Intestinal Permeability and Depression in Patients with Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Questionnaires Used
2.3. Sample Collection and Laboratory Analysis
2.4. Statistical Analysis
3. Results
3.1. Socioeconomic Conditions of the Studied Group
3.2. Central Tendency and Dispersion in Patients’ Health Score, Quality of Life and Biomarkers
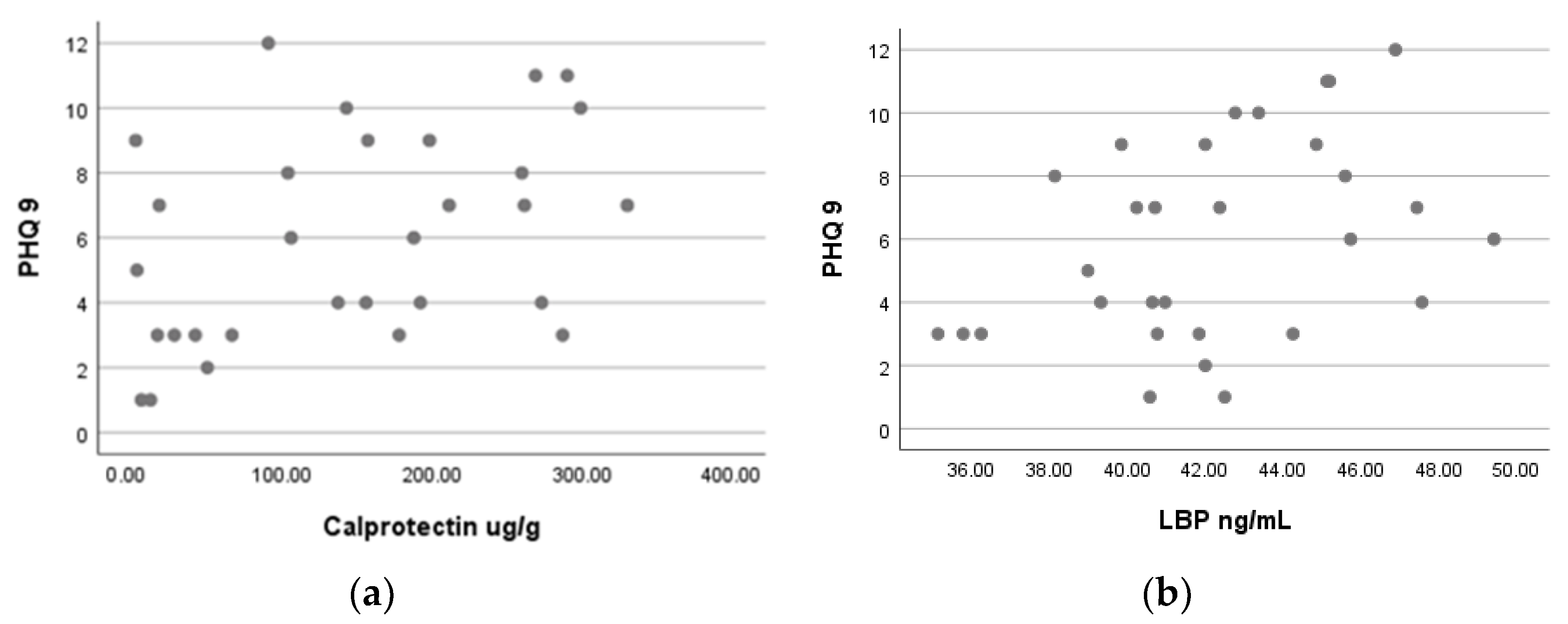
3.3. Correlations between Patients’ Health Score and Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, T.; Lloyd, C.E. Epidemiology of depression and diabetes: A systematic review. J. Affect. Disord. 2012, 142, S8–S21. [Google Scholar] [CrossRef]
- Hare, D.L.; Toukhsati, S.R.; Johansson, P.; Jaarsma, T. Depression and cardiovascular disease: A clinical review. Eur. Heart J. 2014, 35, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Global Health Data Exchange Website. Global Burden of Disease Study 2019 (GBD 2019) Results. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 20 May 2022).
- Khan, A.; Faucett, J.; Lichtenberg, P.; Kirsch, I.; Brown, W.A. A systematic review of comparative efficacy of treatments and controls for depression. PLoS ONE 2012, 7, e41778. [Google Scholar] [CrossRef] [PubMed]
- Nobis, A.; Zalewski, D.; Waszkiewicz, N. Peripheral markers of depression. J. Clin. Med. 2020, 9, 3793. [Google Scholar] [CrossRef] [PubMed]
- Waszkiewicz, N. Ordering knowledge in the markers of psychiatric/mental disorders. J. Clin. Med. 2022, 11, 284. [Google Scholar] [CrossRef]
- Karyotaki, E.; Efthimiou, O.; Miguel, C.; Bermpohl, F.M.G.; Furukawa, T.A.; Cuijpers, P.; Riper, H.; Patel, V.; Mira, A.; Gemmil, A.W.; et al. Internet-based cognitive behavioral therapy for depression: A systematic review and individual patient data network meta-analysis. JAMA Psychiatry 2021, 78, 361–371. [Google Scholar] [CrossRef]
- American Psychiatric Association. DSM-5, Diagnostic Manual and Statistical Classification of Mental Disorders; American Psychiatric Publishing: Washington, DC, USA, 2016. [Google Scholar]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Skonieczna-Żydecka, K.; Marlicz, W.; Misera, A.; Koulaouzidis, A.; Łoniewski, I. Microbiome—The missing link in the gut-brain axis: Focus on its role in gastrointestinal and mental health. J. Clin. Med. 2018, 7, 521. [Google Scholar] [CrossRef]
- Blaga, T.S.; Dumitrascu, D.L.; Pop, A.V.; Grad, S. The interactions between gut and brain in gastrointestinal disorders. In The Complex Interplay Between Gut-Brain, Gut-Liver, and Liver-Brain Axes, 1st ed.; Stasi, C., Ed.; Academic Press: Milano, Italy, 2021; pp. 17–47. [Google Scholar] [CrossRef]
- Vanuytsel, T.; Tack, J.; Farre, R. The role of intestinal permeability in gastrointestinal disorders and current methods of evaluation. Front. Nutr. 2021, 8, 717925. [Google Scholar] [CrossRef]
- Bonaz, B.; Picq, C.; Sinniger, V.; Mayol, J.F.; Clarençon, D. Vagus nerve stimulation: From epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol. Motil. 2013, 25, 208–221. [Google Scholar] [CrossRef]
- Borkent, J.; Ioannou, M.; Laman, J.D.; Haarman, B.C.; Sommer, I.E. Role of the gut microbiome in three major psychiatric disorders. Psychol. Med. 2022, 52, 1222–1242. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Seethaler, B.; Basrai, M.; Neyrinck, A.M.; Nazare, J.A. Biomarkers for assessment of intestinal permeability in clinical practice. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 21, G11–G17. [Google Scholar] [CrossRef]
- Vaos, G.; Kostakis, I.D. The role of calprotectin in pediatric disease. BioMed Res. Int. 2013, 2013, 542363. [Google Scholar] [CrossRef]
- Bjarnason, I. The use of fecal calprotectin in inflammatory bowel disease. Gastroenterol. Hepatol. 2017, 13, 53–56. [Google Scholar]
- Bertani, B.; Ruiz, N. Function and biogenesis of lipopolysaccharides. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef]
- Jang, J.H.; Shin, H.W.; Lee, J.M.; Lee, H.W.; Kim, E.C.; Park, S.H. An overview of pathogen recognition receptors for innate immunity in dental pulp. Mediat. Inflamm. 2015, 2015, 794143. [Google Scholar] [CrossRef]
- Marshall, J.C. Endotoxin in the pathogenesis of sepsis. Endotoxemia Endotoxin Shock 2010, 167, 1–13. [Google Scholar] [CrossRef]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000, 24, 4435–4440. [Google Scholar] [CrossRef]
- Fasano, A. Intestinal permeability and its regulation by zonulin: Diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. 2012, 10, 1096–1100. [Google Scholar] [CrossRef]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; De Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef] [PubMed]
- Malíčková, K.; Francová, I.; Lukáš, M.; Kolář, M.; Králíková, E.; Bortlík, M.; Ďuricová, D.; Štěpánková, L.; Zvolská, K.; Pánková, A.; et al. Fecal zonulin is elevated in Crohn’s disease and in cigarette smokers. Pract. Lab. Med. 2017, 9, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Hotamisligi, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef]
- Ohlsson, L.; Gustafsson, A.; Lavant, E.; Suneson, K.; Brundin, L.; Westrin, Å.; Ljunggren, L.; Lindqvist, D. Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr. Scand. 2019, 139, 185–193. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Group, T.E. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Brooks, R. The EuroQol Group after 25 Years; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar] [CrossRef]
- Alam, R.; Abdolmaleky, H.M.; Zhou, J.R. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 651–660. [Google Scholar] [CrossRef]
- Santosa, C.H.; Galindo, A.C.A.; Silva, B.A.D.; Dantas, C.R.; Guilherme, I.A.D.A.; Gomes, J.E.B.N.; Dallacqua, K.M.; Souza, M.P.D.; Senna, P.S. Comparative analysis of anxiety and depression prevalence between individuals with and without inflammatory bowel disease. J. Coloproctol. 2020, 40, 339–344. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef]
- Moulton, C.D.; Pavlidis, P.; Norton, C.; Norton, S.; Pariante, C.; Hayee, B.; Powell, N. Depressive symptoms in inflammatory bowel disease: An extraintestinal manifestation of inflammation? Clin. Exp. Immunol. 2019, 197, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Frech, M.; Tajik, N.; Frech, M.; Schulz, O.; Schälter, F.; Lucas, S.; Azizov, V.; Dürholz, K.; Steffen, F.; et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020, 11, 1995. [Google Scholar] [CrossRef] [PubMed]
- Maget, A.; Dalkner, N.; Hamm, C.; Bengesser, S.A.; Fellendorf, F.T.; Platzer, M.; Queissner, R.; Birner, A.; Lenger, M.; Mörkl, S.; et al. Sex differences in zonulin in affective disorders and associations with current mood symptoms. J. Affect. Disord. 2021, 294, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, F.; Işık, Ü.; Demirdaş, A.; Doğuç, D.K.; Bozkurt, M. Serum zonulin and claudin-5 levels in patients with bipolar disorder. J. Affect. Disord. 2020, 266, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Asbjornsdottir, B.; Snorradottir, H.; Andresdottir, E.; Fasano, A.; Lauth, B.; Gudmundsson, L.S.; Gottfredsson, M.; Halldorsson, T.I.; Birgisdottir, B.E. Zonulin-dependent intestinal permeability in children diagnosed with mental disorders: A systematic review and meta-analysis. Nutrients 2020, 12, 1982. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, G.J.; Gao, Q.; Li, N.; Wang, R.T. C-type lectin-like receptor 2 and zonulin are associated with mild cognitive impairment and Alzheimer’s disease. Acta Neurol. Scand. 2020, 141, 250–255. [Google Scholar] [CrossRef]
- Liśkiewicz, P.; Kaczmarczyk, M.; Misiak, B.; Wroński, M.; Bąba-Kubiś, A.; Skonieczna-Żydecka, K.; Marlicz, W.; Bieńkowski, P.; Misera, A.; Pełka-Wysiecka, J.; et al. Analysis of gut microbiota and intestinal integrity markers of inpatients with major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 11007. [Google Scholar] [CrossRef]
- Kuzma, J.; Chmelař, D.; Hájek, M.; Lochmanová, A.; Čižnár, I.; Rozložník, M.; Klugar, M. The role of intestinal microbiota in the pathogenesis of colorectal carcinoma. Folia Microbiol. 2020, 65, 17–24. [Google Scholar] [CrossRef]
- Meng, L.; Song, Z.; Liu, A.; Dahmen, U.; Yang, X.; Fang, H. Effects of lipopolysaccharide-binding protein (LBP) single nucleotide polymorphism (SNP) in infections, inflammatory diseases, metabolic disorders and cancers. Front. Immunol. 2021, 12, 2469. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of metabolic endotoxemia in systemic inflammation and potential interventions. Front. Immunol. 2021, 11, 594150. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; de Andrade, N.Q.; Morris, G.; Fernandes, B.S.; Brunoni, A.R.; et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: Systematic review and meta-analysis. Mol. Neurobiol. 2018, 55, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.L.; Majd, M.; Graham-Engeland, J.E.; Smyth, J.M.; Sliwinski, M.J.; Engeland, C.G. Gender differences in the link between depressive symptoms and ex vivo inflammatory responses are associated with markers of endotoxemia. Brain Behav. Immun. Health 2020, 2, 100013. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Regulation of tight intercellular junctions through the zonula ccludes toxin and eukaryotic analogous zonulin. Ann. N. Y. Acad. Sci. 2000, 915, 214–222. [Google Scholar] [CrossRef]
- Ihling, C.H.; Stumvoll, M.; Freire, R.; Fiorentino, M.; Fasano, A.; Kovacs, P. Widely used commercial ELISA does not detect precursor of haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front. Endocrinol. 2018, 9, 22. [Google Scholar] [CrossRef]
- Massier, L.; Chakaroun, R.; Kovacs, P.; Heiker, J.T. Blurring the picture in leaky gut research: How shortcomings of zonulin as a biomarker mislead the field of intestinal permeability. Gut 2021, 70, 1801–1802. [Google Scholar] [CrossRef]
| Parameter | Response | n | Percent (%) |
|---|---|---|---|
| Age (years) * | 20–30 | 5 | 16.67 |
| 30–40 | 6 | 20 | |
| 40–50 | 8 | 26.67 | |
| 50–60 | 7 | 23.33 | |
| 60–70 | 2 | 6.67 | |
| 70–80 | 2 | 6.67 | |
| Sex | Masculine | 15 | 50 |
| Feminine | 15 | 50 | |
| Origin | Urban | 23 | 76.67 |
| Rural | 7 | 23.33 | |
| Marital status | Single | 10 | 33.33 |
| Married | 20 | 66.67 | |
| Education level | Secondary school | 6 | 20 |
| Faculty | 17 | 56.67 | |
| Others | 7 | 23.33 | |
| Occupational status | Full-time employee | 15 | 50 |
| Part-time employee | 2 | 6.67 | |
| Pensioner | 7 | 23.33 | |
| With disabilities | 2 | 6.67 | |
| Others | 4 | 13.33 | |
| Diagnostic | Crohn’s disease | 12 | 40 |
| Ulcerative colitis | 18 | 60 | |
| Smoking status | Smoker | 8 | 26.67 |
| Former smoker | 12 | 40 | |
| Nonsmoker | 10 | 33.33 | |
| Alcohol consumption | Of | 10 | 33.33 |
| Right away | 20 | 66.67 | |
| Physical activity ** | 6–7x/W | 11 | 36.67 |
| 3–4x/S | 5 | 16.67 | |
| 1–2x/S | 8 | 26.67 | |
| Not at all | 6 | 20 | |
| Compliance with the diet | Easy | 9 | 30 |
| Difficult | 21 | 70 |
| Variable | Median | Min | Max | Range | IQR |
|---|---|---|---|---|---|
| PHQ-9 * | 6.50 | 0.00 | 16.00 | 16.00 | 6.00 |
| EQ-5D | 80.00 | 45.00 | 100.00 | 55.00 | 20.00 |
| Calprotectin (ug/g) | 149.64 | 2.80 | 330.21 | 327.41 | 221.42 |
| LBP (ng/mL) | 42.00 | 35.14 | 49.41 | 14.27 | 4.99 |
| Zonulin (ng/mL) | 32.94 | 19.23 | 37.84 | 18.61 | 3.36 |
| IFABP/FABP2 (ng/mL) | 0.87 | 0.35 | 3.00 | 2.65 | 0.65 |
| n | rho | p (2-Tailed) | |
|---|---|---|---|
| Calprotectin (ug/g) | 30 | 0.416 | 0.022 |
| LBP * (ng/mL) | 30 | 0.398 | 0.029 |
| Zonulin (ng/mL) | 30 | 0.016 | 0.934 |
| IFABP/FABP2 (ng/mL) | 30 | −0.059 | 0.755 |
| EQ-5D | 30 | −0.372 | 0.043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iordache, M.M.; Tocia, C.; Aschie, M.; Dumitru, A.; Manea, M.; Cozaru, G.C.; Petcu, L.; Vlad, S.E.; Dumitru, E.; Chisoi, A. Intestinal Permeability and Depression in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2022, 11, 5121. https://doi.org/10.3390/jcm11175121
Iordache MM, Tocia C, Aschie M, Dumitru A, Manea M, Cozaru GC, Petcu L, Vlad SE, Dumitru E, Chisoi A. Intestinal Permeability and Depression in Patients with Inflammatory Bowel Disease. Journal of Clinical Medicine. 2022; 11(17):5121. https://doi.org/10.3390/jcm11175121
Chicago/Turabian StyleIordache, Miorita Melina, Cristina Tocia, Mariana Aschie, Andrei Dumitru, Mihaela Manea, Georgeta Camelia Cozaru, Lucian Petcu, Sabina E. Vlad, Eugen Dumitru, and Anca Chisoi. 2022. "Intestinal Permeability and Depression in Patients with Inflammatory Bowel Disease" Journal of Clinical Medicine 11, no. 17: 5121. https://doi.org/10.3390/jcm11175121
APA StyleIordache, M. M., Tocia, C., Aschie, M., Dumitru, A., Manea, M., Cozaru, G. C., Petcu, L., Vlad, S. E., Dumitru, E., & Chisoi, A. (2022). Intestinal Permeability and Depression in Patients with Inflammatory Bowel Disease. Journal of Clinical Medicine, 11(17), 5121. https://doi.org/10.3390/jcm11175121







