Is Poor Lithium Response in Individuals with Bipolar Disorder Associated with Increased Degradation of Tryptophan along the Kynurenine Pathway? Results of an Exploratory Study
Abstract
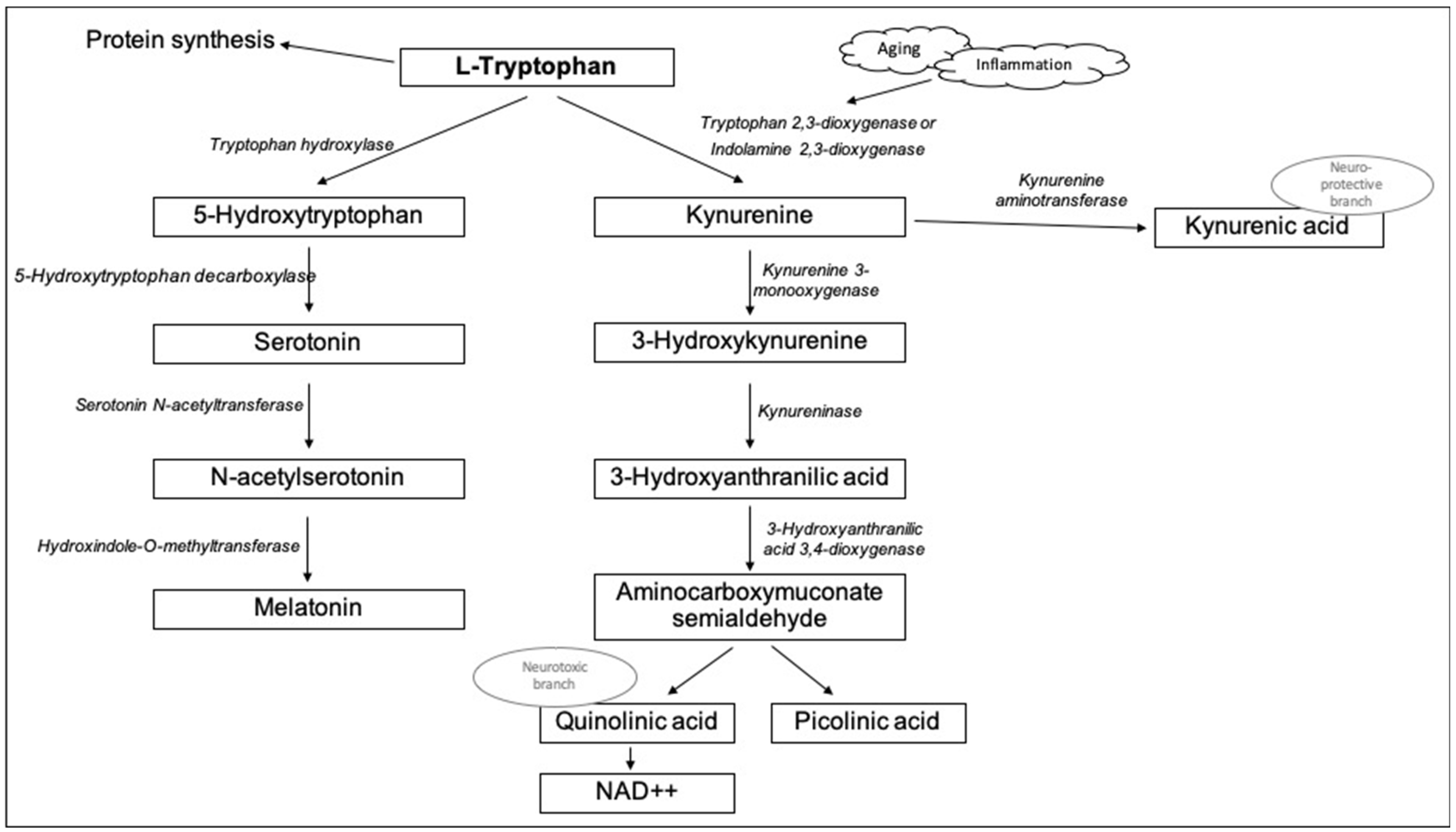
:1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Characterization of Response to Treatment and Psychometric Assessment
2.3. Laboratory Methods
2.4. Statistical Analyses
3. Results
3.1. Comparison of Individuals with Bipolar Disorder and Healthy Controls
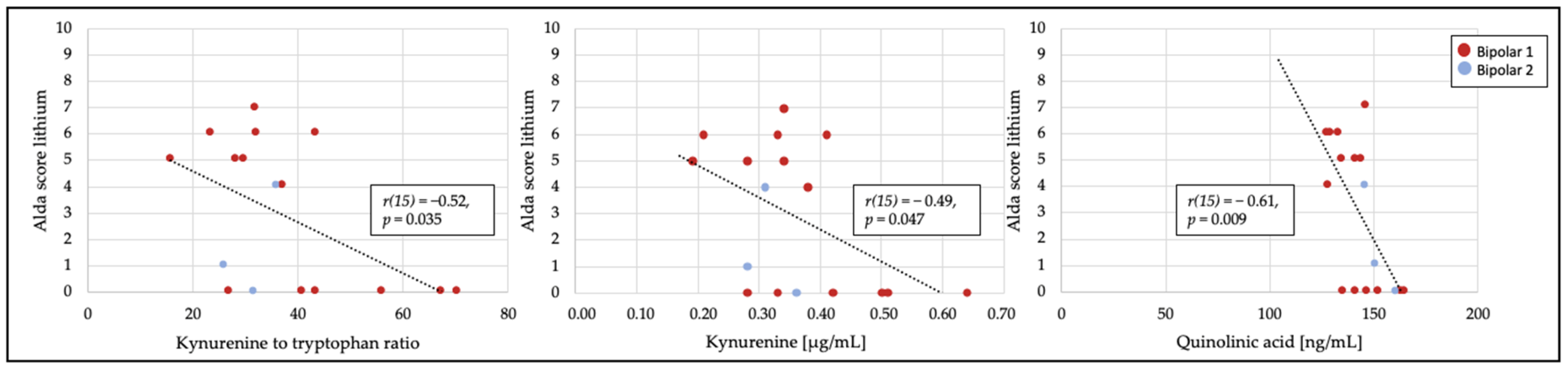
3.2. Relationship of Treatment Response to Tryptophan Catabolites
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BD | bipolar disorder |
| HC | healthy control |
| IDO | indoleamine-2,3-dioxygenase |
| KAT | kynurenine-aminotransferase |
| KMO | kynurenine-3-monooxygenase |
| KYN | kynurenine |
| KYNA | kynurenic acid |
| MLT | melatonin |
| NMDA | n-methyl-d-aspartate |
| TRP | tryptophan |
| TDO | tryptophan 2,3-dioxigenase |
| QA | quinolinic acid |
| 3-HAA | 3-hydroxyanthranilic acid |
| BD | bipolar disorder |
| HC | healthy control |
References
- Goodwin, F.K.; Jamison, K.R. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression; Oxford University Press: Oxford, UK, 2007; Volume 2. [Google Scholar]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Hidalgo-Mazzei, D.; Strawbridge, R.; Young, A.; Resche-Rigon, M.; Etain, B.; Andreassen, O.A.; Bauer, M.; Bennabi, D.; Blamire, A.M.; et al. Prospective cohort study of early biosignatures of response to lithium in bipolar-I-disorders: Overview of the H2020-funded R-LiNK initiative. Int. J. Bipolar Disord. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comai, S.; Bertazzo, A.; Brughera, M.; Crotti, S. Tryptophan in health and disease. Adv. Clin. Chem. 2020, 95, 165–218. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B.; Guillemin, G. The Plasma [Kynurenine]/[Tryptophan] Ratio and Indoleamine 2,3-Dioxygenase: Time for Appraisal. Int. J. Tryptophan Res. 2019, 12, 1178646919868978. [Google Scholar] [CrossRef] [Green Version]
- Messaoud, A.; Rym, M.; Wahiba, D.; Neffati, F.; Najjar, M.F.; Gobbi, G.; Manchia, M.; Valtorta, F.; Lotfi, G.; Comai, S. Investigation of the relationship among cortisol, pro-inflammatory cytokines, and the degradation of tryptophan into kynurenine in patients with major depression and suicidal behavior. Curr. Top. Med. Chem. 2021, 21. online ahead of print. [Google Scholar] [CrossRef]
- Raheja, U.K.; Fuchs, D.; Giegling, I.; Brenner, L.A.; Rovner, S.F.; Mohyuddin, I.; Weghuber, D.; Mangge, H.; Rujescu, D.; Postolache, T.T. In psychiatrically healthy individuals, overweight women but not men have lower tryptophan levels. Pteridines 2015, 26, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Mangge, H.; Summers, K.L.; Meinitzer, A.; Zelzer, S.; Almer, G.; Prassl, R.; Schnedl, W.J.; Reininghaus, E.; Paulmichl, K.; Weghuber, D.; et al. Obesity-related dysregulation of the Tryptophan–Kynurenine metabolism: Role of age and parameters of the metabolic syndrome. Obesity 2014, 22, 195–201. [Google Scholar] [CrossRef]
- Pertovaara, M.; Heliövaara, M.; Raitala, A.; Oja, S.S.; Knekt, P.; Hurme, M. The activity of the immunoregulatory enzyme indoleamine 2,3-dioxygenase is decreased in smokers. Clin. Exp. Immunol. 2006, 145, 469–473. [Google Scholar] [CrossRef]
- Brandacher, G.; Hoeller, E.; Fuchs, D.; Weiss, H. Chronic Immune Activation Underlies Morbid Obesity: Is IDO A Key Player? Curr. Drug Metab. 2007, 8, 289–295. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Wang, L.; Brew, B.J. Quinolinic acid selectively induces apoptosis of human astrocytes: Potential role in AIDS dementia complex. J. Neuroinflamm. 2005, 2, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoga, M.; Oulis, P.; Chatzipanagiotou, S.; Masdrakis, V.G.; Pliatsika, P.; Boufidou, F.; Foteli, S.; Soldatos, C.R.; Nikolaou, C.; Papageorgiou, C. Indoleamine 2,3-dioxygenase and immune changes under antidepressive treatment in major depression in females. In Vivo 2014, 8, 633–638. [Google Scholar]
- Kindler, J.; Lim, C.K.; Weickert, C.S.; Boerrigter, D.; Galletly, C.; Liu, D.; Jacobs, K.R.; Balzan, R.; Bruggemann, J.; O’Donnell, M.; et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatry 2020, 25, 2860–2872. [Google Scholar] [CrossRef] [Green Version]
- Munkholm, K.; Braüner, J.V.; Kessing, L.V.; Vinberg, M. Cytokines in bipolar disorder vs. healthy control subjects: A systematic review and meta-analysis. J. Psychiatr. Res. 2013, 47, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Gostner, J.M.; Geisler, S.; Stonig, M.; Mair, L.; Sperner-Unterweger, B.; Fuchs, D. Tryptophan Metabolism and Related Pathways in Psychoneuroimmunology: The Impact of Nutrition and Lifestyle. Neuropsychobiology 2020, 79, 89–99. [Google Scholar] [CrossRef]
- Musso, T.; Gusella, G.L.; Brooks, A.; Longo, D.L.; Varesio, L. Interleukin-4 inhibits indoleamine 2,3-dioxygenase expression in human monocytes. Blood 1994, 83, 1408–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef]
- Comai, S.; Melloni, E.; Lorenzi, C.; Bollettini, I.; Vai, B.; Zanardi, R.; Colombo, C.; Valtorta, F.; Benedetti, F.; Poletti, S. Selective association of cytokine levels and kynurenine/tryptophan ratio with alterations in white matter microstructure in bipolar but not in unipolar depression. Eur. Neuropsychopharmacol. 2022, 55, 96–109. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Choudhury, S.; Musaelyan, K.; Myint, A.M.; Thuret, S.; Price, J.; Pariante, C.M. Interleukin-1β: A New Regulator of the Kynurenine Pathway Affecting Human Hippocampal Neurogenesis. Neuropsychopharmacology 2012, 37, 939–949. [Google Scholar] [CrossRef] [Green Version]
- O’Farrell, K.; Harkin, A. Stress-related regulation of the kynurenine pathway: Relevance to neuropsychiatric and degenerative disorders. Neuropharmacology 2017, 112, 307–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myint, A.M.; Kim, Y.-K.; Verkerk, R.; Park, S.H.; Scharpé, S.; Steinbusch, H.W.M.; Leonard, B.E. Tryptophan breakdown pathway in bipolar mania. J. Affect. Disord. 2007, 102, 65–72. [Google Scholar] [CrossRef]
- Fellendorf, F.T.; Gostner, J.M.; Lenger, M.; Platzer, M.; Birner, A.; Maget, A.; Queissner, R.; Tmava-Berisha, A.; Pater, C.A.; Ratzenhofer, M.; et al. Tryptophan Metabolism in Bipolar Disorder in a Longitudinal Setting. Antioxidants 2021, 10, 1795. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry 2020, 26, 4158–4178. [Google Scholar] [CrossRef] [PubMed]
- Poletti, S.; Melloni, E.; Aggio, V.; Colombo, C.; Valtorta, F.; Benedetti, F.; Comai, S. Grey and white matter structure associates with the activation of the tryptophan to kynurenine pathway in bipolar disorder. J. Affect. Disord. 2019, 259, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Dantzer, R.; Kelley, K.W.; Lawson, M.A.; Woolwine, B.J.; Vogt, G.; Spivey, J.R.; Saito, K.; Miller, A.H. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: Relationship to CNS immune responses and depression. Mol. Psychiatry 2010, 15, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Abreu, T.; Bragança, M. The bipolarity of light and dark: A review on Bipolar Disorder and circadian cycles. J. Affect. Disord. 2015, 185, 219–229. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Birner, A.; Platzer, M.; Bengesser, S.A.; Dalkner, N.; Fellendorf, F.T.; Queissner, R.; Pilz, R.; Rauch, P.; Maget, A.; Hamm, C.; et al. Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PLoS ONE 2017, 12, e0172699. [Google Scholar] [CrossRef]
- Cipriani, A.; Pretty, H.; Hawton, K.; Geddes, J.R. Lithium in the Prevention of Suicidal Behavior and All-Cause Mortality in Patients With Mood Disorders: A Systematic Review of Randomized Trials. Am. J. Psychiatry 2005, 162, 1805–1819. [Google Scholar] [CrossRef]
- Göttert, R.; Fidzinski, P.; Kraus, L.; Schneider, U.C.; Holtkamp, M.; Endres, M.; Gertz, K.; Kronenberg, G. Lithium inhibits tryptophan catabolism via the inflammation-induced kynurenine pathway in human microglia. Glia 2022, 70, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Geoffroy, P.A. Lithium and bipolar disorder: Impacts from molecular to behavioural circadian rhythms. Chronobiol. Int. 2016, 33, 351–373. [Google Scholar] [CrossRef] [PubMed]
- Hallam, K.T.; Olver, J.S.; Norman, T.R. Effect of Sodium Valproate on Nocturnal Melatonin Sensitivity to Light in Healthy Volunteers. Neuropsychopharmacology 2005, 30, 1400–1404. [Google Scholar] [CrossRef]
- Muneer, A. Kynurenine Pathway of Tryptophan Metabolism in Neuropsychiatric Disorders: Pathophysiologic and Therapeutic Considerations. Clin. Psychopharmacol. Neurosci. 2020, 18, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Drevets, W.; Turecki, G.; Li, Q.S. The relationship between plasma serotonin and kynurenine pathway metabolite levels and the treatment response to escitalopram and desvenlafaxine. Brain. Behav. Immun. 2020, 87, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Manchia, M.; Squassina, A.; Pisanu, C.; Congiu, D.; Garzilli, M.; Guiso, B.; Suprani, F.; Paribello, P.; Pulcinelli, V.; Iaselli, M.N.; et al. Investigating the relationship between melatonin levels, melatonin system, microbiota composition and bipolar disorder psychopathology across the different phases of the disease. Int. J. Bipolar Disord. 2019, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Grof, P.; Duffy, A.; Cavazzoni, P.; Grof, E.; Garnham, J.; MacDougall, M.; O’Donovan, C.; Alda, M. Is Response to Prophylactic Lithium a Familial Trait? J. Clin. Psychiatry 2002, 63, 942–947. [Google Scholar] [CrossRef]
- Manchia, M.; Adli, M.; Akula, N.; Ardau, R.; Aubry, J.-M.; Backlund, L.; Banzato, C.E.; Baune, B.T.; Bellivier, F.; Bengesser, S.; et al. Assessment of Response to Lithium Maintenance Treatment in Bipolar Disorder: A Consortium on Lithium Genetics (ConLiGen) Report. PLoS ONE 2013, 8, e65636. [Google Scholar] [CrossRef] [Green Version]
- Nunes, A.; Trappenberg, T.; Alda, M. Asymmetrical reliability of the Alda score favours a dichotomous representation of lithium responsiveness. PLoS ONE 2020, 15, e0225353. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.W.; Baek, J.H.; Yang, S.-Y.; Kim, Y.; Cho, Y.; Choi, Y.; Lee, K.; Park, T.; Hong, K.S. Long-term response to mood stabilizer treatment and its clinical correlates in patients with bipolar disorders: A retrospective observational study. Int. J. Bipolar Disord. 2017, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Garnham, J.; Munro, A.; Slaney, C.; Macdougall, M.; Passmore, M.; Duffy, A.; O’Donovan, C.; Teehan, A.; Alda, M. Prophylactic treatment response in bipolar disorder: Results of a naturalistic observation study. J. Affect. Disord. 2007, 104, 185–190. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Guy, W. Clinical Global Impression. In ECDEU Assessment Manual for Psychopharmacology; National Institute of Mental Health: Rockville, MD, USA, 1976. [Google Scholar]
- Simonato, M.; Dall’Acqua, S.; Zilli, C.; Sut, S.; Tenconi, R.; Gallo, N.; Sfriso, P.; Sartori, L.; Cavallin, F.; Fiocco, U.; et al. Tryptophan Metabolites, Cytokines, and Fatty Acid Binding Protein 2 in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines 2021, 9, 1724. [Google Scholar] [CrossRef]
- Nazzari, S.; Molteni, M.; Valtorta, F.; Comai, S.; Frigerio, A. Prenatal IL-6 levels and activation of the tryptophan to kynurenine pathway are associated with depressive but not anxiety symptoms across the perinatal and the post-partum period in a low-risk sample. Brain. Behav. Immun. 2020, 89, 175–183. [Google Scholar] [CrossRef]
- Bartoli, F.; Misiak, B.; Callovini, T.; Cavaleri, D.; Cioni, R.M.; Crocamo, C.; Savitz, J.B.; Carrà, G. The kynurenine pathway in bipolar disorder: A meta-analysis on the peripheral blood levels of tryptophan and related metabolites. Mol. Psychiatry 2020, 26, 3419–3429. [Google Scholar] [CrossRef]
- Fuchs, D.; Möller, A.A.; Reibnegger, G.; Stöckle, E.; Werner, E.R.; Wachter, H. Decreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptoms. J. Acquir. Immune Defic. Syndr. 1990, 3, 873–876. [Google Scholar]
- Reininghaus, E.Z.; McIntyre, R.S.; Reininghaus, B.; Geisler, S.; Bengesser, S.A.; Lackner, N.; Hecht, K.; Birner, A.; Kattnig, F.; Unterweger, R.; et al. Tryptophan breakdown is increased in euthymic overweight individuals with bipolar disorder: A preliminary report. Bipolar Disord. 2014, 16, 432–440. [Google Scholar] [CrossRef]
- Trepci, A.; Sellgren, C.M.; Pålsson, E.; Brundin, L.; Khanlarkhani, N.; Schwieler, L.; Landén, M.; Erhardt, S. Central levels of tryptophan metabolites in subjects with bipolar disorder. Eur. Neuropsychopharmacol. 2021, 43, 52–62. [Google Scholar] [CrossRef]
- Mukherjee, D.; Krishnamurthy, V.B.; Millett, C.E.; Reider, A.; Can, A.; Groer, M.; Fuchs, D.; Postolache, T.T.; Saunders, E.F.H. Total sleep time and kynurenine metabolism associated with mood symptom severity in bipolar disorder. Bipolar Disord. 2018, 20, 27–34. [Google Scholar] [CrossRef]
- van den Ameele, S.; Fuchs, D.; Coppens, V.; de Boer, P.; Timmers, M.; Sabbe, B.; Morrens, M. Markers of Inflammation and Monoamine Metabolism Indicate Accelerated Aging in Bipolar Disorder. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, S.K.; Samuelsson, M.; Saetre, P.; Lindström, L.; Jönsson, E.G.; Nordin, C.; Engberg, G.; Erhardt, S.; Landén, M. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J. Psychiatry Neurosci. 2010, 35, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.L.; Llenos, I.C.; Cwik, M.; Walkup, J.; Weis, S. Alterations in kynurenine precursor and product levels in schizophrenia and bipolar disorder. Neurochem. Int. 2008, 52, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M. Bipolar Disorder: Role of Immune-Inflammatory Cytokines, Oxidative and Nitrosative Stress and Tryptophan Catabolites. Curr. Psychiatry Rep. 2015, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Domínguez, A.; Hernández, M.E.; Berlanga, C.; Gutiérrez-Mora, D.; Moreno, J.; Heinze, G.; Pavón, L. Immune variations in bipolar disorder: Phasic differences. Bipolar Disord. 2007, 9, 596–602. [Google Scholar] [CrossRef]
- Bonaccorso, S.; Marino, V.; Puzella, A.; Pasquini, M.; Biondi, M.; Artini, M.; Almerighi, C.; Verkerk, R.; Meltzer, H.; Maes, M. Increased Depressive Ratings in Patients With Hepatitis C Receiving Interferon-α–Based Immunotherapy Are Related to Interferon-α–Induced Changes in the Serotonergic System. J. Clin. Psychopharmacol. 2002, 22, 86–90. [Google Scholar] [CrossRef]
- Lavebratt, C.; Olsson, S.; Backlund, L.; Frisén, L.; Sellgren, C.; Priebe, L.; Nikamo, P.; Träskman-Bendz, L.; Cichon, S.; Vawter, M.P.; et al. The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol. Psychiatry 2014, 19, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Bay-Richter, C.; Linderholm, K.R.; Lim, C.K.; Samuelsson, M.; Träskman-Bendz, L.; Guillemin, G.J.; Erhardt, S.; Brundin, L. A role for inflammatory metabolites as modulators of the glutamate N-methyl-d-aspartate receptor in depression and suicidality. Brain. Behav. Immun. 2015, 43, 110–117. [Google Scholar] [CrossRef]
- Messaoud, A.; Mensi, R.; Douki, W.; Neffati, F.; Najjar, M.F.; Gobbi, G.; Valtorta, F.; Gaha, L.; Comai, S. Reduced peripheral availability of tryptophan and increased activation of the kynurenine pathway and cortisol correlate with major depression and suicide. World J. Biol. Psychiatry 2019, 20, 703–711. [Google Scholar] [CrossRef]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Scott, J.; Etain, B.; Bellivier, F. Can an Integrated Science Approach to Precision Medicine Research Improve Lithium Treatment in Bipolar Disorders? Front. Psychiatry 2018, 9, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amerio, A.; Russo, D.; Miletto, N.; Aguglia, A.; Costanza, A.; Benatti, B.; Odone, A.; Barroilhet, S.A.; Brakoulias, V.; Dell’Osso, B.; et al. Polypharmacy as maintenance treatment in bipolar illness: A systematic review. Acta Psychiatr. Scand. 2021, 144, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Sachs, G.S.; Rush, A.J. Response, remission, and recovery in bipolar disorders: What are the realistic treatment goals? J. Clin. Psychiatry 2003, 64 (Suppl. S6), 18–22; discussion 28. [Google Scholar]
- Müller-Oerlinghausen, B. Lithium long-term treatment--does it act via serotonin? Pharmacopsychiatry 1985, 18, 214–217. [Google Scholar] [CrossRef]
- Bowers, M.B.; Heninger, G.R. Lithium: Clinical effects and cerebrospinal fluid acid monoamine metabolites. Commun. Psychopharmacol. 1977, 1, 135–145. [Google Scholar]
- Sobanski, T.; Bagli, M.; Laux, G.; Rao, M. Serotonin Syndrome after Lithium Add-on Medication to Paroxetine. Pharmacopsychiatry 1997, 30, 106–107. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, X.; Dai, X.; Lu, S.; Gui, B.; Jin, W.; Zhang, S.; Zhang, S.; Qian, Y. Lithium ameliorates lipopolysaccharide-induced microglial activation via inhibition of toll-like receptor 4 expression by activating the PI3K/Akt/FoxO1 pathway. J. Neuroinflamm. 2014, 11, 140. [Google Scholar] [CrossRef] [Green Version]
- Ajmone-Cat, M.A.; D’Urso, M.C.; di Blasio, G.; Brignone, M.S.; De Simone, R.; Minghetti, L. Glycogen synthase kinase 3 is part of the molecular machinery regulating the adaptive response to LPS stimulation in microglial cells. Brain. Behav. Immun. 2016, 55, 225–235. [Google Scholar] [CrossRef]
- Tran, H.-Q.; Shin, E.-J.; Saito, K.; Tran, T.-V.; Phan, D.-H.; Sharma, N.; Kim, D.-W.; Choi, S.Y.; Jeong, J.H.; Jang, C.-G.; et al. Indoleamine-2,3-dioxygenase-1 is a molecular target for the protective activity of mood stabilizers against mania-like behavior induced by d-amphetamine. Food Chem. Toxicol. 2020, 136, 110986. [Google Scholar] [CrossRef]
- Ward, M.E.; Musa, M.N.; Bailey, L. Clinical Pharmacokinetics of Lithium. J. Clin. Pharmacol. 1994, 34, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.E.; Thelwall, P.E.; Necus, J.; Flowers, C.J.; Blamire, A.M.; Cousins, D.A. 3D 7Li magnetic resonance imaging of brain lithium distribution in bipolar disorder. Mol. Psychiatry 2018, 23, 2184–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seggie, J.; Werstiuk, E.S.; Grota, L. Lithium and circadian patterns of melatonin in the retina, hypothalamus, pineal and serum. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1987, 11, 325–334. [Google Scholar] [CrossRef]
- Dallaspezia, S.; Benedetti, F. Melatonin, circadian rhythms, and the clock genes in bipolar disorder. Curr. Psychiatry Rep. 2009, 11, 488–493. [Google Scholar] [CrossRef]
- Maes, M.; Calabrese, J.; Jayathilake, K.; Meltzer, H.Y. Effects of subchronic treatment with valproate on l-5-HTP-induced cortisol responses in mania: Evidence for increased central serotonergic neurotransmission. Psychiatry Res. 1997, 71, 67–76. [Google Scholar] [CrossRef]
- Maciejak, P.; Szyndler, J.; Turzyńska, D.; Sobolewska, A.; Płaźnik, A. Kynurenic acid: A new effector of valproate action? Pharmacol. Rep. 2011, 63, 1569–1573. [Google Scholar] [CrossRef]
- Kocki, T.; Wielosz, M.; Turski, W.A.; Urbanska, E.M. Enhancement of brain kynurenic acid production by anticonvulsants—Novel mechanism of antiepileptic activity? Eur. J. Pharmacol. 2006, 541, 147–151. [Google Scholar] [CrossRef]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood?Brain Barrier Transport of Kynurenines: Implications for Brain Synthesis and Metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef]
| Individuals with BD (n = 48) | HC (n = 48) | Statistics | p | Effect Size | |
|---|---|---|---|---|---|
| Age [years] | 51.59 (±11.13) | 51.71 (±8.47) | t (87,14) = −0.06 | 0.951 | d = 0.01 |
| Sex | 37.5% male 62.5% female | 37.5% male 62.5% female | X2 = 0 | 1.00 | |
| BMI [kg/m2] | 25.33 (±5.24) | 23.60 (±3.42) | t (94) = 1.91 | 0.059 | d = 0.39 |
| Smoking status | 27.1% no 47.9% yes 25.0% ex-smoker | 68.8% no 18.8% yes 12.5% ex-smoker | X2 = 16.82 | <0.001 ** | η2 = 0.419 |
| TRP [μg/mL] | 9.54 (±1.64) | 11.06 (±1.69) | F (1,91) = 18.26 | <0.001 ** | η2 = 0.167 |
| KYN [μg/mL] | 0.35 (±0.10) | 0.38 (±0.14) | F (1,91) = 2.070 | 0.154 | η2 = 0.022 |
| KYN/TRP*1000 ratio | 38.11 (±14.50) | 34.86 (±11.97) | F (1,91) = 0.99 | 0.321 | η2 = 0.011 |
| KYNA [ng/mL] | 9.02 (±4.76) | 10.25 (±8.09) | F (1,91) = 1.26 | 0.264 | η2 = 0.014 |
| 3-HK [ng/mL] | 42.46 (±2.84) | 44.40 (±13.48) | F (1,91) = 0.86 | 0.356 | η2 = 0.009 |
| QA [ng/mL] | 142.62 (±15.13) | 158.73 (±45.89) | F (1,91) = 4.91 | 0.029 * | η2 = 0.051 |
| QA/KYNA ratio | 24.01 (±20.93) | 22.54 (±14.01) | F (1,91) = 0.24 | 0.623 | η2 = 0.003 |
| 5-HTP [ng/mL] | 87.90 (±29.49) | 69.93 (±34.48) | F (1,91) = 5.95 | 0.017 * | η2 = 0.061 |
| 5-HT [ng/mL] | 319.25 (±168.83) | 260.74 (±167.97) | F (1,91) = 3.55 | 0.063 | η2 = 0.038 |
| MLT [pg/mL] | 11.44 (±6.53) | 11.89 (±5.82) | F (1,91) = 0.09 | 0.764 | η2 = 0.001 |
| Individuals with BD (n = 48) | ||||
|---|---|---|---|---|
| Diagnosis | Bipolar I: 72.9% Bipolar II: 27.1% | |||
| Years of illness (Mean ± SD) | 20.82 (±9.72) | |||
| Number of depressions (Mean ± SD) | 6.42 (±9.59) | |||
| - Mild | 4.71 (±4.66) | |||
| - Moderate | 4.87 (±8.93) | |||
| - Severe | 1.56 (±2.03) | |||
| Number of manic episodes (Mean ± SD) | 6.78 (±10.19) | |||
| - Hypomanic | 3.42 (±6.10) | |||
| - Moderate manic | 2.38 (±4.74) | |||
| - Severe manic | 1.13 (±1.85) | |||
| CGI (Mean ± SD) | 2.53 (±0.91) | |||
| HAMD (Mean ± SD) | 4.11 (±3.86) | |||
| YMRS (Mean ± SD) | 1.68 (±2.49) | |||
| HAMA (Mean ± SD) | 6.21 (±5.14) | |||
| Alda Score | ||||
| Lithium | Valproate | Lamotrigine | ||
| Current prophylactic treatment (n, %) | 44 (91.66%) | |||
| - only lithium | 8 (16.67%) | 2.75 (±2.76) | ||
| - only anticonvulsant | 11 (22.92%) | |||
| - only valproate | 8 (16.67)% | 4.00 (±0.82) | ||
| - only lamotrigine | 2 (4.17%) | 1.00 (±1.41) | ||
| - only antipsychotic | 3 (6.25%) | |||
| - lithium + anticonvulsant | 7 (14.58%) | 3.86 (±2.79) | ||
| - with valproate | 3 (6.25%) | 3.67 (±3.21) | 3.67 (±3.21) | |
| - with lamotrigine | 2 (4.17%) | 5.50 (±2.12) | 8 (±0) | |
| - lithium + antipsychotic | 2 (4.17%) | 0 | ||
| - anticonvulsant + antipsychotic | 10 (20.83%) | |||
| - with valproate | 7 (14.58%) | 2.86 (±2.48) | ||
| - with lamotrigine | 1 (2.08%) | 7 | ||
| Other psychopharmaceuticals (n, %) | ||||
| - Antipsychotics in subprophylactic dose (n = 44) | 17 (38.64%) | |||
| - Antidepressants (n = 44) | 11 (25.00%) | |||
| - Benzodiazepines (n = 44) | 23 (52.27%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fellendorf, F.T.; Manchia, M.; Squassina, A.; Pisanu, C.; Dall’Acqua, S.; Sut, S.; Nasini, S.; Congiu, D.; Reininghaus, E.Z.; Garzilli, M.; et al. Is Poor Lithium Response in Individuals with Bipolar Disorder Associated with Increased Degradation of Tryptophan along the Kynurenine Pathway? Results of an Exploratory Study. J. Clin. Med. 2022, 11, 2517. https://doi.org/10.3390/jcm11092517
Fellendorf FT, Manchia M, Squassina A, Pisanu C, Dall’Acqua S, Sut S, Nasini S, Congiu D, Reininghaus EZ, Garzilli M, et al. Is Poor Lithium Response in Individuals with Bipolar Disorder Associated with Increased Degradation of Tryptophan along the Kynurenine Pathway? Results of an Exploratory Study. Journal of Clinical Medicine. 2022; 11(9):2517. https://doi.org/10.3390/jcm11092517
Chicago/Turabian StyleFellendorf, Frederike T., Mirko Manchia, Alessio Squassina, Claudia Pisanu, Stefano Dall’Acqua, Stefania Sut, Sofia Nasini, Donatella Congiu, Eva Z. Reininghaus, Mario Garzilli, and et al. 2022. "Is Poor Lithium Response in Individuals with Bipolar Disorder Associated with Increased Degradation of Tryptophan along the Kynurenine Pathway? Results of an Exploratory Study" Journal of Clinical Medicine 11, no. 9: 2517. https://doi.org/10.3390/jcm11092517
APA StyleFellendorf, F. T., Manchia, M., Squassina, A., Pisanu, C., Dall’Acqua, S., Sut, S., Nasini, S., Congiu, D., Reininghaus, E. Z., Garzilli, M., Guiso, B., Suprani, F., Paribello, P., Pulcinelli, V., Iaselli, M. N., Pinna, I., Somaini, G., Arru, L., Corrias, C., ... Comai, S. (2022). Is Poor Lithium Response in Individuals with Bipolar Disorder Associated with Increased Degradation of Tryptophan along the Kynurenine Pathway? Results of an Exploratory Study. Journal of Clinical Medicine, 11(9), 2517. https://doi.org/10.3390/jcm11092517











