Response to Treatment with Melatonin and Clonazepam versus Placebo in Patients with Burning Mouth Syndrome
Abstract
:1. Introduction
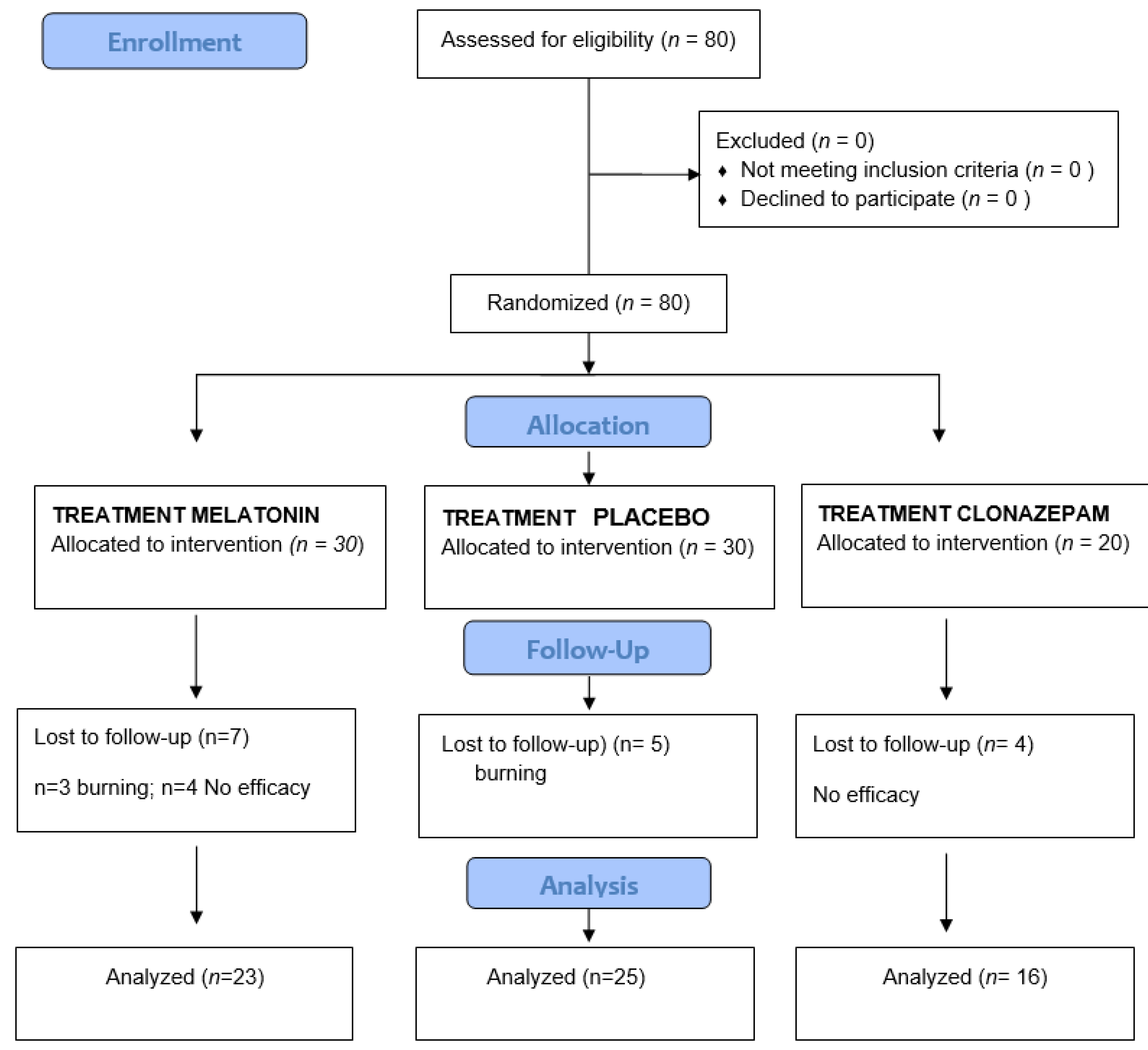
2. Material and Methods
2.1. Study Protocol: Clinical Variables and Data Compilation
- Burning sensation visual analog scale (VAS pain): the patient scores burning sensation on a VAS from 0 to 10, where 0 = no burning sensation and 10 = maximum burning sensation [38].
- Xerostomia score (VAS xeros): the patient scores xerostomia (dry mouth) on a VAS from 0 to 10, where 0 = no xerostomia and 10 = maximum xerostomia.
- Oral Health Impact Profile 14 (OHIP-14)(Spanish version): this questionnaire is composed of 7 dimensions: functional limitation, physical discomfort, psychological discomfort, physical disability, psychological disability, social disability, and handicaps. Each dimension comprises two questions (with a total of 14 questions), and the answers are scored from 0 to 4 (0 = lowest level and 4 = highest level). The higher the score, the poorer the oral quality of life of the patient [39].
- Hospital Anxiety and Depression Scale (HADS): this instrument consists of two subscales that respectively assess anxiety state (HADS-A) and depressive state (HADS-D). Each subscale has 7 items scored from 0 to 3, where a total score of over 10 reflects the presence of anxiety or depression, scores between 8 and 10 are borderline, and a score of under 7 indicates the absence of anxiety or depression [40].
- Pittsburg Sleep Quality Index: this instrument consists of 19 questions divided into 7 sections, with each section addressing a specific characteristic of the patient sleep pattern: subjective quality of sleep, latency of sleep, duration of sleep, usual efficiency of sleep, alterations of sleep, use of medication to sleep, and daytime dysfunction. Each section receives a score of 0–3, where 0 = no problems and 3 = great problems. The final score corresponds to the sum of the scores of the 7 sections, yielding a maximum score of 21 points. A score of 5 or less is indicative of satisfactory quality of sleep, while scores of over 5 are indicative of sleep disorders [41].
- Epworth daytime sleepiness scale: this scale comprises 8 questions that simulate situations in which the patient is asked to score the probability of experiencing sleepiness. The result of the questionnaire is the sum of the individual scores of the 8 questions, and higher scores are indicative of a greater probability of daytime sleepiness [42].
2.2. Sialometry
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jääskeläinen, S.K.; Woda, A. Burning mouth syndrome. Cephalalgia 2017, 37, 627–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scala, A.; Checchi, L.; Montevecchi, M.; Marini, I.; Giamberardino, M.A. Update on Burning Mouth Syndrome: Overview and Patient Management. Crit. Rev. Oral Biol. Med. 2003, 14, 275–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohorst, J.J.; Bruce, A.J.; Torgerson, R.R.; Schenck, L.A.; Davis, M.D.P. The prevalence of burning mouth syndrome: A population-based study. Br. J. Dermatol. 2015, 172, 1654–1656. [Google Scholar] [CrossRef] [Green Version]
- Orofacial Pain Classification Committee. International Classification of Orofacial Pain, 1st edition (ICOP). Cephalalgia 2020, 40, 129–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forssell, H.; Teerijoki-Oksa, T.; Kotiranta, U.; Kantola, R.; Bäck, M.; Vuorjoki-Ranta, T.-R.; Siponen, M.; Leino, A.; Puukka, P.; Estlander, A.-M. Pain and pain behavior in burning mouth syndrome: A pain diary study. J. Orofac. Pain 2012, 26, 117–125. [Google Scholar] [PubMed]
- Schiavone, V.; Adamo, D.; Ventrella, G.; Morlino, M.; De Notaris, E.B.; Ravel, M.G.; Kusmann, F.; Piantadosi, M.; Pollio, A.; Fortuna, G.; et al. Anxiety, Depression, and Pain in Burning Mouth Syndrome: First Chicken or Egg? Headache 2012, 52, 1019–1025. [Google Scholar] [CrossRef]
- Silvestre, F.J.; Silvestre-Rangil, J.; López-Jornet, P. Burning mouth syndrome: A review and update. Rev. Neurol. 2015, 60, 457–463. [Google Scholar]
- Tan, H.L.; Smith, J.G.; Hoffmann, J.; Renton, T. A systematic review of treatment for patients with burning mouth syndrome. Cephalalgia 2022, 42, 128–161. [Google Scholar] [CrossRef]
- Klasser, G.D.; Fischer, D.J.; Epstein, J.B. Burning Mouth Syndrome: Recognition, Understanding, and Management. Oral Maxillofac. Surg. Clin. N. Am. 2008, 20, 255–271. [Google Scholar] [CrossRef]
- López-Jornet, P.; Camacho-Alonso, F.; Andujar-Mateos, P.; Sanchez-Siles, M.; Gomez-Garcia, F. Burning mouth syndrome: An update. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e562–e568. [Google Scholar] [CrossRef]
- Ariyawardana, A.; Chmieliauskaite, M.; Farag, A.M.; Albuquerque, R.; Forssell, H.; Nasri-Heir, C.; Klasser, G.D.; Sardella, A.; Mignogna, M.D.; Ingram, M.; et al. World Workshop on Oral Medicine VII: Burning mouth syndrome: A systematic review of disease definitions and diagnostic criteria utilized in randomized clinical trials. Oral Dis. 2019, 25, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jornet, P.; Felipe, C.C.; Pardo-Marin, L.; Ceron, J.J.; Pons-Fuster, E.; Tvarijonaviciute, A. Salivary Biomarkers and Their Correlation with Pain and Stress in Patients with Burning Mouth Syndrome. J. Clin. Med. 2020, 9, 929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-I.; Kim, Y.-Y.; Chang, J.-Y.; Ko, J.-Y.; Kho, H.-S. Salivary cortisol, 17β-estradiol, progesterone, dehydroepiandrosterone, and α-amylase in patients with burning mouth syndrome. Oral Dis. 2012, 18, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Carreño-Hernández, I.; Cassol-Spanemberg, J.; Rodríguez de Rivera-Campillo, E.; Estrugo-Devesa, A.; López-López, J. Is Burning Mouth Syndrome a Neuropathic Pain Disorder? A Systematic Review. J. Oral Facial Pain Headache 2021, 35, 218–229. [Google Scholar] [CrossRef]
- Lee, G.S.; Kim, H.K.; Kim, M.E. Relevance of sleep, pain cognition, and psychological distress with regard to pain in patients with burning mouth syndrome. Cranio 2022, 40, 79–87. [Google Scholar] [CrossRef]
- Ritchie, A.; Kramer, J. Recent Advances in the Etiology and Treatment of Burning Mouth Syndrome. J. Dent. Res. 2018, 97, 1193–1199. [Google Scholar] [CrossRef]
- Lee, Y.C.; Hong, I.K.; Na, S.Y.; Eun, Y.G. Evaluation of salivary function in patients with burning mouth syndrome. Oral Dis. 2015, 21, 308–313. [Google Scholar] [CrossRef]
- Franco-Martínez, L.; Tecles, F.; Torres-Cantero, A.; Bernal, E.; San Lázaro, I.; Alcaraz, M.J.; Vicente-Romero, M.R.; Lamy, E.; Sánchez-Resalt, C.; Rubio, C.P.; et al. Analytical validation of an automated assay for the measurement of adenosine deaminase (ADA) and its isoenzymes in saliva and a pilot evaluation of their changes in patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2021, 59, 1592–1599. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Chaisiwamongkhol, K.; Batchelor-Mcauley, C.; Compton, R.G. Chemical analysis in saliva and the search for salivary biomarkers—A tutorial review. Analyst 2018, 143, 81–99. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Zamora, C.; Martinez-Subiela, S.; Tecles, F.; Pina, F.; Lopez-Jornet, P. Salivary adiponectin, but not adenosine deaminase, correlates with clinical signs in women with Sjögren’s syndrome: A pilot study. Clin. Oral Investig. 2019, 23, 1407–1414. [Google Scholar] [CrossRef]
- Orbach, H.; Zandman-Goddard, G.; Amital, H.; Barak, V.; Szekanecz, Z.; Szucs, G.; Danko, K.; Nagy, E.; Csepany, T.; Carvalho, J.F.; et al. Novel Biomarkers in Autoimmune Diseases: Prolactin, Ferritin, Vitamin D, and TPA Levels in Autoimmune Diseases. Ann. N. Y. Acad. Sci. 2007, 1109, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Mera, J.C.; Molano, M.A.; López, C.C.; Triana, C.A.; Cotrina, J.M. Discussions and perspectives regarding oxytocin as a biomarker in human investigations. Heliyon 2021, 7, e08289. [Google Scholar] [CrossRef] [PubMed]
- Franco-Martínez, L.; Tvarijonaviciute, A.; Martínez-Subiela, S.; Márquez, G.; Martínez Díaz, N.; Cugat, R.; Cerón, J.J.; Jiménez-Reyes, P. Changes in lactate, ferritin, and uric acid in saliva after repeated explosive effort sequences. J. Sports Med. Phys. Fit. 2019, 59, 902–909. [Google Scholar] [CrossRef]
- Nater, U.M.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Lokesh Sundaram, B.; Rathnavelu, V.; Sabesan, M.; Ganesh, A.; Anandan, S. A Study to Assess the Levels of Salivary Ferritin in Iron Deficiency Anemia Subjects and Healthy Subjects. Cureus 2021, 13, e17241. [Google Scholar] [CrossRef]
- McMillan, R.; Forssell, H.; Buchanan, J.A.; Glenny, A.M.; Weldon, J.C.; Zakrzewska, J.M. Interventions for treating burning mouth syndrome. Cochrane Database Syst. Rev. 2016, 11, CD002779. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Xu, H.; Chen, F.M.; Liu, J.L.; Jiang, L.; Zhou, Y.; Chen, Q.M. Efficacy evaluation of clonazepam for symptom remission in burning mouth syndrome: A meta-analysis. Oral Dis. 2016, 22, 503–511. [Google Scholar] [CrossRef]
- Liu, Y.F.; Kim, Y.; Yoo, T.; Han, P.; Inman, J.C. Burning mouth syndrome: A systematic review of treatments. Oral Dis. 2018, 24, 325–334. [Google Scholar] [CrossRef]
- Arduino, P.G.; Cafaro, A.; Garrone, M.; Gambino, A.; Cabras, M.; Romagnoli, E.; Broccoletti, R. A randomized pilot study to assess the safety and the value of low-level laser therapy versus clonazepam in patients with burning mouth syndrome. Lasers Med. Sci. 2016, 31, 811–816. [Google Scholar] [CrossRef]
- Varoni, E.M.; Soru, C.; Pluchino, R.; Intra, C.; Iriti, M. The Impact of Melatonin in Research. Molecules 2016, 21, 240. [Google Scholar] [CrossRef] [Green Version]
- Abdel Moneim, A.E.; Guerra-Librero, A.; Florido, J.; Shen, Y.Q.; Fernández-Gil, B.; Acuña-Castroviejo, D.; Escames, G. Oral Mucositis: Melatonin Gel an Effective New Treatment. Int. J. Mol. Sci. 2017, 18, 1003. [Google Scholar] [CrossRef]
- Carpentieri, A.R.; Peralta Lopez, M.E.; Aguilar, J.; Solá, V.M. Melatonin and periodontal tissues: Molecular and clinical perspectives. Pharmacol. Res. 2017, 125, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Elsabagh, H.H.; Moussa, E.; Mahmoud, S.A.; Elsaka, R.O.; Abdelrahman, H. Efficacy of Melatonin in prevention of radiation-induced oral mucositis: A randomized clinical trial. Oral Dis. 2020, 26, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.A.; Liu, X.Y.; Acuña-Castroviejo, D.; Escames, G.; Tan, D.-X. Melatonin in the oral cavity: Physiological and pathological implications. J. Periodontal Res. 2015, 50, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Permuy, M.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz, F. Melatonin: A Review of Its Potential Functions and Effects on Dental Diseases. Int. J. Mol. Sci. 2017, 18, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuthati, Y.; Lin, S.; Chen, I.; Wong, C. Melatonin and their analogs as a potential use in the management of Neuropathic pain. J. Formos. Med Assoc. 2018, 118, 1177–1186. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lo Faro, A.F.; Lodi, G.; Carrassi, A.; Iriti, M.; Sardella, A. Melatonin Treatment in Patients with Burning Mouth Syndrome: A Triple-Blind, Placebo-Controlled, Crossover Randomized Clinical Trial. J. Oral Facial Pain Headache 2018, 32, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, A.M. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 1983, 16, 87–101. [Google Scholar] [CrossRef]
- Montero-Martín, J.; Bravo-Pérez, M.; Albaladejo-Martínez, A.; Hernández-Martín, L.A. Validation the Oral Health Impact Profile (OHIP-14sp) for adults in Spain. Med. Oral Patol. Oral Cir. Bucal 2009, 14, 44–50. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Patton, L.L.; Siegel, M.A.; Benoliel, R.; De Laat, A. Management of burning mouth syndrome: Systematic review and management recommendations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 103, S39.e1–S39.e13. [Google Scholar] [CrossRef] [PubMed]
- Kuten-Shorrer, M.; Treister, N.S.; Stock, S.; Kelley, J.M.; Ji, Y.D.; Woo, S.B.; Lerman, M.A.; Palmason, S.; Sonis, S.T.; Villa, A. Topical Clonazepam Solution for the Management of Burning Mouth Syndrome: A Retrospective Study. J. Oral Facial Pain Headache 2017, 31, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vecchierini, M.F.; Kilic-Huck, U.; Quera-Salva, M.A. Melatonin (MEL) and its use in neurological diseases and insomnia: Rec-ommendations of the French Medical and Research Sleep Society (SFRMS). Rev. Neurol. 2021, 177, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Rossella, I.; Alessandro, V.; Naman, R.; Gary, K.; Hervé, S.Y. Topical clonazepam for burning mouth syndrome: Is it efficacious in patients with anxiety or depression? J. Oral Rehabil. 2022, 49, 54–61. [Google Scholar] [CrossRef]
- Kuten-Shorrer, M.; Kelley, J.M.; Sonis, S.T.; Treister, N.S. Placebo effect in burning mouth syndrome: A systematic review. Oral Dis. 2014, 20, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.B.; Lentino, C.V.; Boyd, C.C.; O’connell, M.L.; Crawford, C.C.; Sprengel, M.L.; Deuster, P.A. The effectiveness of melatonin for promoting healthy sleep: A rapid evidence assessment of the literature. Nutr. J. 2014, 13, 106. [Google Scholar] [CrossRef] [Green Version]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; Manzano-Moreno, F.J.; Ruiz, C.; Illescas-Montes, R. Salivary Biomarkers and Their Application in the Diagnosis and Monitoring of the Most Common Oral Pathologies. Int. J. Mol. Sci. 2020, 21, 5173. [Google Scholar] [CrossRef]
- Nagler, R.M.; Hershkovich, O. Sialochemical and gustatory analysis in patients with oral sensory complaints. J. Pain 2004, 5, 56–63. [Google Scholar] [CrossRef]
- Imura, H.; Shimada, M.; Yamazaki, Y.; Sugimoto, K. Characteristic changes of saliva and taste in burning mouth syndrome pa-tients. J. Oral Pathol. Med. 2016, 45, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Tartar, J.L.; Hiffernan, F.S.; Freitas, K.E.; Fins, A.I.; Banks, J.B. A Functional Adenosine Deaminase Polymorphism Associates with Evening Melatonin Levels and Sleep Quality. J. Circadian Rhythm. 2021, 19, 5. [Google Scholar] [CrossRef] [PubMed]
| Melatonin | Clonazepam | Placebo | p | |
|---|---|---|---|---|
| Gender (%) FemaleMale | 19 (82.61) 4 (17.39) | 15 (92) 1 (6.25) | 23 (92) 2 (8) | p > 0.05 |
| Age (mean;SD) | 57.68 (10.32) | 63.81 (10.69) | 60.40 (13.8) | p > 0.05 |
| Smoking n (%) Smoking No Smoking Ex Smoking | 6 (26.1) 13 (56.5) 4 (17.4) | 3 (18.8) 13 (81.3) 0 (0) | 4 (16) 17 (68) 4 (16) | p > 0.05 |
| Alcohol n (%) less than once a week Once a day weekends | 11 (47.8) 6 (26.1) 6 (26.1) | 15 (93.7) 0 (0) 1 (6.3) | 20 (80) 2 (8) 3 (12) | p < 0.05 |
| Oral hygiene (brushing) n (%) No Once a day Twice a day Three times a day | 0 (0) 13 (59.9) 0(0) 9 (39.1) | 1 (6.3) 7 (43.8) 1 (6.3) 7 (43.8) | 1(4) 10 (40) 1 (4) 13 (52) | p > 0.05 |
| Melatonin | p | Placebo | p | Clonazepam | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | ||||
| Oxytocin | 804.4 (562–1902) | 1581 (544.6–2128) | 0.339 | 1417 (907.6–2491) | 1627 (768.4–2388) | 0.953 | 804.4 (562–3065) | 1581 (544.6–2467) | 0.910 |
| ADA | 3.1 (0.75–5.7) | 2.8 (0.75–6.575) | 0.718 | 3.8 (1.3–11.1) | 3.7 (1.4–11.5) | 0.992 | 3.1 (0.75–7.525) | 2.8 (0.75–5.65) | 0.945 |
| Ferritin | 6$(2–13.78) | 5.75 (3.075–22.18) | 0.266 | 9 (4.25–12.33) | 8.45 (2.925–19.7) | 0.252 | 6 (2–22.63) | 5.75 (3.075–17.55) | 0.219 |
| SAA (U/L) | 198,760 (132,680–368,600) | 196,720 (111,080–359,160) | 0.266 | 181,520 (97,400–364,520) | 166,960 (81,680–232,920) | 0.891 | 198,760 (132,680–395,180) | 196,720 (111,080–382,800) | 0.910 |
| PT | 121.9 (78.35–208.4) | 149.6 (101.2–222.1) | 0.043 | 185.9 (99.38–309.1) | 130 (90.16–332.2) | 0.679 | 121.9 (78.35–183.2) | 149.6 (101.2–217.5) | 0.250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Felipe, C.; Tvarijonaviciute, A.; López-Arjona, M.; Pardo-Marin, L.; Pons-Fuster, E.; López-Jornet, P. Response to Treatment with Melatonin and Clonazepam versus Placebo in Patients with Burning Mouth Syndrome. J. Clin. Med. 2022, 11, 2516. https://doi.org/10.3390/jcm11092516
Castillo-Felipe C, Tvarijonaviciute A, López-Arjona M, Pardo-Marin L, Pons-Fuster E, López-Jornet P. Response to Treatment with Melatonin and Clonazepam versus Placebo in Patients with Burning Mouth Syndrome. Journal of Clinical Medicine. 2022; 11(9):2516. https://doi.org/10.3390/jcm11092516
Chicago/Turabian StyleCastillo-Felipe, Candela, Asta Tvarijonaviciute, Marina López-Arjona, Luis Pardo-Marin, Eduardo Pons-Fuster, and Pia López-Jornet. 2022. "Response to Treatment with Melatonin and Clonazepam versus Placebo in Patients with Burning Mouth Syndrome" Journal of Clinical Medicine 11, no. 9: 2516. https://doi.org/10.3390/jcm11092516
APA StyleCastillo-Felipe, C., Tvarijonaviciute, A., López-Arjona, M., Pardo-Marin, L., Pons-Fuster, E., & López-Jornet, P. (2022). Response to Treatment with Melatonin and Clonazepam versus Placebo in Patients with Burning Mouth Syndrome. Journal of Clinical Medicine, 11(9), 2516. https://doi.org/10.3390/jcm11092516







