2005 [49] | 131 | Head injury requiring at least 6 h of ICP, CPP, and MAP data recorded within 96 h of injury | ICP-targeted therapy with albumin infusion to maintain adequate COP, stable MAP, and CVP ≤ 5 mmHg * vs. CPP-targeted therapy |
| Liu et al. 2010 [47] | 68 | Head injury and mean GCS of 5.8 | ICP-targeted therapy with albumin infusion to maintain serum albumin ≤ 40 g·L−1 vs. CPP-targeted therapy |
| Dizdarevic et al. 2012 [48] | 30 | Isolated head injury and intradural focal lesions with GCS ≤ 8 and secondary brain ischemia | ICP-targeted therapy with albumin infusion to maintain a serum albumin of approximately 40 g·L−1 vs. CPP-targeted therapy |
| Study | Males, n (%) | Age (y) * | GCS * | |||
|---|---|---|---|---|---|---|
| ICP-Targeted with Albumin Infusion | CPP-Targeted | ICP-Targeted with Albumin Infusion | CPP-Targeted | ICP-Targeted with Albumin Infusion | CPP-Targeted | |
| Eker et al. 1998 [23,24,43,44,45] | n.d. | 30 (78.9) | Grouped † | 20 (7–59) ‡ | <8 | coma > 6 h |
| Howells et al. 2005 [49] | n.d. | n.d. | 40 ± 18 | 39 ± 18 | 4.5 ± 1.1†† | 3.5 ± 1.6 †† |
| Liu et al. 2010 [47] | 17 (56.7) | 28 (73.7) | 53.3 ± 20.3 | 55.6 ± 19.8 | 5.9 ± 1.4 | 5.7 ± 1.3 |
| Dizdarevic et al. 2012 [48] | 10 (66.7) | 12 (80.0) | 35.7 ± 17.7 | 43.0 ± 14.8 | 5 § | 5 § |
| Criteria | Eker et al. 1998 [23,24,43,44,45] | Howells et al. 2005 [49] | Liu et al. 2010 [47] | Dizdarevic et al. 2012 [48] | |
|---|---|---|---|---|---|
| 1. | Was the study described as randomized, a randomized trial, a randomized clinical trial, or an RCT? | No | No | No | Yes |
| 2. | Was the method of randomization adequate (i.e., use of randomly generated assignment)? | NA | NA | NA | Yes |
| 3. | Was the treatment allocation concealed (so that assignments could not be predicted)? | NA | NA | NA | Yes |
| 4. | Were study participants and providers blinded to treatment group assignment? | No | No | No | No |
| 5. | Were the people assessing the outcomes blinded to the participants’ group assignments? | No | No | No | No |
| 6. | Were the groups similar at baseline on important characteristics that could affect outcomes (e.g., demographics, risk factors, co-morbid conditions)? | Yes | Yes | Yes | Yes |
| 7. | Was the overall drop-out rate from the study at endpoint 20% or lower of the number allocated to treatment? | NR | NR | NR | NR |
| 8. | Was the differential drop-out rate (between treatment groups) at endpoint 15 percentage points or lower? | NR | NR | NR | NR |
| 9. | Was there high adherence to the intervention protocols for each treatment group? | NR | NR | NR | NR |
| 10. | Were other interventions avoided or similar in the groups (e.g., similar background treatments)? | No | No | No | No |
| 11. | Were outcomes assessed using valid and reliable measures, implemented consistently across all study participants? | Yes | Yes | Yes | Yes |
| 12. | Did the authors report that the sample size was sufficiently large to be able to detect a difference in the main outcome between groups with at least 80% power? | No | No | No | No |
| 13. | Were outcomes reported or subgroups analyzed prespecified (i.e., identified before analyses were conducted)? | Yes | Yes | Yes | Yes |
| 14. | Were all randomized participants analyzed in the group to which they were originally assigned, i.e., did they use an intention-to-treat analysis? | NA | NA | NA | No |
| Risk of bias | High | High | High | High | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiedermann, C.J. Use of Hyperoncotic Human Albumin Solution in Severe Traumatic Brain Injury Revisited—A Narrative Review and Meta-Analysis. J. Clin. Med. 2022, 11, 2662. https://doi.org/10.3390/jcm11092662
Wiedermann CJ. Use of Hyperoncotic Human Albumin Solution in Severe Traumatic Brain Injury Revisited—A Narrative Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(9):2662. https://doi.org/10.3390/jcm11092662
Chicago/Turabian StyleWiedermann, Christian J. 2022. "Use of Hyperoncotic Human Albumin Solution in Severe Traumatic Brain Injury Revisited—A Narrative Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 9: 2662. https://doi.org/10.3390/jcm11092662
APA StyleWiedermann, C. J. (2022). Use of Hyperoncotic Human Albumin Solution in Severe Traumatic Brain Injury Revisited—A Narrative Review and Meta-Analysis. Journal of Clinical Medicine, 11(9), 2662. https://doi.org/10.3390/jcm11092662






