Negative-Pressure Ventilation in Neuromuscular Diseases in the Acute Setting
Abstract
1. Introduction
2. NPV Interfaces
2.1. Iron Lung
2.2. Poncho
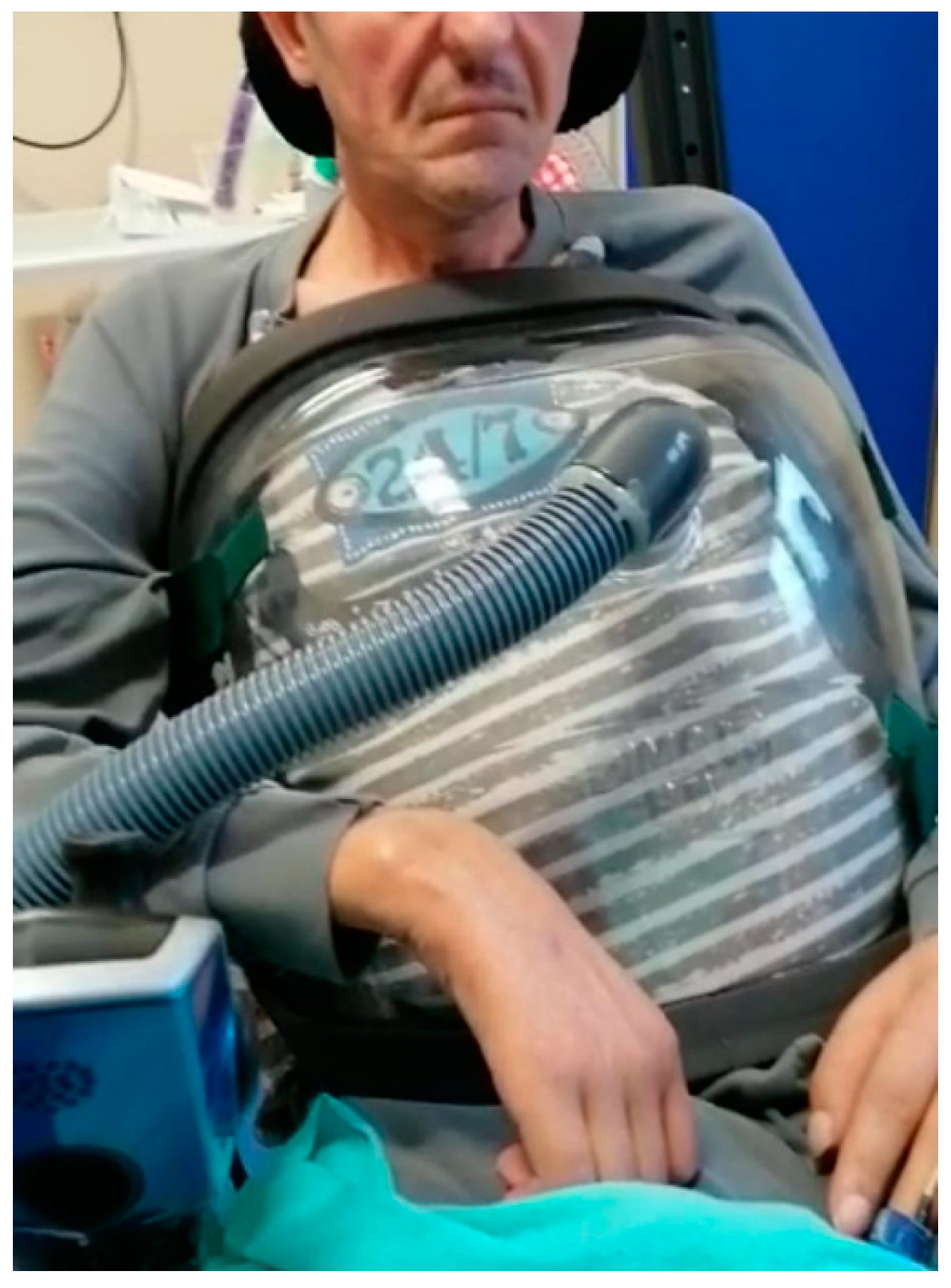
2.3. Cuirass
3. NPV Mode
4. NPV Candidacy
5. NPV in Acute Settings in Neuromuscular Diseases
6. Advantages and Limitations of NPV in Acute Setting
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drinker, P.A.; McKhann, C.F. The Iron Lung: First practical means of respiratory support. JAMA 1986, 255, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Emerson, J.H. The Evolution of Iron Lungs; JH Emerson Co.: Cambridge, MA, USA, 1978. [Google Scholar]
- Kinnear, W.; Petch, M.; Taylor, G.; Shneerson, J. Assisted ventilation using cuirass respirators. Eur. Respir. J. 1988, 1, 198–203. [Google Scholar] [PubMed]
- Corrado, A.; Gorini, M.; Villella, G.; De Paola, E. Negative pressure ventilation in the treatment of acute respiratory failure: An old noninvasive technique reconsidered. Eur. Respir. J. 1996, 9, 1531–1544. [Google Scholar] [CrossRef]
- Kinnear, W.; Hockley, S.; Harvey, J.; Shneerson, J. The effects of one year of nocturnal cuirass-assisted ventilation in chest wall disease. Eur. Respir. J. 1988, 1, 204–208. [Google Scholar]
- Easa, D.; Mundie, T.G.; Finn, K.C.; Hashiro, G.; Balaraman, V. Continuous negative extrathoracic pressure versus positive end-expiratory pressure in piglets after saline lung lavage. Pediatr. Pulmonol. 1994, 17, 161–168. [Google Scholar] [CrossRef]
- Annunziata, A.; Coppola, A.; Polistina, G.E.; Imitazione, P.; Simioli, F.; Lanza, M.; Cauteruccio, R.; Fiorentino, G. Daytime alternatives for non-invasive mechanical ventilation in neuromuscular disorders. Acta Myol. 2021, 40, 51–60. [Google Scholar] [CrossRef]
- Voulgaris, A.; Antoniadou, M.; Agrafiotis, M.; Steiropoulos, P. Respiratory Involvement in Patients with Neuromuscular Diseases: A Narrative Review. Pulm. Med. 2019, 2019, 2734054. [Google Scholar] [CrossRef]
- Wilson, J.L. Acute anterior poliomyelitis. N. Engl. J. Med. 1932, 206, 887–893. [Google Scholar] [CrossRef][Green Version]
- Crone, N.L. The Treatment of Acute Poliomyelitis with the Respirator. N. Engl. J. Med. 1934, 210, 621–623. [Google Scholar] [CrossRef]
- Brahdy, M.B.; Lenarsky, M. Treatment of Respiratory Failure in Acute Epidemic Poliomyelitis. Arch. Pediatr. Adolesc. Med. 1933, 46, 705–729. [Google Scholar] [CrossRef]
- Libby, D.M.; Briscoe, W.A.; Boyce, B.; Smith, J.P. Acute respiratory failure in scoliosis or kyphosis: Prolonged survival and treatment. Am. J. Med. 1982, 73, 532–538. [Google Scholar] [CrossRef]
- Braun, S.R.; Sufit, R.L.; Giovannoni, R.; O’Connor, M.; Peters, H. Intermittent negative pressure ventilation in the treatment of respiratory failure in progressive neuromuscular disease. Neurology 1987, 37, 1874. [Google Scholar] [CrossRef] [PubMed]
- Garay, S.M.; Turino, G.M.; Goldring, R.M. Sustained reversal of chronic hypercapnia in patients with alveolar hypoventilation syndromes: Long-term maintenance with noninvasive nocturnal mechanical ventilation. Am. J. Med. 1981, 70, 269–274. [Google Scholar] [CrossRef]
- Del Bufalo, C.; Fasano, L.; Quarta, C.C.; Nastasi, M.; Gunilla, G. Use of extrathoracic negative pressure ventilation in weaning COPD and Kyphoscoliotic patients from mechanical ventilation. Respir. Care 1994, 39, 21–29. [Google Scholar]
- Corrado, A.; Gorini, M.; De Paola, E. Alternative Techniques for Managing Acute Neuromuscular Respiratory Failure. Skull Base 1995, 15, 84–89. [Google Scholar] [CrossRef]
- Hino, H.; Suzuki, Y.; Ishii, E.; Fukuda, M. Biphasic cuirass ventilation for treatment of an air leak after pneumothorax in a patient with nemaline myopathy: A case report. J. Anesth. 2016, 30, 1087–1090. [Google Scholar] [CrossRef]
- Imitazione, P.; Annunziata, A.; Lanza, M.; Fiorentino, G. Combined high flow nasal cannula and negative pressure ventilation as a novel respiratory approach in a patient with acute respiratory failure and limb-girdle muscular dystrophy. Acta Myol. 2021, 40, 101–104. [Google Scholar]
- Mohr, C.H.; Hill, N.S. Long-Term Follow-up of Nocturnal Ventilatory Assistance in Patients with Respiratory Failure Due to Duchenne-Type Muscular Dystrophy. Chest 1990, 97, 91–96. [Google Scholar] [CrossRef]
- Corrado, A.; Gorini, M. Negative-pressure ventilation: Is there still a role? Eur. Respir. J. 2002, 20, 187–197. [Google Scholar] [CrossRef]
- Hassinger, A.B.; Breuer, R.K.; Nutty, K.; Ma, C.X.; Al Ibrahim, O.S. Negative-Pressure Ventilation in Pediatric Acute Respiratory Failure. Respir. Care 2017, 62, 1540–1549. [Google Scholar] [CrossRef]
- Nunez, C.A.; Hassinger, A.B. Predictors of Negative Pressure Ventilation Response in Pediatric Acute Respiratory Failure. Respir. Care 2019, 65, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.S.; Redline, S.; Carskadon, M.A.; Curran, F.J.; Millman, R.P. Sleep-Disordered Breathing in Patients with Duchenne Muscular Dystrophy Using Negative Pressure Ventilators. Chest 1992, 102, 1656–1662. [Google Scholar] [CrossRef]
- Bach, J.R.; Penek, J. Obstructive Sleep Apnea Complicating Negative-Pressure Ventilatory Support in Patients with Chronic Paralytic/Restrictive Ventilatory Dysfunction. Chest 1991, 99, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Jawad, M.H.; Noyes, J.; Samuels, M.P.; Southall, D.P. Negative extrathoracic pressure ventilation in central hypoventilation syndrome. Arch. Dis. Child. 1994, 70, 418–423. [Google Scholar] [CrossRef] [PubMed]
| Indications | Contraindications |
|---|---|
| Severe facial decubitus | Sleep apnea syndrome |
| Interface intolerance | Severe obesity |
| Facial deformity | Severe kyphoscoliosis |
| Severe respiratory acidosis | Rib fractures |
| Severe hypercapnic encephalopathy | Recent abdominal surgery |
| Reference | Study Design | Sample Size (N°) | Age Mean | Neuromuscular Diseases | NPV | Findings |
|---|---|---|---|---|---|---|
| Libby et al. 1982 [12] | Case series | 20 | 52 years (13–78) | Severe scoliosis or kyphosis with ARF (6 poliomyelitis, 1 AMC) | Iron lung | The only poliomyelitis patient treated with NPV avoided EI |
| Braun et al. 1987 [13] | Case series | 5 | 49 years (19–70) | 3 ALS, 2 DMD | Intermittent Pneumowrap | 1 ALS patient avoided EI, 3 ALS patients slowed pulmonary deterioration, 2 DMD patients were weaned from PPV |
| Garay et al. 1981 [14] | Case series | 8 | (33–71) | Alveolar hypoventilation (3 kyphoscoliosis, 2 postpoliomyelitis, 1 MD, 1 1° alveolar hypoventilation, 1 pneumonectomy with phrenic nerve crush) | iron lung (nocturnal NPV + daytime MPV) | All patients discharged and home treatment during 10 years |
| Corrado et al. 1995 [16] | Retrospective study | 15 | - | 7 ALS, 5 MD, 2 MG, 1 MS | Iron lung | Successful treatment in 12 patients |
| Hino et al. 2016 [17] | Case Report | 1 | 11 years | Nemaline myopathy complicated by tension pneumothorax undergoing NIPPV | Biphasic cuirass ventilation | Successful treatment, air leak resolution |
| Imitazione et al. 2021 [18] | Case Report | 1 | 56 years | Limb–girdle muscular dystrophy (LGMDs) | Poncho NPV + HFNC | Successful treatment in hospital and at home after refusing NIV. |
| Hassinger et al. 2017 [21] | Retrospective chart review | 233 | 15.5 months (7.6–39.6) | 15 neuromuscular diseases | Cuirass (CNEP or biphasic) | 8 patient responders |
| Nunez et al. 2019 [22] | Retrospective cohort study | 118 | 12 months | 3 neuromuscular diseases | Biphasic cuirass ventilation | 2 patient responders 1 nonresponder |
| Side Effects |
|---|
| Apneas Intolerance of corset Severe decubitus Lack of protection of the upper airway Musculoskeletal pain |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annunziata, A.; Calabrese, C.; Simioli, F.; Coppola, A.; Flora, M.; Marotta, A.; Di Spirito, V.; Didonna, F.; Cicalese, M.; Fiorentino, G. Negative-Pressure Ventilation in Neuromuscular Diseases in the Acute Setting. J. Clin. Med. 2022, 11, 2589. https://doi.org/10.3390/jcm11092589
Annunziata A, Calabrese C, Simioli F, Coppola A, Flora M, Marotta A, Di Spirito V, Didonna F, Cicalese M, Fiorentino G. Negative-Pressure Ventilation in Neuromuscular Diseases in the Acute Setting. Journal of Clinical Medicine. 2022; 11(9):2589. https://doi.org/10.3390/jcm11092589
Chicago/Turabian StyleAnnunziata, Anna, Cecilia Calabrese, Francesca Simioli, Antonietta Coppola, Martina Flora, Antonella Marotta, Valentina Di Spirito, Francesco Didonna, Marcellino Cicalese, and Giuseppe Fiorentino. 2022. "Negative-Pressure Ventilation in Neuromuscular Diseases in the Acute Setting" Journal of Clinical Medicine 11, no. 9: 2589. https://doi.org/10.3390/jcm11092589
APA StyleAnnunziata, A., Calabrese, C., Simioli, F., Coppola, A., Flora, M., Marotta, A., Di Spirito, V., Didonna, F., Cicalese, M., & Fiorentino, G. (2022). Negative-Pressure Ventilation in Neuromuscular Diseases in the Acute Setting. Journal of Clinical Medicine, 11(9), 2589. https://doi.org/10.3390/jcm11092589







