Use of CPAP Failure Score to Predict the Risk of Helmet-CPAP Support Failure in COVID-19 Patients: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Stabilized IPTW Effect
3.2. CPAP-FS
3.3. Diagnostic Ability
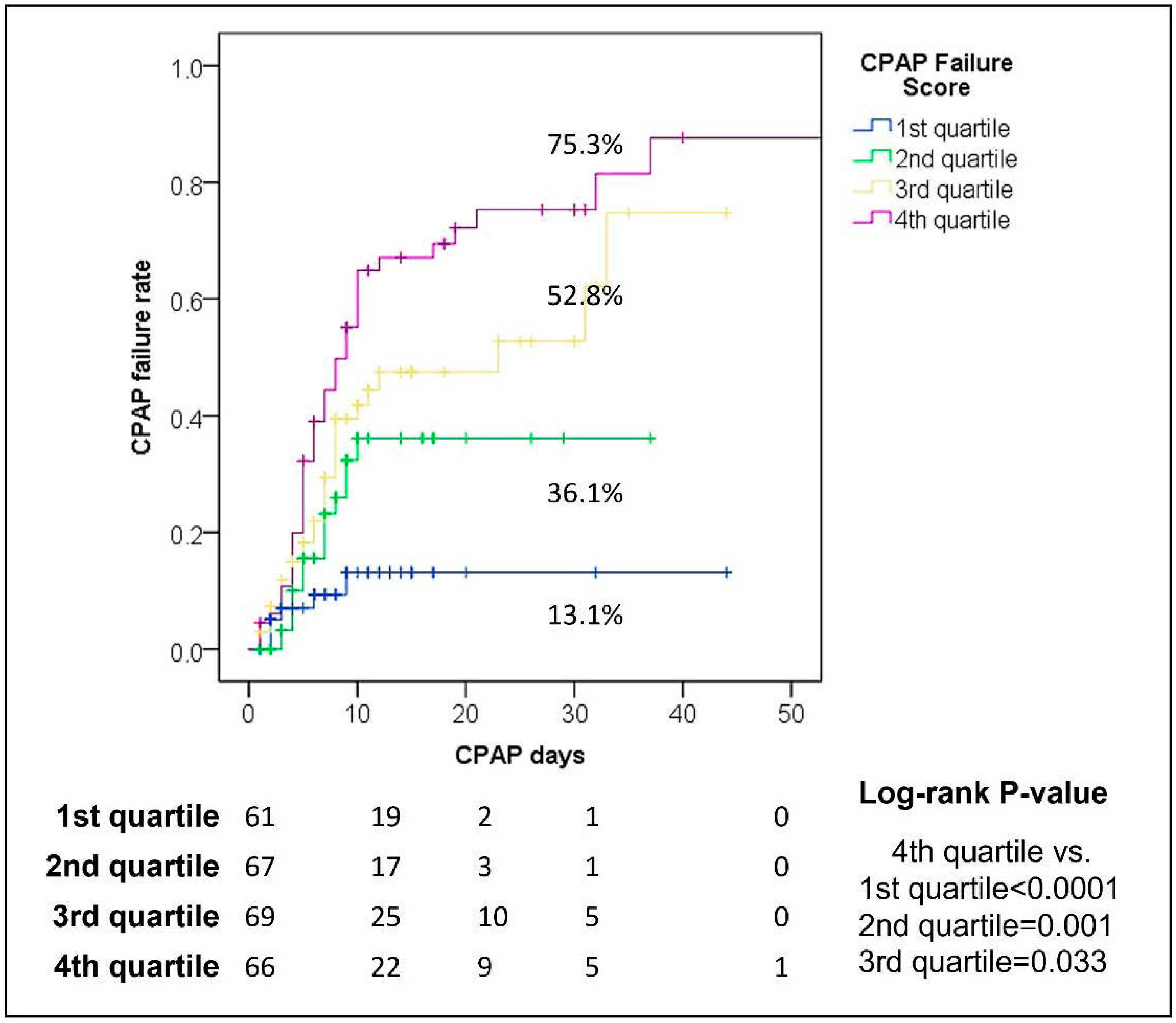
3.4. CPAP-FS and CPAP Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Camporota, L.; Chiumello, D.; Busana, M.; Romitti, F.; Gattinoni, L. From phenotypes to black holes… and back. Intensive Care Med. 2020, 46, 1498–1499. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329–2330. [Google Scholar] [CrossRef]
- Papoutsi, E.; Giannakoulis, V.G.; Xourgia, E.; Routsi, C.; Kotanidou, A.; Siempos, I.I. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: A systematic review and meta-analysis of non-randomized cohort studies. Crit. Care 2021, 25, 121. [Google Scholar] [CrossRef]
- Pandya, A.; Kaur, N.A.; Sacher, D.; O’Corragain, O.; Salerno, D.; Desai, P.; Criner, G.J. Ventilatory mechanics in early vs late intubation in a cohort of coronavirus disease 2019 patients with ARDS: A single center’s experience. Chest 2021, 159, 653–656. [Google Scholar] [CrossRef]
- Hernandez-Romieu, A.C.; Adelman, M.W.; Hockstein, M.A.; Robichaux, C.J.; Edwards, J.A.; Fazio, J.C.; Auld, S.C. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: A single-center cohort study. Crit. Care Med. 2020, 48, e1045–e1053. [Google Scholar] [CrossRef]
- Menga, L.S.; Cese, L.D.; Bongiovanni, F.; Lombardi, G.; Michi, T.; Luciani, F.; Cicetti, M.; Timpano, J.; Ferrante, M.C.; Cesarano, M.; et al. High failure rate of noninvasive oxygenation strategies in critically ill subjects with acute hypoxemic respiratory failure due to COVID-19. Respir. Care 2021, 66, 705–714. [Google Scholar] [CrossRef]
- Alviset, S.; Riller, Q.; Aboab, J.; Dilworth, K.; Billy, P.A.; Lombardi, Y.; Ioos, V. Continuous Positive Airway Pressure (CPAP) face-mask ventilation is an easy and cheap option to manage a massive influx of patients presenting acute respiratory failure during the SARS-CoV-2 outbreak: A retrospective cohort study. PLoS ONE 2020, 15, e0240645. [Google Scholar] [CrossRef]
- Battaglini, D.; Robba, C.; Ball, L.; Silva, P.L.; Cruz, F.F.; Pelosi, P.; Rocco, P.R. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: A narrative review. Br. J. Anaesth. 2021, 127, 353–364. [Google Scholar] [CrossRef]
- Carrillo, A.; Lopez, A.; Carrillo, L.; Caldeira, V.; Guia, M.; Alonso, N.; Esquinas, A. Validity of a clinical scale in predicting the failure of non-invasive ventilation in hypoxemic patients. J. Crit. Care 2020, 60, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, A.; Singh, G.P.; Rath, G.P.; Chaturvedi, A. The “COVID-19 score” can predict the need for tracheal intubation in critically ill COVID-19 patients da hypothesis. Med. Hypotheses 2020, 144, 110292. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, J.; Wu, W.; Chen, H.; Li, S.; He, H.; Yu, Y.; Hu, M.; Li, J.; Zheng, R.; et al. A simple nomogram for predicting failure of non-invasive respiratory strategies in adults with COVID-19: A retrospective multicentre study. Lancet Digit. Health 2021, 3, e166–e174. [Google Scholar] [CrossRef]
- Zhang, Z. Missing data imputation: Focusing on single imputation. Ann. Transl. Med. 2016, 4, 9. [Google Scholar] [PubMed]
- McCaffrey, D.F.; Griffin, B.A.; Almirall, D.; Slaughter, M.E.; Ramchand, R.; Burgette, L.F. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat. Med. 2013, 32, 3388–3414. [Google Scholar] [CrossRef] [Green Version]
- Burnand, B.; Kernan, W.N.; Feinstein, A.R. Indexes and boundaries for ‘‘quantitative significance’’ in statistical decisions. J. Clin. Epidemiol. 1990, 43, 1273–1284. [Google Scholar] [CrossRef]
- Bellani, G.; Patroniti, N.; Greco, M.; Foti, G.; Pesenti, A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008, 74, 651–656. [Google Scholar]
- Foti, G.; Sangalli, F.; Berra, L.; Sironi, S.; Cazzaniga, M.; Rossi, G.P.; Pesenti, A. Is helmet CPAP first line pre-hospital treatment of presumed severe acute pulmonary edema? Intensive Care Med. 2009, 35, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Pisani, L.; Mega, C.; Vaschetto, R.; Bellone, A.; Scala, R.; Cosentini, R.; Musti, M.; Del Forno, M.; Grassi, M.; Fasano, L.; et al. Oronasal mask versus helmet in acute hypercapnic respiratory failure. Eur. Respir. J. 2015, 45, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Bellani, G.; Laffey, J.G.; Pham, T.; Madotto, F.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Bumbasirevic, V.; Piquilloud, L.; et al. LUNG SAFE Investigators; ESICM Trials Group. Noninvasive ventilation of patients with acute respiratory distress syndrome: Insights from the LUNG SAFE Study. Am. J. Respir. Crit. Care Med. 2017, 195, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Ferreyro, B.L.; Angriman, F.; Munshi, L.; Del Sorbo, L.; Ferguson, N.D.; Rochwerg, B.; Scales, D.C. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: A systematic review and meta-analysis. JAMA 2020, 324, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Grieco, D.L.; Menga, L.S.; Cesarano, M.; Rosà, T.; Spadaro, S.; Bitondo, M.M. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: The HENIVOT randomized clinical trial. JAMA 2021, 325, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Guia, M.F.; Boléo-Tomé, J.P.; Imitazione, P.; Polistina, G.E.; Alves, C.; Ishikawa, O.; Ballenberger, M.; Mina, B.; Fiorentino, G.; Esquinas, A.; et al. Usefulness of the HACOR score in predicting success of CPAP in COVID-19-related hypoxemia. Respir. Med. 2021, 187, 106550. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Zhang, D.; Xu, J.; Chen, Z.; Yang, T.; Zhao, P.; Chen, G.; Cheng, G.; Wang, Y.; Bi, J.; et al. Prediction for progression risk in patients with COVID-19 pneumonia: The CALL Score. Clin. Infect. Dis. 2020, 71, 1393–1399. [Google Scholar] [CrossRef] [Green Version]
- Stawicki, S.P.; Jeanmonod, R.; Miller, A.C.; Paladino, L.; Gaieski, D.F.; Yaffee, A.Q.; De Wulf, A.; Grover, J.; Papadimos, T.J.; Bloem, C.; et al. The 2019–2020 Novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic: A joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group Consensus Paper. J. Glob. Infect. Dis. 2020, 12, 47–93. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Cruces, P.; Retamal, J.; Hurtado, D.E.; Erranz, B.; Iturrieta, P.; González, C.; Díaz, F. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit. Care 2020, 24, 494. [Google Scholar] [CrossRef]
| Variable | Entire Population (N = 263, 100.0%) | Short-CPAP (N = 191, 72.6%) | Long-CPAP (N = 72, 27.4%) | p-Value |
|---|---|---|---|---|
| Median (IQR) or N (%) | ||||
| COVID-19 first wave | 89 (33.8) | 68 (35.6) | 21 (29.2) | 0.38 |
| Age, years | 72 (62–81) | 72 (61–80) | 71 (62–83) | 0.68 |
| Male sex | 176 (66.9) | 127 (66.5) | 49 (68.1) | 0.88 |
| Arterial hypertension | 65 (24.7) | 53 (27.7) | 12 (16.7) | 0.08 |
| T2DM | 51 (19.4) | 33 (17.3) | 18 (25.0) | 0.17 |
| Cardiovascular comorbidity | 59 (22.4) | 45 (23.6) | 14 (19.4) | 0.51 |
| Liver comorbidity | 6 (2.3) | 5 (2.6) | 1 (1.4) | 1.00 |
| Asthma | 6 (2.3) | 6 (3.1) | 0 (0.0) | 0.19 |
| Chronic lung disease | 31 (11.8) | 19 (9.9) | 12 (16.7) | 0.14 |
| Renal comorbidity | 19 (7.2) | 13 (6.8) | 6 (8.3) | 0.79 |
| Neurological comorbidity | 33 (12.5) | 25 (13.1) | 8 (11.1) | 0.84 |
| Obesity | 18 (6.8) | 12 (6.3) | 6 (8.3) | 0.59 |
| HIV or malignancy | 10 (3.8) | 8 (4.2) | 2 (2.8) | 0.73 |
| Any comorbidity | 172 (65.4) | 124 (64.9) | 48 (66.7) | 0.89 |
| Hospital stay, days | 19 (11–30) | 16 (10–24) | 31 (20–37) | <0.0001 |
| Need for ICU stay | 64 (24.3) | 56 (29.3) | 8 (11.1) | 0.002 |
| CPAP use days | 7 (4–11) | 5 (4–8) | 17 (13–27) | <0.0001 |
| CT scan lungs mean damage % | 33 (20–50) | 33 (18–55) | 33 (20–45) | 0.42 |
| P/F ratio | 242 (177–290) | 242 (178–290) | 242 (170–295) | 0.80 |
| Lymphocytes, 103 cells/μL | 0.75 (0.51–1.10) | 0.76 (0.53–1.10) | 0.74 (0.50–1.11) | 0.72 |
| LDH, mU/mL | 366 (291–452) | 366 (283–453) | 364 (294–444) | 0.68 |
| Call Score | 10 (8–12) | 10 (8–12) | 10 (9–12) | 0.42 |
| SpO2 | 94 (90–96) | 93 (89–95) | 95 (91–97) | 0.02 |
| C-reactive protein, mmol/L | 8195 (1487–48,000) | 8195 (1438–48,000) | 9018 (1737–49,350) | 0.59 |
| D-dimer, ng/mL | 1089 (633–2140) | 1089 (627–1858) | 1132 (638–3085) | 0.44 |
| Orotracheal intubation | 43 (16.3) | 41 (21.5) | 2 (2.8) | <0.0001 |
| Death | 92 (35.0) | 81 (42.4) | 11 (15.3) | <0.0001 |
| CPAP failure (intubation and/or death) | 96 (36.5) | 85 (44.5) | 11 (15.3) | <0.0001 |
| Variables | Pre-IPTW | Post-IPTW | ||||
|---|---|---|---|---|---|---|
| Short-CPAP (N = 191) | Long-CPAP (N = 72) | Cohen’s D-Value | Short-CPAP (N = 97) | Long-CPAP (N = 71) | Cohen’s D-Value | |
| Mean ± SD | Mean ± SD | |||||
| COVID-19 first wave | 0.36 ± 0.48 | 0.29 ± 0.46 | 0.14 | 0.34 ± 0.48 | 0.30 ± 0.46 | 0.09 |
| Age | 70.27 ± 13.40 | 70.92 ± 13.72 | −0.05 | 70.64 ± 13.43 | 69.81 ± 14.28 | 0.06 |
| Male sex | 0.66 ± 0.47 | 0.68 ± 0.47 | −0.03 | 0.67 ± 0.47 | 0.59 ± 0.50 | 0.16 |
| Arterial hypertension | 0.28 ± 0.45 | 0.17 ± 0.38 | 0.28 | 0.25 ± 0.43 | 0.20 ± 0.41 | 0.10 |
| T2DM | 0.17 ± 0.38 | 0.25 ± 0.44 | −0.18 | 0.21 ± 0.41 | 0.18 ± 0.39 | 0.06 |
| Cardiovascular disease | 0.24 ± 0.43 | 0.19 ± 0.40 | 0.10 | 0.22 ± 0.42 | 0.17 ± 0.38 | 0.13 |
| Liver disease | 0.03 ± 0.16 | 0.01 ± 0.12 | 0.09 | 0.02 ± 0.15 | 0.02 ± 0.12 | 0.05 |
| Asthma | 0.03 ± 0.17 | 0.00 ± 0.00 | 0.34 | 0.02 ± 0.15 | 0.00 ± 0.00 | 0.23 |
| Chronic lung disease | 0.10 ± 0.30 | 0.17 ± 0.38 | −0.19 | 0.12 ± 0.33 | 0.12 ± 0.33 | 0.01 |
| Renal comorbidity | 0.07 ± 0.25 | 0.08 ± 0.28 | −0.06 | 0.07 ± 0.26 | 0.07 ± 0.27 | −0.01 |
| Neurological disease | 0.13 ± 0.34 | 0.11 ± 0.32 | 0.06 | 0.13 ± 0.33 | 0.11 ± 0.31 | 0.05 |
| Obesity | 0.06 ± 0.24 | 0.08 ± 0.28 | −0.08 | 0.06 ± 0.23 | 0.04 ± 0.19 | 0.10 |
| HIV or malignancy | 0.04 ± 0.20 | 0.03 ± 0.17 | 0.08 | 0.04 ± 0.19 | 0.03 ± 0.17 | 0.05 |
| Need for ICU stay | 0.29 ± 0.46 | 0.11 ± 0.32 | 0.51 | 0.24 ± 0.43 | 0.27 ± 0.45 | −0.07 |
| CT scan lung damage % | 36.69 ± 22.80 | 33.59 ± 20.11 | 0.15 | 36.09 ± 22.41 | 32.06 ± 20.28 | 0.19 |
| P/F ratio | 236.84 ± 79.59 | 240.42 ± 82.51 | −0.04 | 237.87 ± 79.29 | 232.38 ± 80.31 | 0.07 |
| Call Score | 9.88 ± 2.08 | 10.06 ± 2.23 | −0.08 | 9.94 ± 2.08 | 9.50 ± 2.35 | 0.20 |
| SpO2 | 91.84 ± 5.29 | 92.93 ± 5.86 | −0.19 | 92.06 ± 5.12 | 90.34 ± 9.56 | 0.21 |
| C-reactive protein | 40,073.96 ± 86,244.73 | 35,689.07 ± 59,617.40 | 0.06 | 38,824.67 ± 83,007.51 | 36,246.87 ± 62,108.37 | 0.04 |
| D-dimer | 1742.88 ± 1939.86 | 3839.19 ± 10,909.58 | −0.22 | 2007.53 ± 2622.81 | 2385.01 ± 6264.52 | −0.07 |
| Variables | Beta | SE | Wald | OR | 95.0% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| SpO2 | −0.153 | 0.050 | 3.17 | 0.86 | 0.79 | 0.93 | 0.001 |
| P/F ratio | −0.008 | 0.004 | 3.41 | 0.99 | 0.985 | 0.998 | 0.008 |
| Call Score | 0.365 | 0.189 | 0.99 | 1.44 | 1.09 | 1.91 | 0.02 |
| Chronic lung disease | 1.124 | 0.995 | 5.79 | 3.08 | 0.93 | 10.14 | 0.057 |
| Age | 0.044 | 0.031 | 6.54 | 1.05 | 0.99 | 1.10 | 0.10 |
| Male sex | 0.512 | 0.598 | 13.00 | 1.67 | 0.61 | 4.59 | 0.33 |
| Constant | 7.315 | 4.721 | 3.01 | 1502.64 | - | - | 0.051 |
| −2Log likelihood: 119.81; Hosmer-Lemeshow Test: 0.97 | |||||||
| Calculation of the CPAP Failure Score 7.315 + 0.512 (if male) + 0.044 × age + 1.124 (if chronic lung disease) + 0.365 × Call Score − 0.153 × SpO2 − 0.008 × P/F ratio | |||||||
| Variable | No CPAP Failure (n = 167, 63.5%) | CPAP Failure (n = 96, 36.5%) | p-Value |
|---|---|---|---|
| Median (IQR) or n (%) | |||
| COVID-19 first wave | 53 (31.7) | 36 (37.5) | 0.35 |
| Age, years | 70 (57–78) | 75 (68–84) | <0.0001 |
| Male sex | 106 (63.5) | 70 (72.9) | 0.14 |
| Arterial hypertension | 37 (22.2) | 28 (29.2) | 0.24 |
| T2DM | 27 (16.2) | 24 (25.0) | 0.11 |
| Cardiovascular comorbidity | 24 (14.4) | 35 (36.5) | <0.0001 |
| Liver comorbidity | 3 (1.8) | 3 (3.1) | 0.67 |
| Asthma | 4 (2.4) | 2 (2.1) | 1.00 |
| Chronic lung disease | 15 (9.0) | 16 (16.7) | 0.08 |
| Renal comorbidity | 11 (6.6) | 8 (8.3) | 0.63 |
| Neurological comorbidity | 16 (9.6) | 17 (17.7) | 0.04 |
| Obesity | 13 (7.8) | 5 (5.2) | 0.61 |
| HIV or malignancy | 4 (2.4) | 6 (6.3) | 0.18 |
| Any comorbidity | 93 (55.7) | 79 (82.3) | <0.0001 |
| Hospital stay, days | 23 (17–33) | 11 (7–20) | <0.0001 |
| Need for ICU stay | 20 (12.0) | 44 (45.8) | <0.0001 |
| CPAP lenght days | 8 (5–14) | 6 (4–9) | 0.001 |
| CT scan lungs mean damage % | 30 (20–45) | 38 (18–64) | 0.02 |
| p/f ratio | 252 (210–310) | 224 (131–243) | <0.0001 |
| Lymphocytes, 103 cells/μL | 0.77 (0.53–1.08) | 0.74 (0.49–1.16) | 0.81 |
| LDH, mU/mL | 366 (295–431) | 376 (274–500) | 0.26 |
| Call Score | 9 (8–12) | 11 (10–12) | <0.0001 |
| SpO2 | 94 (91–96) | 91 (87–95) | <0.0001 |
| C-reactive protein, mmol/L | 8195 (2000–50,100) | 8142 (1276–47,075) | 0.35 |
| D-dimer, ng/mL | 958 (552–1871) | 1264 (844–2913) | 0.007 |
| Orotracheal intubation | 0 (-) | 43 (44.8) | <0.0001 |
| Death | 0 (-) | 92 (95.8) | <0.0001 |
| CPAP failure (intubation and/or death) | 0 (-) | 96 (100.0) | <0.0001 |
| Post-IPTW (N = 168) | Pre-IPTW (N = 263) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | AUC | SE | 95.0% CI | p-Value | AUC | SE | 95.0% CI | p-Value | ||
| CPAP Failure Score | 0.87 | 0.03 | 0.81 | 0.93 | <0.0001 | 0.78 | 0.03 | 0.72 | 0.83 | <0.0001 |
| Age | 0.77 | 0.04 | 0.69 | 0.85 | <0.0001 | 0.66 | 0.03 | 0.59 | 0.73 | <0.0001 |
| D-dimer | 0.73 | 0.05 | 0.64 | 0.82 | <0.0001 | 0.60 | 0.04 | 0.53 | 0.67 | 0.007 |
| 1-(P/F ratio) | 0.71 | 0.04 | 0.62 | 0.79 | <0.0001 | 0.68 | 0.04 | 0.61 | 0.74 | <0.0001 |
| Call Score | 0.69 | 0.04 | 0.60 | 0.79 | <0.0001 | 0.68 | 0.03 | 0.61 | 0.74 | <0.0001 |
| 1-(SpO2) | 0.69 | 0.05 | 0.59 | 0.79 | <0.0001 | 0.65 | 0.04 | 0.58 | 0.72 | <0.0001 |
| Comorbidity | 0.63 | 0.05 | 0.53 | 0.72 | 0.01 | 0.63 | 0.04 | 0.57 | 0.70 | <0.0001 |
| Chronic lung disease | 0.59 | 0.05 | 0.49 | 0.69 | 0.08 | 0.54 | 0.04 | 0.47 | 0.61 | 0.30 |
| C-reactive protein | 0.43 | 0.05 | 0.33 | 0.53 | 0.15 | 0.47 | 0.04 | 0.39 | 0.54 | 0.35 |
| Male sex | 0.45 | 0.05 | 0.35 | 0.55 | 0.31 | 0.55 | 0.04 | 0.48 | 0.62 | 0.20 |
| CT scan lung damage % | 0.53 | 0.06 | 0.41 | 0.65 | 0.52 | 0.59 | 0.04 | 0.51 | 0.66 | 0.02 |
| 1st (N = 200) | 2nd (N = 200) | 3rd (N = 200) | 4th (N = 200) | 5th (N = 200) | 6th (N = 200) | 7th (N = 200) | 8th (N = 200) | 9th (N = 200) | 10th (N = 200) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | AUC | P | AUC | P | AUC | P | AUC | P | AUC | P | AUC | P | AUC | P | AUC | P | AUC | P | AUC | P |
| CPAP Failure Score | 0.80 | <0.0001 | 0.76 | <0.0001 | 0.77 | <0.0001 | 0.76 | <0.0001 | 0.77 | <0.0001 | 0.77 | <0.0001 | 0.80 | <0.0001 | 0.77 | <0.0001 | 0.78 | <0.0001 | 0.77 | <0.0001 |
| Age | 0.71 | <0.0001 | 0.64 | 0.002 | 0.65 | 0.001 | 0.68 | <0.0001 | 0.64 | 0.001 | 0.66 | <0.0001 | 0.67 | <0.0001 | 0.64 | 0.001 | 0.66 | <0.0001 | 0.67 | <0.0001 |
| D-dimer | 0.60 | 0.02 | 0.63 | 0.003 | 0.61 | 0.01 | 0.57 | 0.12 | 0.62 | 0.006 | 0.60 | 0.02 | 0.63 | 0.004 | 0.62 | 0.003 | 0.61 | 0.01 | 0.61 | 0.01 |
| 1-(p/f ratio) | 0.68 | <0.0001 | 0.65 | <0.0001 | 0.68 | <0.0001 | 0.66 | <0.0001 | 0.65 | <0.0001 | 0.68 | <0.0001 | 0.69 | <0.0001 | 0.69 | <0.0001 | 0.67 | <0.0001 | 0.68 | <0.0001 |
| Call Score | 0.69 | <0.0001 | 0.68 | <0.0001 | 0.66 | <0.0001 | 0.65 | <0.0001 | 0.66 | <0.0001 | 0.66 | <0.0001 | 0.69 | <0.0001 | 0.67 | <0.0001 | 0.68 | <0.0001 | 0.69 | <0.0001 |
| 1-(SpO2) | 0.66 | <0.0001 | 0.65 | <0.0001 | 0.68 | <0.0001 | 0.64 | 0.001 | 0.64 | 0.001 | 0.63 | 0.003 | 0.67 | <0.0001 | 0.67 | <0.0001 | 0.64 | 0.001 | 0.63 | 0.002 |
| Comorbidity | 0.64 | 0.001 | 0.64 | 0.002 | 0.63 | 0.002 | 0.62 | 0.006 | 0.64 | 0.001 | 0.60 | 0.03 | 0.65 | 0.001 | 0.63 | 0.002 | 0.63 | 0.002 | 0.63 | 0.003 |
| Chronic lung disease | 0.56 | 0.16 | 0.54 | 0.37 | 0.55 | 0.22 | 0.53 | 0.45 | 0.55 | 0.23 | 0.53 | 0.55 | 0.56 | 0.15 | 0.55 | 0.26 | 0.55 | 0.26 | 0.53 | 0.47 |
| C-reactive protein | 0.46 | 0.37 | 0.48 | 0.65 | 0.45 | 0.24 | 0.47 | 0.50 | 0.48 | 0.65 | 0.48 | 0.57 | 0.43 | 0.12 | 0.46 | 0.34 | 0.47 | 0.47 | 0.47 | 0.44 |
| Male sex | 0.54 | 0.38 | 0.54 | 0.33 | 0.53 | 0.47 | 0.54 | 0.34 | 0.57 | 0.12 | 0.56 | 0.19 | 0.55 | 0.29 | 0.53 | 0.43 | 0.57 | 0.10 | 0.53 | 0.55 |
| CT scan lungs damage % | 0.57 | 0.12 | 0.60 | 0.02 | 0.56 | 0.14 | 0.53 | 0.47 | 0.55 | 0.21 | 0.60 | 0.02 | 0.63 | 0.004 | 0.60 | 0.02 | 0.61 | 0.009 | 0.57 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessandri, F.; Tosi, A.; De Lazzaro, F.; Andreoli, C.; Cicchinelli, A.; Carrieri, C.; Lai, Q.; Pugliese, F.; on behalf of the Policlinico Umberto I COVID-19 Group. Use of CPAP Failure Score to Predict the Risk of Helmet-CPAP Support Failure in COVID-19 Patients: A Retrospective Study. J. Clin. Med. 2022, 11, 2593. https://doi.org/10.3390/jcm11092593
Alessandri F, Tosi A, De Lazzaro F, Andreoli C, Cicchinelli A, Carrieri C, Lai Q, Pugliese F, on behalf of the Policlinico Umberto I COVID-19 Group. Use of CPAP Failure Score to Predict the Risk of Helmet-CPAP Support Failure in COVID-19 Patients: A Retrospective Study. Journal of Clinical Medicine. 2022; 11(9):2593. https://doi.org/10.3390/jcm11092593
Chicago/Turabian StyleAlessandri, Francesco, Antonella Tosi, Francesco De Lazzaro, Chiara Andreoli, Andrea Cicchinelli, Cosima Carrieri, Quirino Lai, Francesco Pugliese, and on behalf of the Policlinico Umberto I COVID-19 Group. 2022. "Use of CPAP Failure Score to Predict the Risk of Helmet-CPAP Support Failure in COVID-19 Patients: A Retrospective Study" Journal of Clinical Medicine 11, no. 9: 2593. https://doi.org/10.3390/jcm11092593
APA StyleAlessandri, F., Tosi, A., De Lazzaro, F., Andreoli, C., Cicchinelli, A., Carrieri, C., Lai, Q., Pugliese, F., & on behalf of the Policlinico Umberto I COVID-19 Group. (2022). Use of CPAP Failure Score to Predict the Risk of Helmet-CPAP Support Failure in COVID-19 Patients: A Retrospective Study. Journal of Clinical Medicine, 11(9), 2593. https://doi.org/10.3390/jcm11092593







